It Takes Two to Tango: Potential Prognostic Impact of Circulating TGF-Beta and PD-L1 in Pancreatic Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Detection of Soluble PD-L1 (sPD-L1) and Soluble TGF-Beta (sTGF-Beta) Levels
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
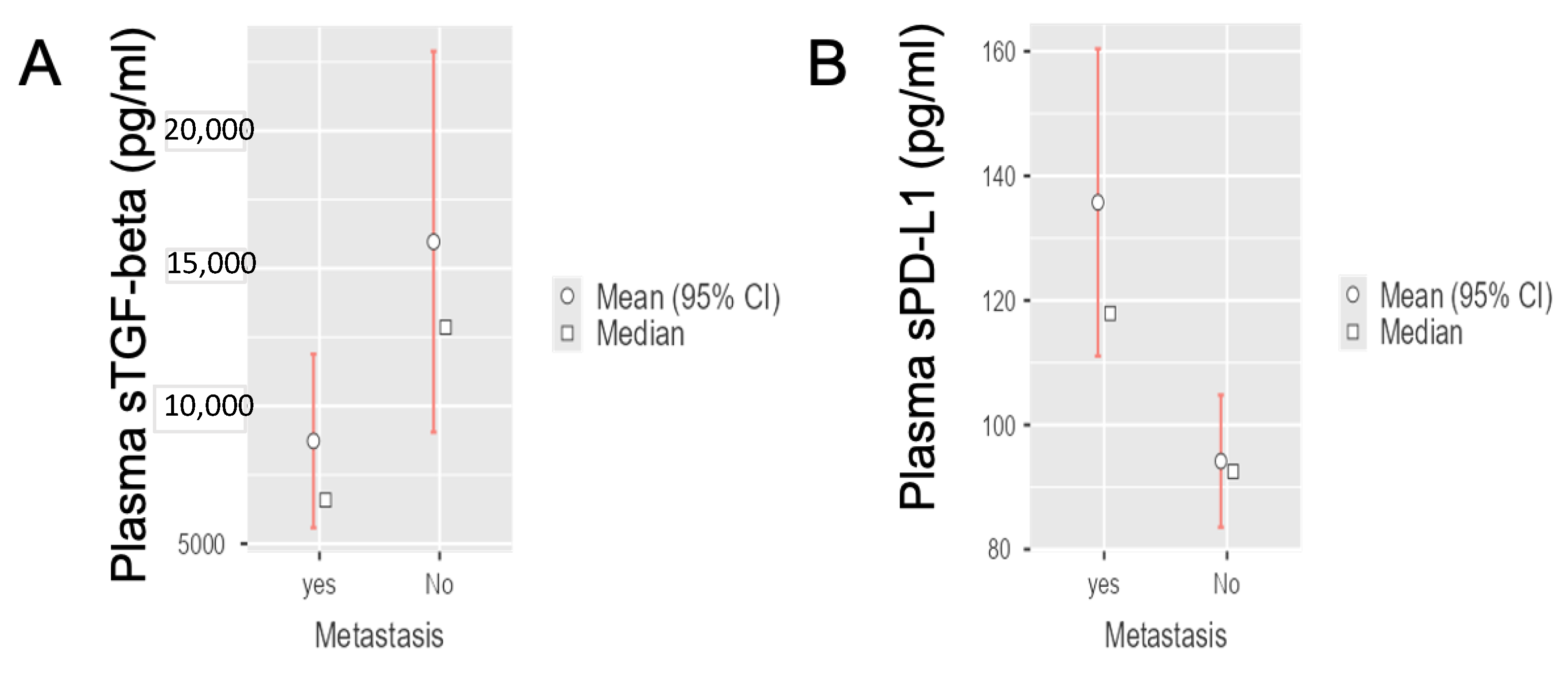
3.2. Circulating sTGF-Beta and sPD-L1 Levels in Radically-Resected and Metastatic PDAC Patients
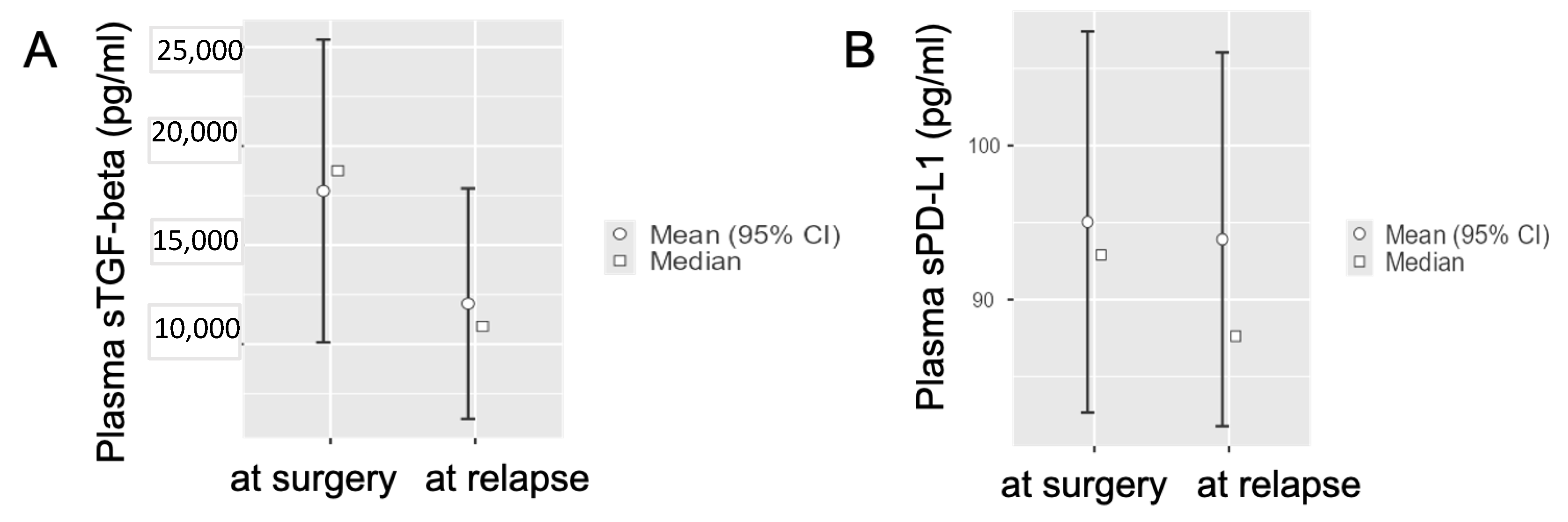
3.3. Circulating TGF-Beta and PD-L1 Levels in Radically-Resected PDAC Patients at Disease Relapse
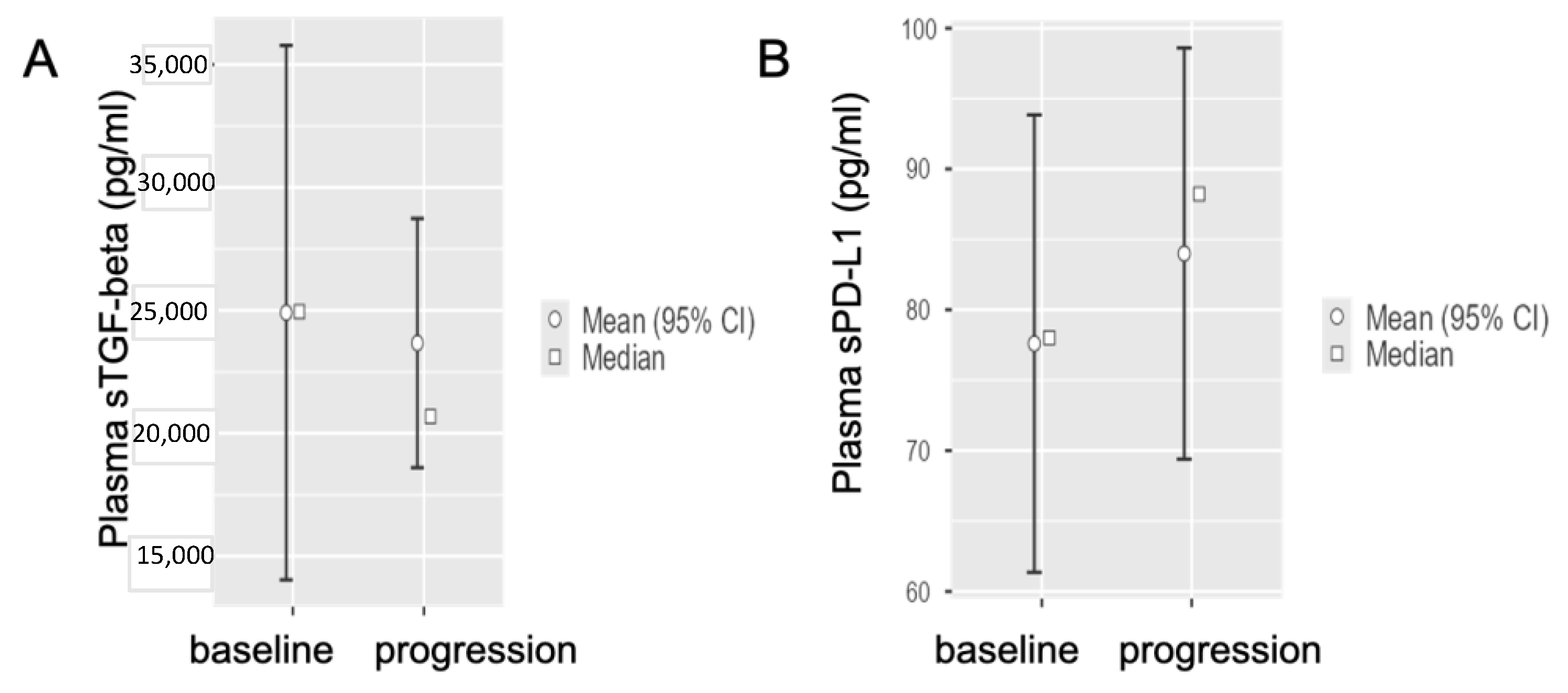
3.4. Circulating sTGF-Beta and sPD-L1 Baseline Levels in Metastatic PDAC Patients at Disease Progression after First-Line Chemotherapy
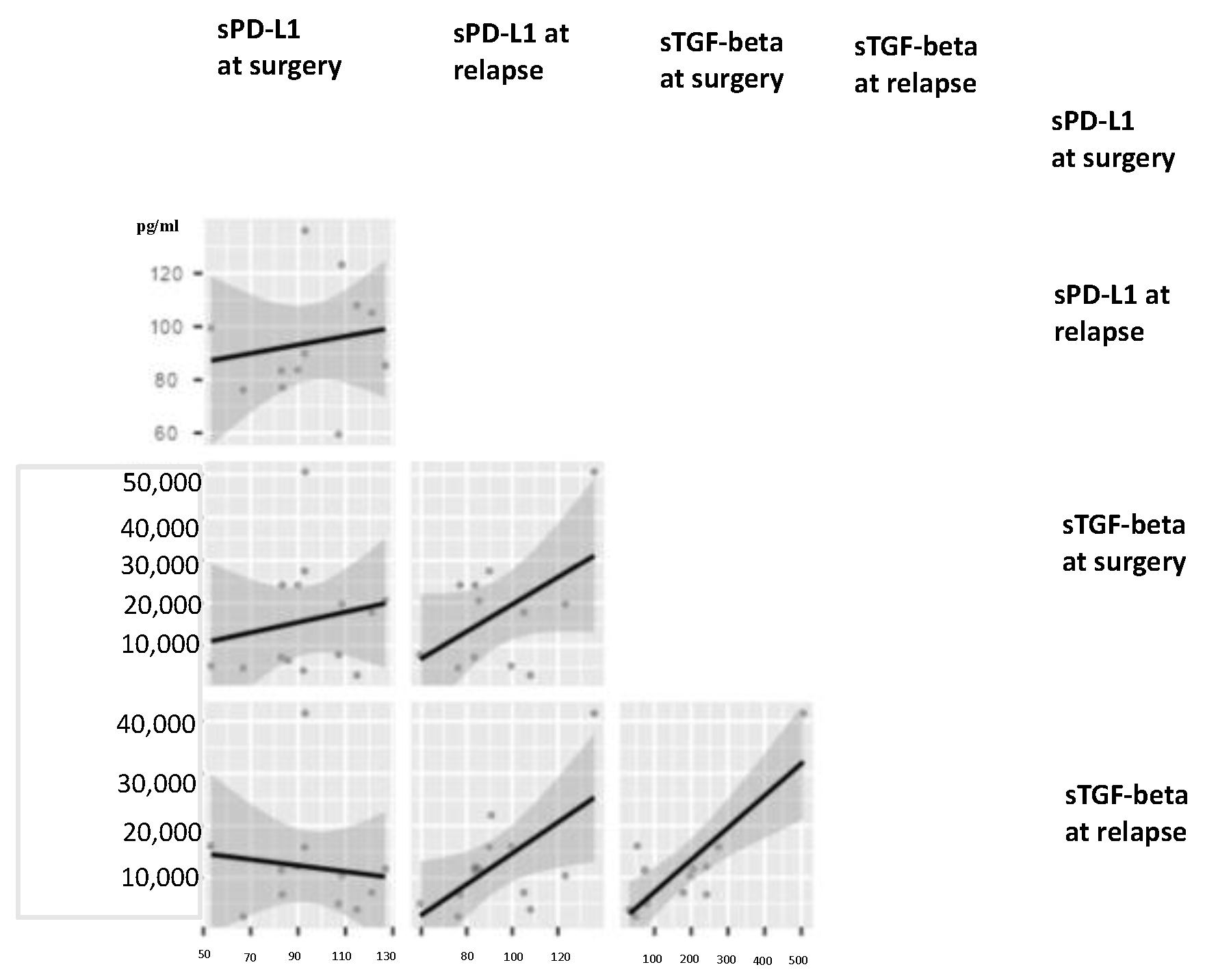
3.5. Circulating TGF-Beta and PD-L1 and Proportions of Different Immune Cells
3.6. Circulating TGF-Beta and PD-L1 and Clinical Outcome
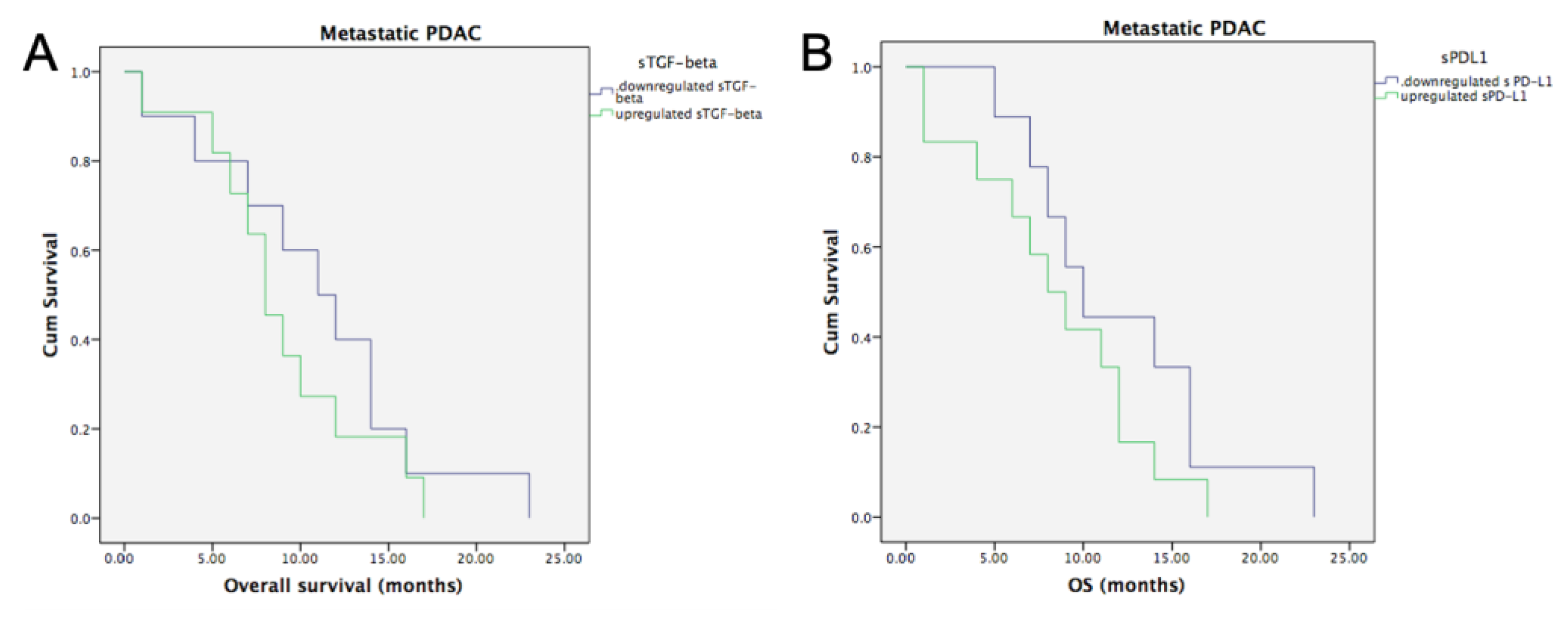
3.6.1. Metastatic PDAC and Baseline sTGF-Beta and sPD-L1
3.6.2. Radically Resected PDAC and Baseline sTGF-Beta and sPD-L1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nevala-Plagemann, C.; Hidalgo, M.; Garrido-Laguna, I. From State-of-the-Art Treatments to Novel Therapies for Advanced-Stage Pancreatic Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Balsano, R.; Tommasi, C.; Garajova, I. State of the Art for Metastatic Pancreatic Cancer Treatment: Where Are We Now?*. Anticancer. Res. 2019, 39, 3405–3412. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; van Dam, J.L.; Suker, M.; Janssen, Q.P.; Groothuis, K.B.C.; Besselink, M.G.H.; Busch, O.R.C.; Bonsing, B.A.; Groot Koerkamp, B.; de Hingh, I.H.J.T.; et al. Improved Overall Survival in Pancreatic Cancer with Preoperative Chemoradiotherapy: Long-Term Results of the PREOPANC Trial. HPB 2021, 23, S672–S673. [Google Scholar] [CrossRef]
- Boyd, L.N.C.; Andini, K.D.; Peters, G.J.; Kazemier, G.; Giovannetti, E. Heterogeneity and Plasticity of Cancer-Associated Fibroblasts in the Pancreatic Tumor Microenvironment. Semin. Cancer Biol. 2021, 82, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, E.; van der Borden, C.L.; Frampton, A.E.; Ali, A.; Firuzi, O.; Peters, G.J. Never Let It Go: Stopping Key Mechanisms Underlying Metastasis to Fight Pancreatic Cancer. Semin. Cancer Biol. 2017, 44, 43–59. [Google Scholar] [CrossRef]
- Singh, H.; Perez, K.; Wolpin, B.M.; Aguirre, A.J. Beyond the Front Line: Emerging Data for Maintenance Therapy in Pancreatic Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 3199–3206. [Google Scholar] [CrossRef]
- Pecoraro, C.; Faggion, B.; Balboni, B.; Carbone, D.; Peters, G.J.; Diana, P.; Assaraf, Y.G.; Giovannetti, E. GSK3β as a Novel Promising Target to Overcome Chemoresistance in Pancreatic Cancer. Drug Resist. Updates 2021, 58, 100799. [Google Scholar] [CrossRef]
- Beatty, G.L.; Werba, G.; Lyssiotis, C.A.; Simeone, D.M. The Biological Underpinnings of Therapeutic Resistance in Pancreatic Cancer. Genes Dev. 2021, 35, 940–962. [Google Scholar] [CrossRef]
- Digiacomo, G.; Volta, F.; Garajova, I.; Balsano, R.; Cavazzoni, A. Biological Hallmarks and New Therapeutic Approaches for the Treatment of Pdac. Life 2021, 11, 843. [Google Scholar] [CrossRef]
- Garajová, I.; le Large, T.Y.; Frampton, A.E.; Rolfo, C.; Voortman, J.; Giovannetti, E. Molecular Mechanisms Underlying the Role of MicroRNAs in the Chemoresistance of Pancreatic Cancer. BioMed Res. Int. 2014, 2014, 678401. [Google Scholar] [CrossRef]
- Crinò, S.F.; di Mitri, R.; Nguyen, N.Q.; Tarantino, I.; de Nucci, G.; Deprez, P.H.; Carrara, S.; Kitano, M.; Shami, V.M.; Fernández-Esparrach, G.; et al. Endoscopic Ultrasound–Guided Fine-Needle Biopsy With or Without Rapid On-Site Evaluation for Diagnosis of Solid Pancreatic Lesions: A Randomized Controlled Non-Inferiority Trial. Gastroenterology 2021, 161, 899–909.e5. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Carnevale, I.; Danen, E.H.J.; Peters, G.J.; Schmidt, T.; Assaraf, Y.G.; Giovannetti, E. A Mechanopharmacology Approach to Overcome Chemoresistance in Pancreatic Cancer. Drug Resist. Updates 2017, 31, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Olive, K.P. Stroma, Stroma Everywhere (Far More than You Think). Clin. Cancer Res. 2015, 21, 3366–3368. [Google Scholar] [CrossRef] [Green Version]
- Meijer, L.; Garajová, I.; Caparello, C.; le Large, T.; Funel, N.; Vasile, E.; Zonderhuis, B.; Daams, F.; Giovannetti, E.; Kazemier, G. Circulating MicroRNAs as Dynamic Biomarkers of Response to Treatment with FOLFIRINOX in Advanced Pancreatic Ductal Adenocarcinoma. HPB 2019, 21, S1019–S1020. [Google Scholar] [CrossRef] [Green Version]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and Future Perspectives of Liquid Biopsies in Genomics-Driven Oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef] [PubMed]
- el Hassouni, B.; Li Petri, G.; Liu, D.S.K.; Cascioferro, S.; Parrino, B.; Hassan, W.; Diana, P.; Ali, A.; Frampton, A.E.; Giovannetti, E. Pharmacogenetics of Treatments for Pancreatic Cancer. Expert Opin. Drug Metab. Toxicol. 2019, 15, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Cardona, A.F.; Cristofanilli, M.; Paz-Ares, L.; Diaz Mochon, J.J.; Duran, I.; Raez, L.E.; Russo, A.; Lorente, J.A.; Malapelle, U.; et al. Challenges and Opportunities of CfDNA Analysis Implementation in Clinical Practice: Perspective of the International Society of Liquid Biopsy (ISLB). Crit. Rev. Oncol. Hematol. 2020, 151, 102978. [Google Scholar] [CrossRef]
- Pisapia, P.; Costa, J.L.; Pepe, F.; Russo, G.; Gragnano, G.; Russo, A.; Iaccarino, A.; de Miguel-Perez, D.; Serrano, M.J.; Denninghoff, V.; et al. Next Generation Sequencing for Liquid Biopsy Based Testing in Non-Small Cell Lung Cancer in 2021. Crit. Rev. Oncol. Hematol. 2021, 161, 103311. [Google Scholar] [CrossRef]
- Modi, S.; Kir, D.; Saluja, A.K. Old Dog, New Tricks: Use of CA 19-9 for Early Diagnosis of Pancreatic Cancer. Gastroenterology 2021, 160, 1019–1021. [Google Scholar] [CrossRef]
- Tsen, A.; Barbara, M.; Rosenkranz, L. Dilemma of Elevated CA 19-9 in Biliary Pathology. Pancreatology 2018, 18, 862–867. [Google Scholar] [CrossRef]
- Skulimowski, A.; Durczyński, A.; Strzelczyk, J.; Hogendorf, P. Comparison of Clinical Usefulness of Serum Ca125 and CA19-9 in Pancreatic Adenocarcinoma Diagnosis: Meta-Analysis and Systematic Review of Literature. Biomarkers 2021, 26, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Maisey, N.R.; Norman, A.R.; Hill, A.; Massey, A.; Oates, J.; Cunningham, D. CA19-9 as a Prognostic Factor in Inoperable Pancreatic Cancer: The Implication for Clinical Trials. Br. J. Cancer 2005, 93, 740–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- le Large, T.Y.S.; Meijer, L.L.; Paleckyte, R.; Boyd, L.N.C.; Kok, B.; Wurdinger, T.; Schelfhorst, T.; Piersma, S.R.; Pham, T.V.; van Grieken, N.C.T.; et al. Combined Expression of Plasma Thrombospondin-2 and CA19-9 for Diagnosis of Pancreatic Cancer and Distal Cholangiocarcinoma: A Proteome Approach. Oncologist 2020, 25, e634–e643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, H.B. Predicting Clinical Outcomes Using Molecular Biomarkers. Biomark. Cancer 2016, 8, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Drabsch, Y.; ten Dijke, P. TGF-β Signalling and Its Role in Cancer Progression and Metastasis. Cancer Metastasis Rev. 2012, 31, 553–568. [Google Scholar] [CrossRef]
- Cave, D.D.; di Guida, M.; Costa, V.; Sevillano, M.; Ferrante, L.; Heeschen, C.; Corona, M.; Cucciardi, A.; Lonardo, E. TGF-Β1 Secreted by Pancreatic Stellate Cells Promotes Stemness and Tumourigenicity in Pancreatic Cancer Cells through L1CAM Downregulation. Oncogene 2020, 39, 4271–4285. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Bang, J.H.; Nam, A.R.; Park, J.E.; Jin, M.H.; Bang, Y.J.; Oh, D.Y. The Prognostic Role of Soluble TGF-Beta and Its Dynamics in Unresectable Pancreatic Cancer Treated with Chemotherapy. Cancer Med. 2020, 9, 43–51. [Google Scholar] [CrossRef]
- Ahmed, S.; Bradshaw, A.D.; Gera, S.; Zahidunnabi Dewan, M.; Xu, R. The TGF-β/Smad4 Signaling Pathway in Pancreatic Carcinogenesis and Its Clinical Significance. J. Clin. Med. 2017, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Principe, D.R.; DeCant, B.; Mascariñas, E.; Wayne, E.A.; Diaz, A.M.; Akagi, N.; Hwang, R.; Pasche, B.; Dawson, D.W.; Fang, D.; et al. TGFβ Signaling in the Pancreatic Tumor Microenvironment Promotes Fibrosis and Immune Evasion to Facilitate Tumorigenesis. Cancer Res. 2016, 76, 2525–2539. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Sung, C.O. PD-L1 Expression and Surgical Outcomes of Adenosquamous Carcinoma of the Pancreas in a Single-Centre Study of 56 Lesions: PD-L1 Expression in Adenosquamous Carcinoma of the Pancreas. Pancreatology 2021, 21, 920–927. [Google Scholar] [CrossRef]
- Leonetti, A.; Wever, B.; Mazzaschi, G.; Assaraf, Y.G.; Rolfo, C.; Quaini, F.; Tiseo, M.; Giovannetti, E. Molecular Basis and Rationale for Combining Immune Checkpoint Inhibitors with Chemotherapy in Non-Small Cell Lung Cancer. Drug Resist. Updates 2019, 46, 100644. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.; Santos, J.M.O.; Gil da Costa, R.M.; Medeiros, R. Impact of Immune Cells on the Hallmarks of Cancer: A Literature Review. Crit. Rev. Oncol. Hematol. 2021, 168, 103541. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Jung, M.Y.; Choudhury, M.; Leof, E.B. Transforming Growth Factor Beta Induces Fibroblasts to Express and Release the Immunomodulatory Protein PD-L1 into Extracellular Vesicles. FASEB J. 2020, 34, 2213–2226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, Y.D.; Pillarisetty, V.G. T-Cell Programming in Pancreatic Adenocarcinoma: A Review. Cancer Gene Ther. 2017, 24, 106–113. [Google Scholar] [CrossRef] [PubMed]
- le Large, T.Y.S.; Bijlsma, M.F.; Kazemier, G.; van Laarhoven, H.W.M.; Giovannetti, E.; Jimenez, C.R. Key Biological Processes Driving Metastatic Spread of Pancreatic Cancer as Identified by Multi-Omics Studies. Semin. Cancer Biol. 2017, 44, 153–169. [Google Scholar] [CrossRef]
- Zhu, X.; Lang, J. Soluble PD-1 and PD-L1: Predictive and Prognostic Significance in Cancer. Oncotarget 2017, 8, 97671–97682. [Google Scholar] [CrossRef] [Green Version]
- Birnbaum, D.J.; Finetti, P.; Lopresti, A.; Gilabert, M.; Poizat, F.; Turrini, O.; Raoul, J.L.; Delpero, J.R.; Moutardier, V.; Birnbaum, D.; et al. Prognostic Value of PDL1 Expression in Pancreatic Cancer. Oncotarget 2016, 7, 71198–71210. [Google Scholar] [CrossRef] [Green Version]
- Bailly, C.; Thuru, X.; Quesnel, B. Soluble Programmed Death Ligand-1 (Spd-L1): A Pool of Circulating Proteins Implicated in Health and Diseases. Cancers 2021, 13, 3034. [Google Scholar] [CrossRef]
- Johansson, H.; Andersson, R.; Bauden, M.; Hammes, S.; Holdenrieder, S.; Ansari, D. Immune Checkpoint Therapy for Pancreatic Cancer. World J. Gastroenterol. 2016, 22, 9457–9476. [Google Scholar] [CrossRef]
- del Re, M.; Vivaldi, C.; Rofi, E.; Salani, F.; Crucitta, S.; Catanese, S.; Fontanelli, L.; Massa, V.; Cucchiara, F.; Fornaro, L.; et al. Gemcitabine plus Nab-Paclitaxel Induces PD-L1 MRNA Expression in Plasma-Derived Microvesicles in Pancreatic Cancer. Cancers 2021, 13, 3738. [Google Scholar] [CrossRef]
- Principe, D.R.; Park, A.; Dorman, M.J.; Kumar, S.; Viswakarma, N.; Rubin, J.; Torres, C.; Mckinney, R.; Munshi, H.G.; Grippo, P.J.; et al. TGFb Blockade Augments PD-1 Inhibition to Promote T-Cell-Mediated Regression of Pancreatic Cancer. Mol. Cancer Ther. 2019, 18, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Feng, M.; Xiong, G.; Cao, Z.; Yang, G.; Zheng, S.; Song, X.; You, L.; Zheng, L.; Zhang, T.; Zhao, Y. PD-1/PD-L1 and Immunotherapy for Pancreatic Cancer. Cancer Lett. 2017, 407, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Izeradjene, K.; Combs, C.; Best, M.; Gopinathan, A.; Wagner, A.; Grady, W.M.; Deng, C.X.; Hruban, R.H.; Adsay, N.V.; Tuveson, D.A.; et al. KrasG12D and Smad4/Dpc4 Haploinsufficiency Cooperate to Induce Mucinous Cystic Neoplasms and Invasive Adenocarcinoma of the Pancreas. Cancer Cell 2007, 11, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, M.; Matsuzaki, K.; Date, M.; Watanabe, T.; Shibano, K.; Nakagawa, T.; Yanagitani, S.; Amoh, Y.; Takemoto, H.; Ogata, N.; et al. Down-Regulation of TGF-β Receptors in Human Colorectal Cancer: Implications for Cancer Development. Br. J. Cancer 1999, 80, 194–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, C.; Oh, D.Y.; Choi, H.J.; Kudo, M.; Ueno, M.; Kondo, S.; Chen, L.T.; Osada, M.; Helwig, C.; Dussault, I.; et al. Phase i Study of Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Patients with Pretreated Biliary Tract Cancer. J. ImmunoTherapy Cancer 2020, 8, e000564. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Metastatic No, % | Radically Resected No, % |
|---|---|---|
| No. Patients | 23 | 15 |
| Age (median), y | 69 (48–83%) | 69 (43–82%) |
| Sex | ||
| Male | 10 (43.5%) | 8 (53%) |
| Female | 13 (56.5%) | 7 (47%) |
| Tumor location | head: 16 (70%) | head: 10 (67%) |
| body-tail: 5 (30%) | body-tail: 5 (23%) | |
| TNM in resected PDAC pts | ||
| T | T1N0 (1 pt), T2N0 (5 pts), unknown (1 pt) | |
| N | T1-3N+ (8 pts) | |
| CEA elevated after surgery | ||
| no | 11 (73%) | |
| yes | 4 (27%) | |
| CA 19-9 elevated after surgery | ||
| no | 12 (80%) | |
| yes | 3 (20%) | |
| Adjuvant therapy | gemcitabine-based regimen: 6 (40%) | |
| 5-FU based regimen: 7 (47%) | ||
| no adjuvant therapy: 2 (13%) | ||
| Relapse after surgery | ||
| no | 5 (33%) | |
| yes | 10 (67%) | |
| Median DFS | 8 (4–18 months) | |
| CEA elevated at diagnosis of metastatic disease | ||
| no | 10 (43.5%) | |
| yes | 13 (56.5%) | |
| CA 19-9 elevated at diagnosis of metastatic disease | ||
| no | 3 (13%) | |
| yes | 20 (87%) | |
| Location of metastases | liver only: 8 (35%) | |
| lung only: 1 (4%) | ||
| two or more sites: 14 (61%) | ||
| Palliative chemotherapy | gemcitabine-based: 14 (61%) | |
| 5FU-based: 7 (30%) | ||
| no therapy: 2 (9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garajová, I.; Cavazzoni, A.; Verze, M.; Minari, R.; Pedrazzi, G.; Balsano, R.; Gelsomino, F.; Valle, R.D.; Digiacomo, G.; Giovannetti, E.; et al. It Takes Two to Tango: Potential Prognostic Impact of Circulating TGF-Beta and PD-L1 in Pancreatic Cancer. Life 2022, 12, 960. https://doi.org/10.3390/life12070960
Garajová I, Cavazzoni A, Verze M, Minari R, Pedrazzi G, Balsano R, Gelsomino F, Valle RD, Digiacomo G, Giovannetti E, et al. It Takes Two to Tango: Potential Prognostic Impact of Circulating TGF-Beta and PD-L1 in Pancreatic Cancer. Life. 2022; 12(7):960. https://doi.org/10.3390/life12070960
Chicago/Turabian StyleGarajová, Ingrid, Andrea Cavazzoni, Michela Verze, Roberta Minari, Giuseppe Pedrazzi, Rita Balsano, Fabio Gelsomino, Raffaele Dalla Valle, Graziana Digiacomo, Elisa Giovannetti, and et al. 2022. "It Takes Two to Tango: Potential Prognostic Impact of Circulating TGF-Beta and PD-L1 in Pancreatic Cancer" Life 12, no. 7: 960. https://doi.org/10.3390/life12070960







