Heterogeneities in Ventricular Conduction Following Treatment with Heptanol: A Multi-Electrode Array Study in Langendorff-Perfused Mouse Hearts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solutions
2.2. Preparation of Langendorff-Perfused Mouse Hearts
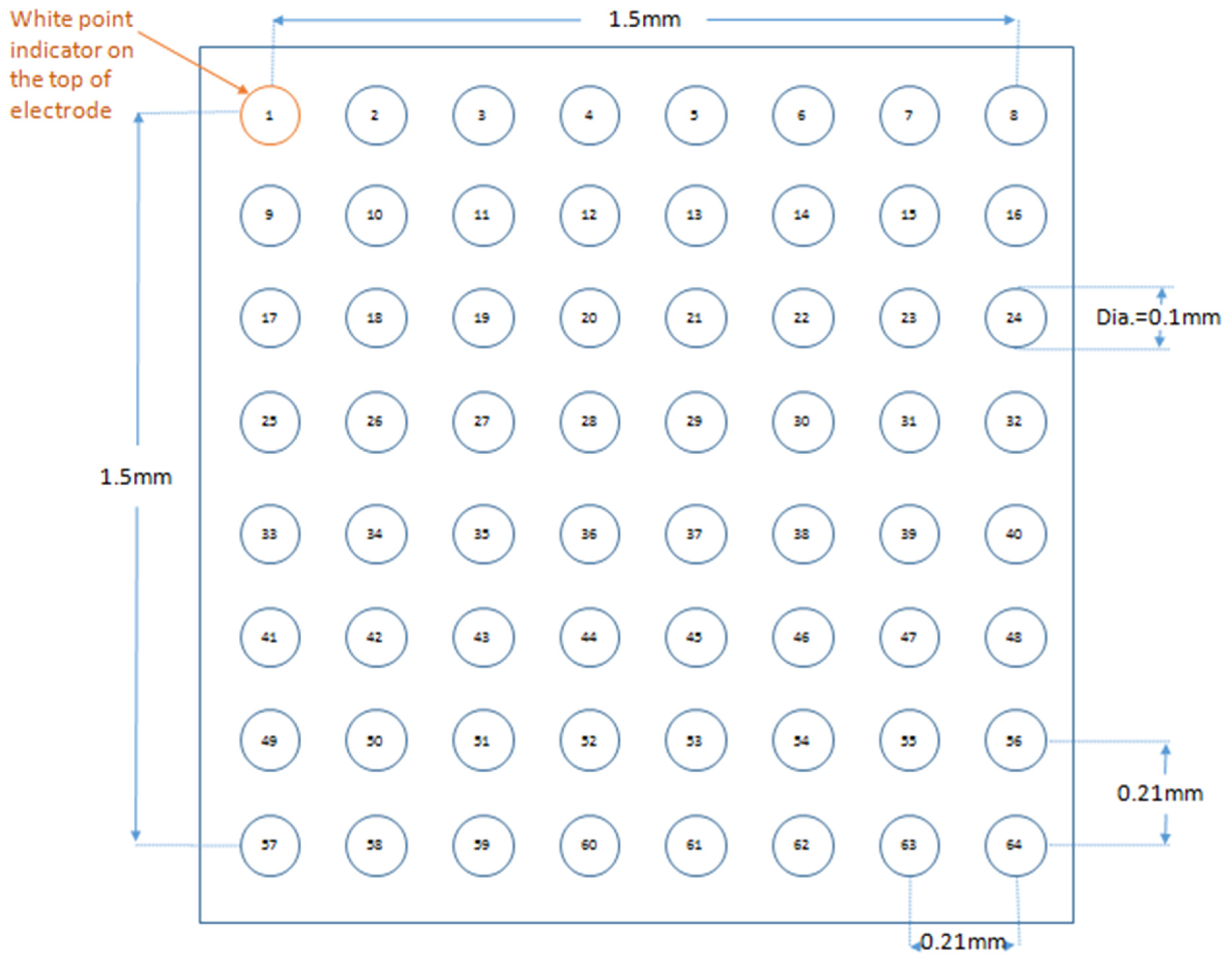
2.3. Stimulating and Recording Procedures
2.4. Statistical Analysis
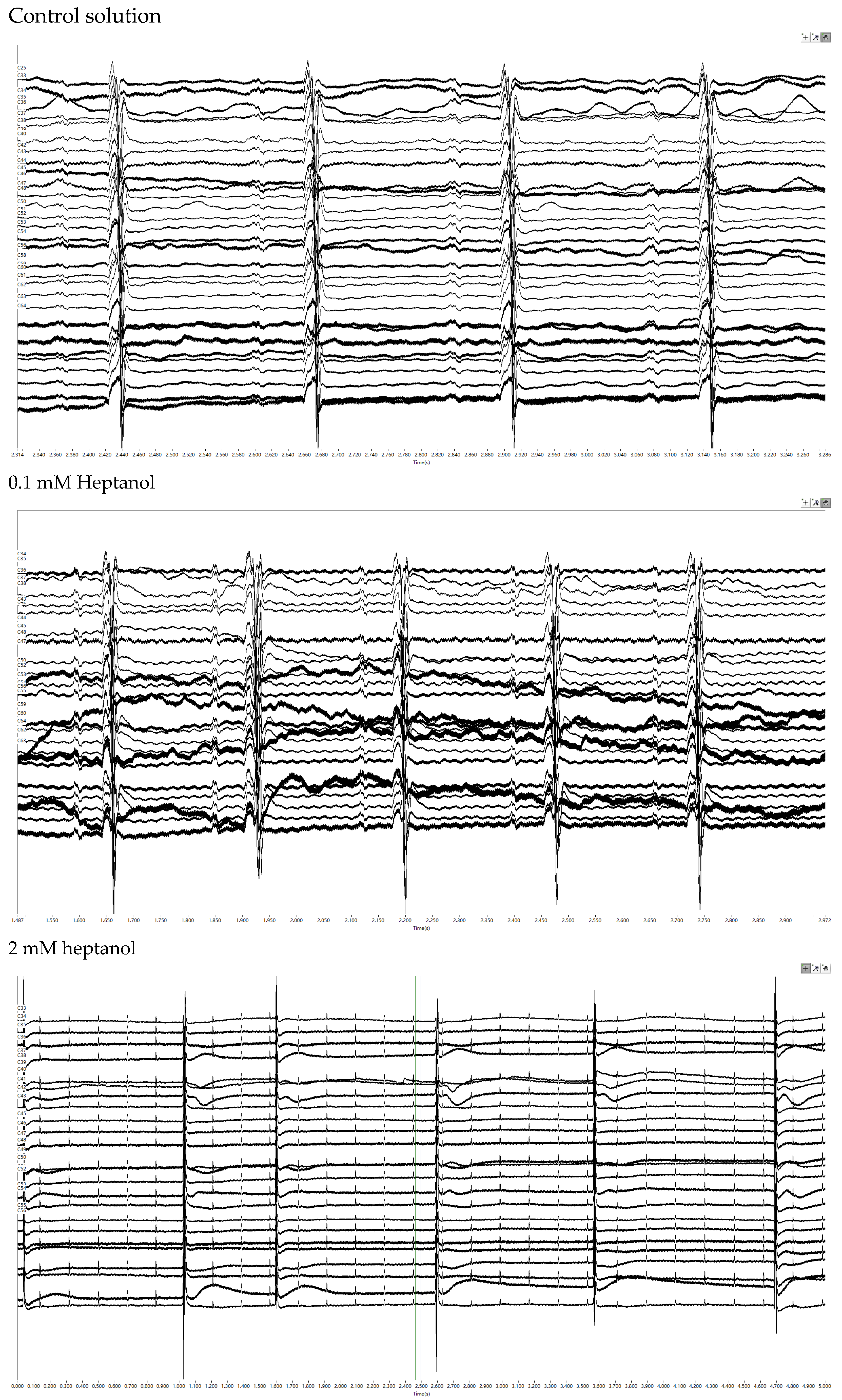
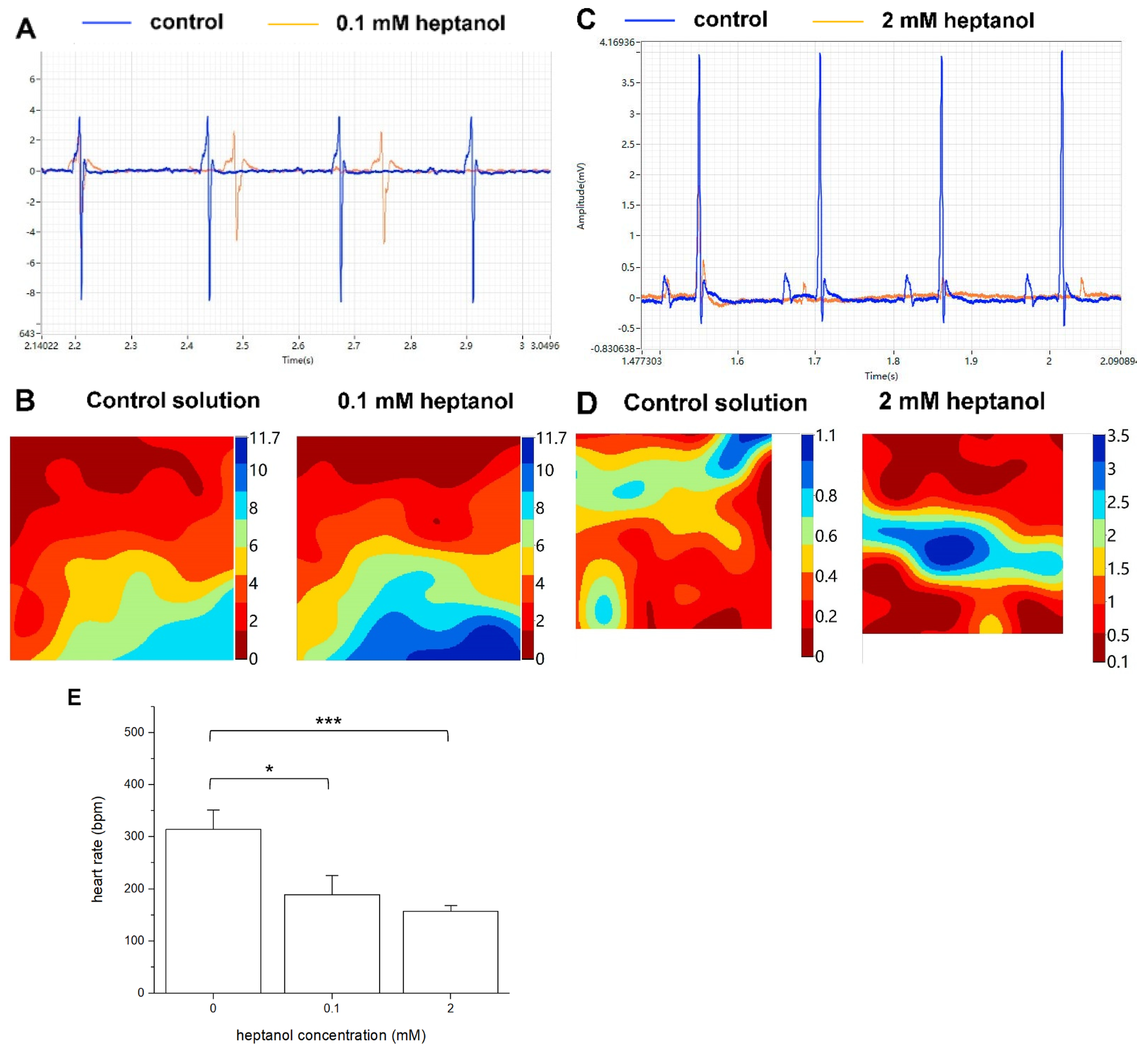
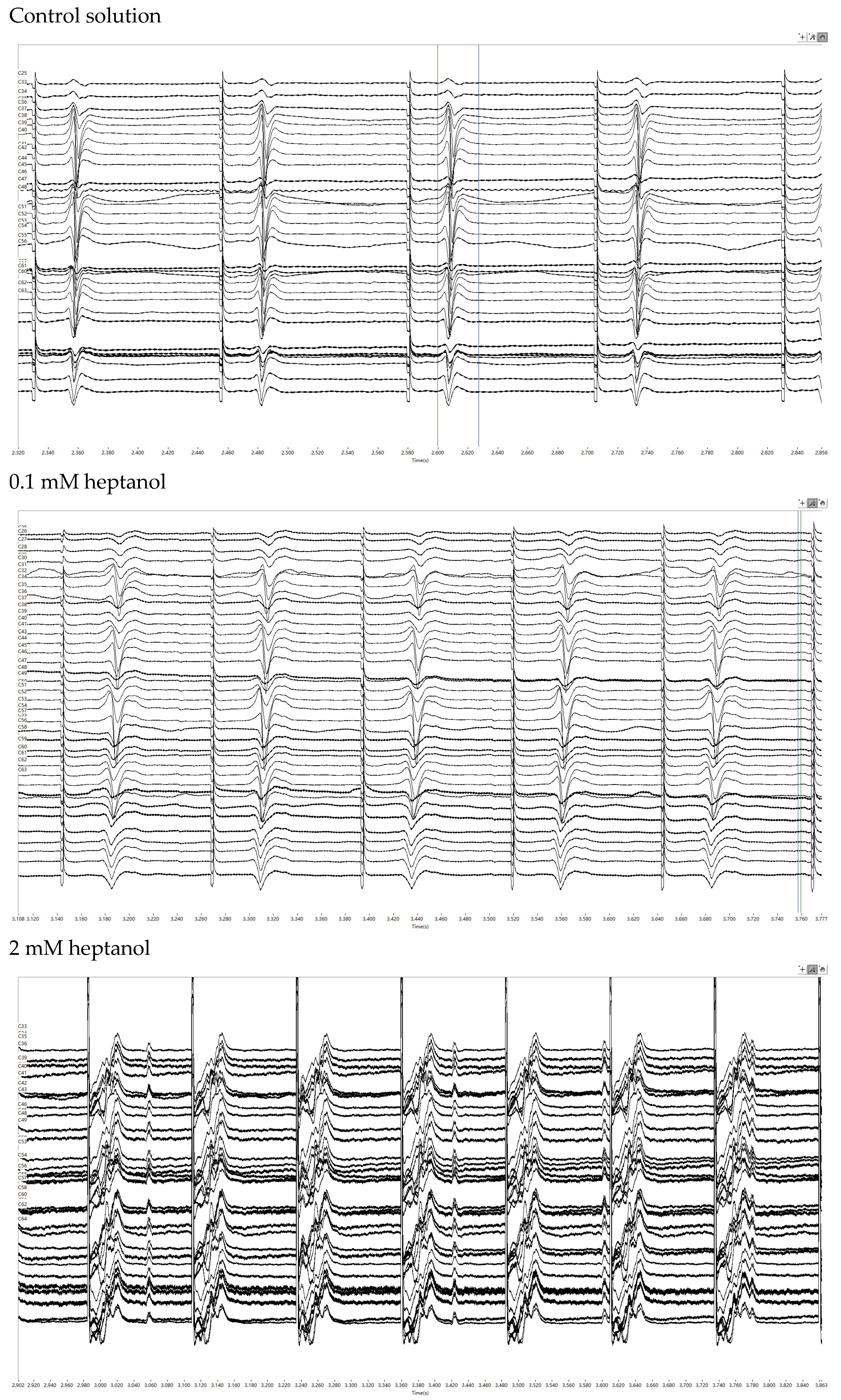
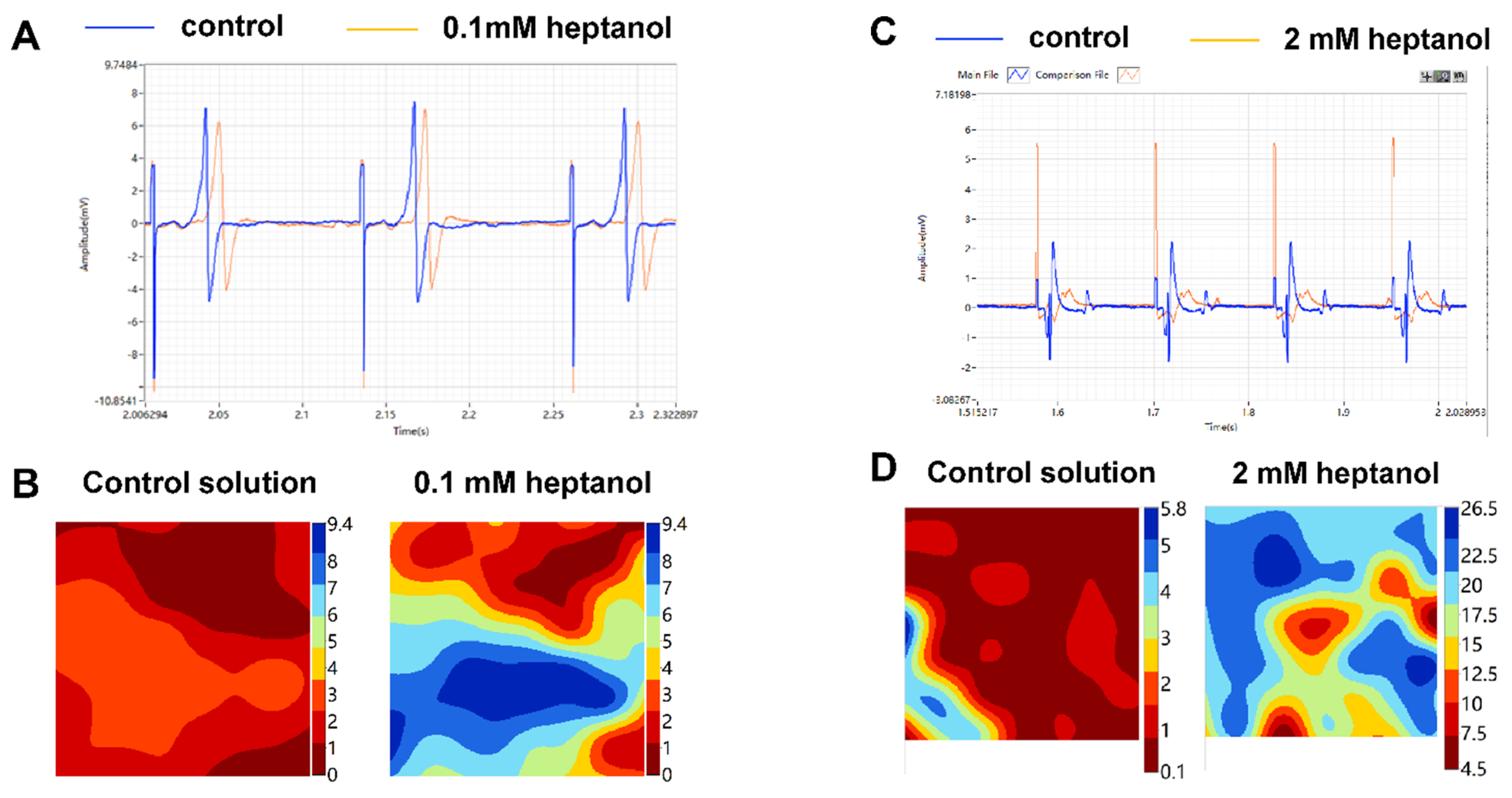
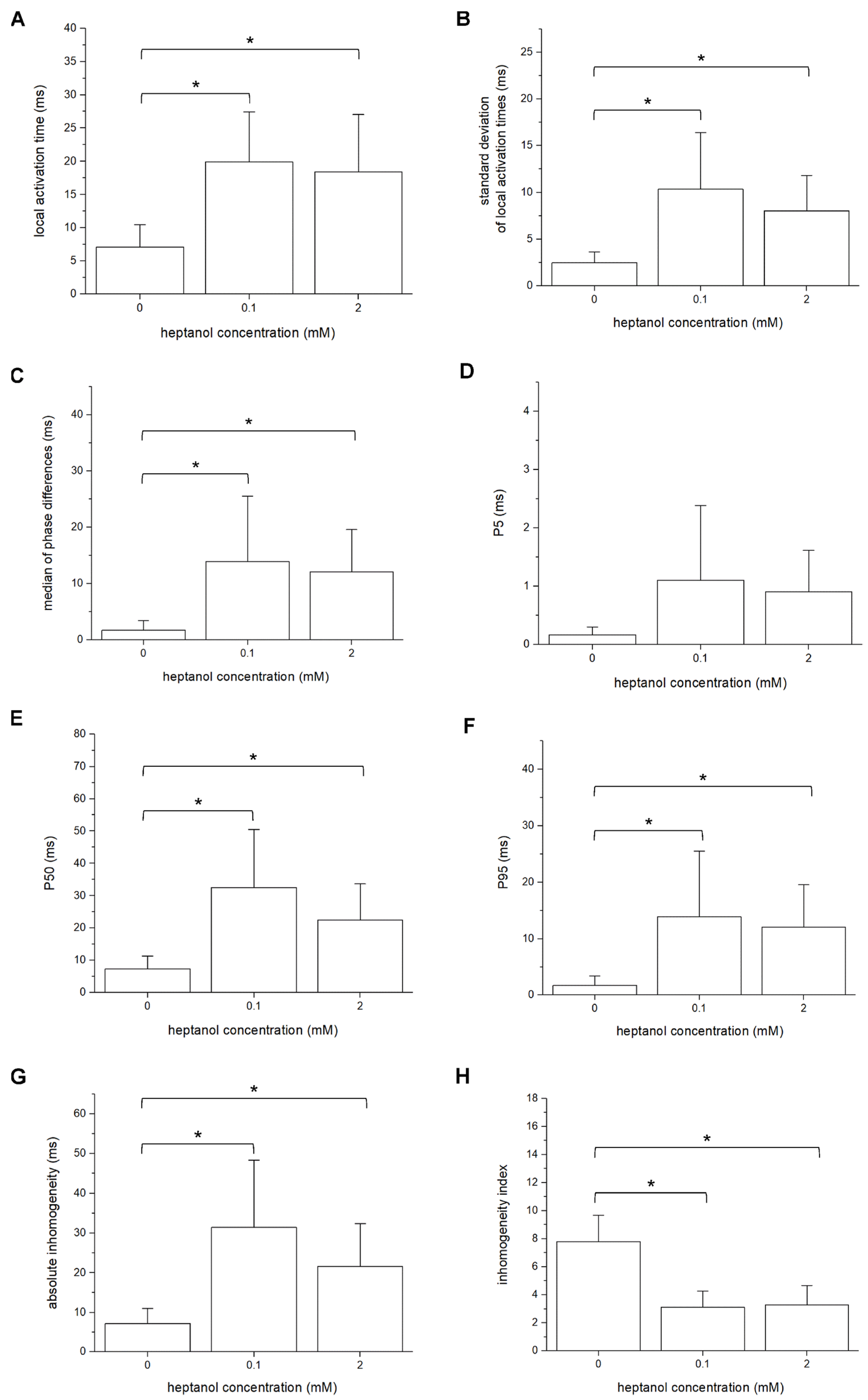
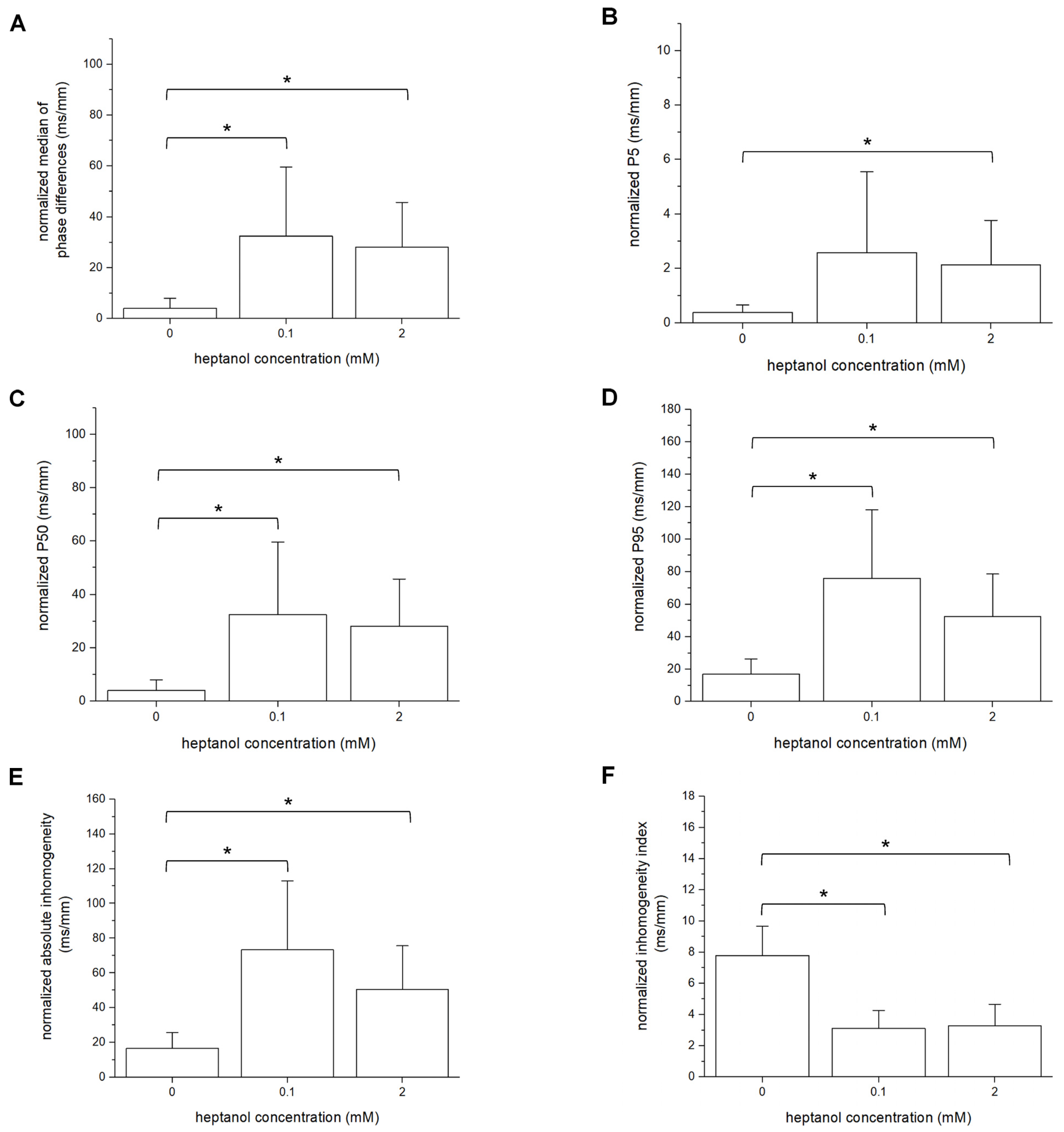
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antzelevitch, C.; Burashnikov, A. Overview of Basic Mechanisms of Cardiac Arrhythmia. Card. Electrophysiol. Clin. 2011, 3, 23–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotadia, I.D.; Whitaker, J.; Roney, C.H.; Niederer, S.; O’Neill, M.; Bishop, M. Anisotropic Cardiac Conduction. Arrhythmia Electrophysiol. Rev. 2020, 9, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.; Strunets, A.; Shen, W.-K.; Jahangir, A. Mechanisms of arrhythmias and conduction disorders in older adults. Clin. Geriatr. Med. 2012, 28, 555–573. [Google Scholar] [CrossRef] [Green Version]
- Tolppanen, H.; Siirila-Waris, K.; Harjola, V.P.; Marono, D.; Parenica, J.; Kreutzinger, P.; Nieminen, T.; Pavlusova, M.; Tarvasmaki, T.; Twerenbold, R.; et al. Ventricular conduction abnormalities as predictors of long-term survival in acute de novo and decompensated chronic heart failure. ESC Heart Fail. 2016, 3, 35–43. [Google Scholar] [CrossRef]
- Tse, G.; Lai, E.T.; Tse, V.; Yeo, J.M. Molecular and Electrophysiological Mechanisms Underlying Cardiac Arrhythmogenesis in Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 2848759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, M.B.; Poelzing, S.; Weinberg, S.H. Mechanisms underlying age-associated manifestation of cardiac sodium channel gain-of-function. J. Mol. Cell Cardiol. 2021, 153, 60–71. [Google Scholar] [CrossRef]
- Letsas, K.P.; Vlachos, K.; Conte, G.; Efremidis, M.; Nakashima, T.; Duchateau, J.; Bazoukis, G.; Frontera, A.; Mililis, P.; Tse, G.; et al. Right ventricular outflow tract electroanatomical abnormalities in asymptomatic and high-risk symptomatic patients with Brugada syndrome: Evidence for a new risk stratification tool? J. Cardiovasc. Electrophysiol. 2021, 32, 2997–3007. [Google Scholar] [CrossRef]
- Letsas, K.P.; Efremidis, M.; Asvestas, D.; Vlachos, K.; Georgopoulos, S.; Tse, G.; Liu, T.; Bazoukis, G.; Sideris, A.; Baranchuk, A.; et al. Right Ventricular Outflow Tract Electroanatomical Abnormalities Predict Ventricular Fibrillation Inducibility in Brugada Syndrome. Circ. Arrhythm. Electrophysiol. 2018, 11, e005928. [Google Scholar] [CrossRef]
- Palatinus, J.A.; Gourdie, R.G. Diabetes Increases Cryoinjury Size with Associated Effects on Cx43 Gap Junction Function and Phosphorylation in the Mouse Heart. J. Diabetes Res. 2016, 2016, 8789617. [Google Scholar] [CrossRef] [Green Version]
- Veeraraghavan, R.; Lin, J.; Hoeker, G.S.; Keener, J.P.; Gourdie, R.G.; Poelzing, S. Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: An experimental and modeling study. Pflug. Arch. 2015, 467, 2093–2105. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.J.; Cheng, C.C.; Chen, Y.C.; Kao, Y.H.; Chen, S.A.; Chen, Y.J. Gap junction modifiers regulate electrical activities of the sinoatrial node and pulmonary vein: Therapeutic implications in atrial arrhythmogenesis. Int. J. Cardiol. 2016, 221, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, R.D. Gap junction heterogeneity in reentrant ventricular tachycardia. Cardiovasc. Res. 2006, 72, 196–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, W.L.; Makielski, J.C. Block of sodium current by heptanol in voltage-clamped canine cardiac Purkinje cells. Circ. Res. 1991, 68, 977–983. [Google Scholar] [CrossRef] [Green Version]
- Callans, D.J.; Moore, E.N.; Spear, J.F. Effect of coronary perfusion of heptanol on conduction and ventricular arrhythmias in infarcted canine myocardium. J. Cardiovasc. Electrophysiol. 1996, 7, 1159–1171. [Google Scholar] [CrossRef]
- Tse, G.; Hothi, S.S.; Grace, A.A.; Huang, C.L. Ventricular arrhythmogenesis following slowed conduction in heptanol-treated, Langendorff-perfused mouse hearts. J. Physiol. Sci. 2012, 62, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.; Yeo, J.M.; Tse, V.; Kwan, J.; Sun, B. Gap junction inhibition by heptanol increases ventricular arrhythmogenicity by reducing conduction velocity without affecting repolarization properties or myocardial refractoriness in Langendorff-perfused mouse hearts. Mol. Med. Rep. 2016, 14, 4069–4074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tse, G.; Liu, T.; Li, G.; Keung, W.; Yeo, J.M.; Fiona Chan, Y.W.; Yan, B.P.; Chan, Y.S.; Wong, S.H.; Li, R.A.; et al. Effects of pharmacological gap junction and sodium channel blockade on S1S2 restitution properties in Langendorff-perfused mouse hearts. Oncotarget 2017, 8, 85341–85352. [Google Scholar] [CrossRef] [Green Version]
- Tse, G.; Hao, G.; Lee, S.; Zhou, J.; Zhang, Q.; Du, Y.; Liu, T.; Cheng, S.H.; Wong, W.T. Measures of repolarization variability predict ventricular arrhythmogenesis in heptanol-treated Langendorff-perfused mouse hearts. Curr. Res. Physiol. 2021, 4, 125–134. [Google Scholar] [CrossRef]
- Tse, G.; Du, Y.; Hao, G.; Li, K.H.C.; Chan, F.Y.W.; Liu, T.; Li, G.; Bazoukis, G.; Letsas, K.P.; Wu, W.K.K.; et al. Quantification of Beat-To-Beat Variability of Action Potential Durations in Langendorff-Perfused Mouse Hearts. Front. Physiol. 2018, 9, 1578. [Google Scholar] [CrossRef] [Green Version]
- Spira, M.E.; Hai, A. Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 2013, 8, 83–94. [Google Scholar] [CrossRef]
- Issa, Z.F.; Miller, J.M.; Zipes, D.P. Chapter 6—Advanced Mapping and Navigation Modalities. In Clinical Arrhythmology and Electrophysiology: A Companion to Braunwald’s Heart Disease, 2nd ed.; Issa, Z.F., Miller, J.M., Zipes, D.P., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2012; pp. 111–143. [Google Scholar] [CrossRef]
- Yeo, J.M.; Tse, V.; Kung, J.; Lin, H.Y.; Lee, Y.T.; Kwan, J.; Yan, B.P.; Tse, G. Isolated heart models for studying cardiac electrophysiology: A historical perspective and recent advances. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tse, G.; Wong, S.T.; Tse, V.; Yeo, J.M. Monophasic action potential recordings: Which is the recording electrode? J. Basic Clin. Physiol. Pharmacol. 2016, 27, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Podesser, B.K.; Lim, C.C. The continuing evolution of the Langendorff and ejecting murine heart: New advances in cardiac phenotyping. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H156–H167. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.M.; Mocanu, M.M.; Yellon, D.M. Retrograde heart perfusion: The Langendorff technique of isolated heart perfusion. J. Mol. Cell Cardiol. 2011, 50, 940–950. [Google Scholar] [CrossRef]
- Lammers, W.J.; Schalij, M.J.; Kirchhof, C.J.; Allessie, M.A. Quantification of spatial inhomogeneity in conduction and initiation of reentrant atrial arrhythmias. Am. J. Physiol. 1990, 259, H1254–H1263. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Jin, J.; Shen, W.; Tsui, H.; Shi, Y.; Wang, Y.; Zhang, Y.; Hao, G.; Wu, J.; Chen, S.; et al. Mkk4 is a negative regulator of the transforming growth factor beta 1 signaling associated with atrial remodeling and arrhythmogenesis with age. J. Am. Heart Assoc. 2014, 3, e000340. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Guzadhur, L.; Jeevaratnam, K.; Salvage, S.C.; Matthews, G.D.K.; Lammers, W.J.; Lei, M.; Huang, C.L.H.; Fraser, J.A. Arrhythmic substrate, slowed propagation and increased dispersion in conduction direction in the right ventricular outflow tract of murine Scn5a+/− hearts. Acta Physiol. 2014, 211, 559–573. [Google Scholar] [CrossRef] [Green Version]
- Bastiaanse, E.M.; Jongsma, H.J.; van der Laarse, A.; Takens-Kwak, B.R. Heptanol-induced decrease in cardiac gap junctional conductance is mediated by a decrease in the fluidity of membranous cholesterol-rich domains. J. Membr. Biol. 1993, 136, 135–145. [Google Scholar] [CrossRef]
- Takens-Kwak, B.R.; Jongsma, H.J.; Rook, M.B.; Van Ginneken, A.C. Mechanism of heptanol-induced uncoupling of cardiac gap junctions: A perforated patch-clamp study. Am. J. Physiol. 1992, 262, C1531–C1538. [Google Scholar] [CrossRef]
- Burt, J.M.; Spray, D.C. Single-channel events and gating behavior of the cardiac gap junction channel. Proc. Natl. Acad. Sci. USA 1988, 85, 3431–3434. [Google Scholar] [CrossRef] [Green Version]
- Rudisuli, A.; Weingart, R. Electrical properties of gap junction channels in guinea-pig ventricular cell pairs revealed by exposure to heptanol. Pflug. Arch. 1989, 415, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Keevil, V.L.; Huang, C.L.; Chau, P.L.; Sayeed, R.A.; Vandenberg, J.I. The effect of heptanol on the electrical and contractile function of the isolated, perfused rabbit heart. Pflug. Arch. 2000, 440, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Burnham, M.P.; Sharpe, P.M.; Garner, C.; Hughes, R.; Pollard, C.E.; Bowes, J. Investigation of connexin 43 uncoupling and prolongation of the cardiac QRS complex in preclinical and marketed drugs. Br. J. Pharmacol. 2014, 171, 4808–4819. [Google Scholar] [CrossRef] [PubMed]
- Caillier, B.; Pilote, S.; Castonguay, A.; Patoine, D.; Menard-Desrosiers, V.; Vigneault, P.; Hreiche, R.; Turgeon, J.; Daleau, P.; De Koninck, Y.; et al. QRS widening and QT prolongation under bupropion: A unique cardiac electrophysiological profile. Fundam. Clin. Pharmacol. 2012, 26, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Haydon, D.A.; Urban, B.W. The action of alcohols and other non-ionic surface active substances on the sodium current of the squid giant axon. J. Physiol. 1983, 341, 411–427. [Google Scholar] [CrossRef] [Green Version]
- Niggli, E.; Rudisuli, A.; Maurer, P.; Weingart, R. Effects of general anesthetics on current flow across membranes in guinea pig myocytes. Am. J. Physiol. 1989, 256, C273–C281. [Google Scholar] [CrossRef]
- Spray, D.C.; Burt, J.M. Structure-activity relations of the cardiac gap junction channel. Am. J. Physiol. 1990, 258, C195–C205. [Google Scholar] [CrossRef]
- Dhein, S. Pharmacology of gap junctions in the cardiovascular system. Cardiovasc. Res. 2004, 62, 287–298. [Google Scholar] [CrossRef]
- Balke, C.W.; Lesh, M.D.; Spear, J.F.; Kadish, A.; Levine, J.H.; Moore, E.N. Effects of cellular uncoupling on conduction in anisotropic canine ventricular myocardium. Circ. Res. 1988, 63, 879–892. [Google Scholar] [CrossRef] [Green Version]
- Delmar, M.; Michaels, D.C.; Johnson, T.; Jalife, J. Effects of increasing intercellular resistance on transverse and longitudinal propagation in sheep epicardial muscle. Circ. Res. 1987, 60, 780–785. [Google Scholar] [CrossRef] [Green Version]
- Jalife, J.; Sicouri, S.; Delmar, M.; Michaels, D.C. Electrical uncoupling and impulse propagation in isolated sheep Purkinje fibers. Am. J. Physiol. 1989, 257, H179–H189. [Google Scholar] [CrossRef] [PubMed]
- Brugada, J.; Mont, L.; Boersma, L.; Kirchhof, C.; Allessie, M.A. Differential effects of heptanol, potassium, and tetrodotoxin on reentrant ventricular tachycardia around a fixed obstacle in anisotropic myocardium. Circulation 1991, 84, 1307–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Varma, P.; Newman, D.; Dorian, P. Gap junction blockers decrease defibrillation thresholds without changes in ventricular refractoriness in isolated rabbit hearts. Circulation 2001, 104, 1544–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, J.J.; Schoff, K.L.; Loeb, J.M.; Wiegert, N.A. Regional gap junction inhibition increases defibrillation thresholds. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H10–H16. [Google Scholar] [CrossRef] [Green Version]
- Saltman, A.E.; Aksehirli, T.O.; Valiunas, V.; Gaudette, G.R.; Matsuyama, N.; Brink, P.; Krukenkamp, I.B. Gap junction uncoupling protects the heart against ischemia. J. Thorac. Cardiovasc. Surg. 2002, 124, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.P.; Mao, H.J.; Fan, F.Y.; Bruce, I.C.; Xia, Q. Delayed uncoupling contributes to the protective effect of heptanol against ischaemia in the rat isolated heart. Clin. Exp. Pharmacol. Physiol. 2005, 32, 655–662. [Google Scholar] [CrossRef]
- Ohara, T.; Qu, Z.; Lee, M.H.; Ohara, K.; Omichi, C.; Mandel, W.J.; Chen, P.S.; Karagueuzian, H.S. Increased vulnerability to inducible atrial fibrillation caused by partial cellular uncoupling with heptanol. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1116–H1122. [Google Scholar] [CrossRef] [Green Version]
- Tse, G.; Tse, V.; Yeo, J.M.; Sun, B. Atrial Anti-Arrhythmic Effects of Heptanol in Langendorff-Perfused Mouse Hearts. PLoS ONE 2016, 11, e0148858. [Google Scholar] [CrossRef] [Green Version]
- Jeevaratnam, K.; Poh Tee, S.; Zhang, Y.; Rewbury, R.; Guzadhur, L.; Duehmke, R.; Grace, A.A.; Lei, M.; Huang, C.L. Delayed conduction and its implications in murine Scn5a(+/−) hearts: Independent and interacting effects of genotype, age, and sex. Pflug. Arch. 2011, 461, 29–44. [Google Scholar] [CrossRef] [Green Version]
- Boineau, J.P.; Schuessler, R.B.; Mooney, C.R.; Miller, C.B.; Wylds, A.C.; Hudson, R.D.; Borremans, J.M.; Brockus, C.W. Natural and evoked atrial flutter due to circus movement in dogs. Role of abnormal atrial pathways, slow conduction, nonuniform refractory period distribution and premature beats. Am. J. Cardiol. 1980, 45, 1167–1181. [Google Scholar] [CrossRef]
- Allessie, M.A.; Bonke, F.I.; Schopman, F.J. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. II. The role of nonuniform recovery of excitability in the occurrence of unidirectional block, as studied with multiple microelectrodes. Circ. Res. 1976, 39, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brack, K.E.; Narang, R.; Winter, J.; Ng, G.A. The mechanical uncoupler blebbistatin is associated with significant electrophysiological effects in the isolated rabbit heart. Exp. Physiol. 2013, 98, 1009–1027. [Google Scholar] [CrossRef] [PubMed]
- Rajendran Pradeep, S.; Ajijola Olujimi, A.; Vetter, R.; Snellings, A.; Tompkins John, D.; Deb, A.; Kipke Daryl, R.; Shivkumar, K.; Ardell Jeffrey, L. Abstract 16717: Customizable High-density Microelectrode Arrays for Murine Cardiac Electrophysiology. Circulation 2016, 134, A16717. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Tse, G.; Hao, G.; Du, Y. Heterogeneities in Ventricular Conduction Following Treatment with Heptanol: A Multi-Electrode Array Study in Langendorff-Perfused Mouse Hearts. Life 2022, 12, 996. https://doi.org/10.3390/life12070996
Dong X, Tse G, Hao G, Du Y. Heterogeneities in Ventricular Conduction Following Treatment with Heptanol: A Multi-Electrode Array Study in Langendorff-Perfused Mouse Hearts. Life. 2022; 12(7):996. https://doi.org/10.3390/life12070996
Chicago/Turabian StyleDong, Xiuming, Gary Tse, Guoliang Hao, and Yimei Du. 2022. "Heterogeneities in Ventricular Conduction Following Treatment with Heptanol: A Multi-Electrode Array Study in Langendorff-Perfused Mouse Hearts" Life 12, no. 7: 996. https://doi.org/10.3390/life12070996





