Metformin-Treatment Option for Social Impairment? An Open Clinical Trial to Elucidate the Effects of Metformin Treatment on Steroid Hormones and Social Behavior
Abstract
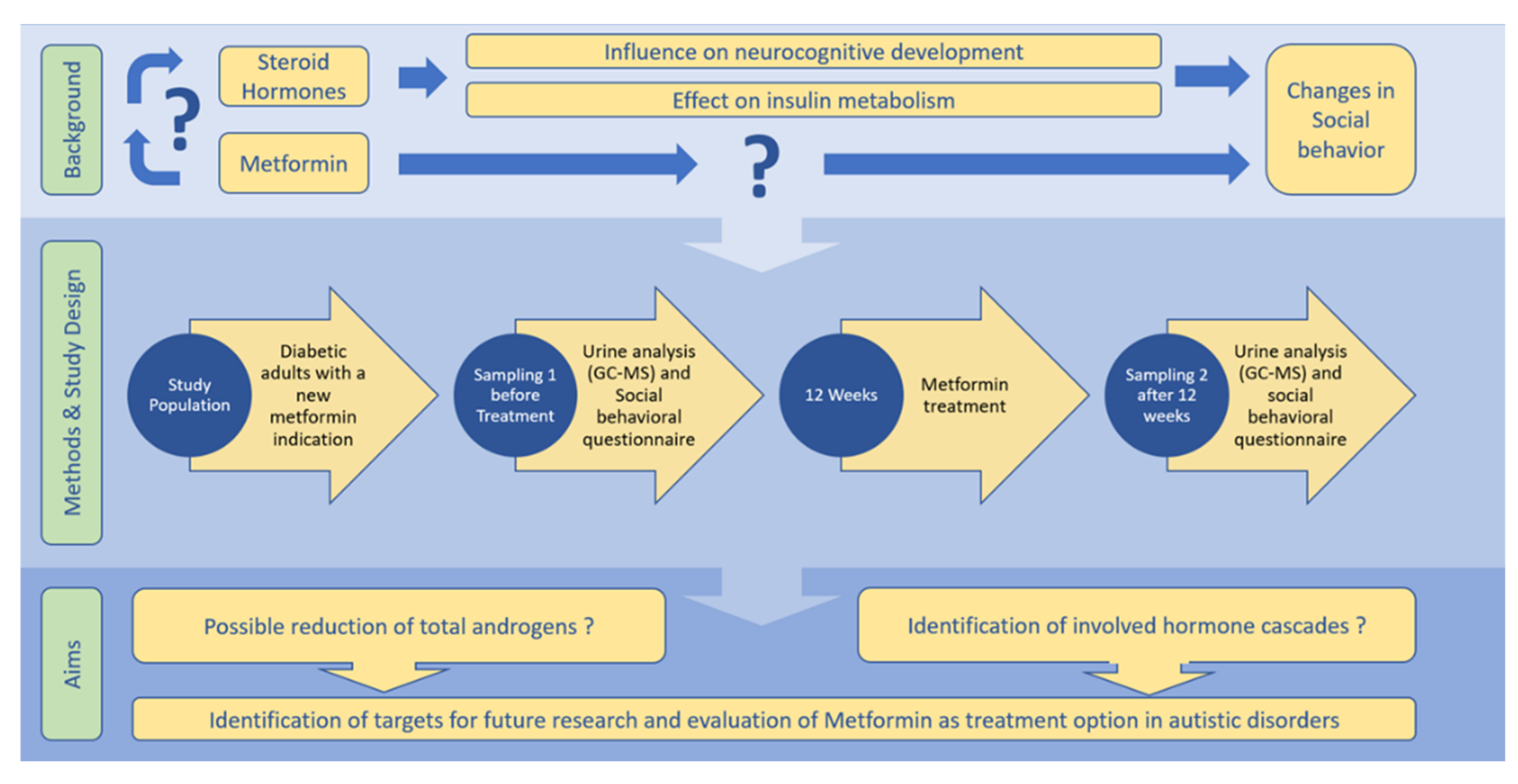
:1. Background and Need for This Study
2. Hypothesis
2.1. Primary Research Hypothesis
2.2. Secondary Research Hypothesis
3. Material and Methods
3.1. Trial Design
3.2. Objectives
3.2.1. Primary Research Objective
3.2.2. Secondary Research Objective
3.3. Endpoints
3.3.1. Primary Research Endpoint
3.3.2. Secondary Research Endpoint
3.4. Eligibility Criteria
3.4.1. Inclusion Criteria
3.4.2. Exclusion Criteria
- Patients under 18 years of age.
- Clinically significant concomitant disease (e.g., advanced renal failure, hepatic dysfunction, neoplasia).
- Significant musculoskeletal disease.
- Active infection during sample collection.
- Immunosuppressive medical therapy.
- Hormonal/steroid treatment.
- Pregnancy.
- Psychiatric disease and known social-behavior-altering medication (e.g., antipsychotic medication).
- Known or suspected malcompliance, drug or alcohol abuse.
- Inability to follow the procedures of the study, e.g., due to insufficient language skills, severe dementia.
- Life expectancy less than 6 months.
- Poor tolerability to metformin treatment with following treatment discontinuation within duration of follow-up.
- Pharmaceutical treatment with insulin or another pharmaceutical with a known strong effect on blood sugar levels.
3.5. Intervention and Follow-Up
3.6. Recruitment
4. Data Collection Methods
4.1. Steroid Measurement Procedures
4.2. Social Behavior Measurement Methods
4.3. Data Collection, Management and Retention
4.4. Sample Size
5. Statistical Analysis
6. Discussion
7. Ethics and Dissemination Policy
7.1. Ethics and Dissemination
7.2. Trial Status
7.3. Ethics Approval and Consent to Participate
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- American Diabetes Association 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S111–S124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, B.; Yang, Y.; Wang, Y.; Zhao, Z.; Miao, Z.; Zhu, J. Metformin exerts antidepressant effects by regulated DNA hydroxymethylation. Epigenomics 2019, 11, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Guigas, B.; Garcia, N.S.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 2012, 122, 253–270. [Google Scholar] [CrossRef] [Green Version]
- King, P.; Peacock, I.; Donnelly, R. The UK Prospective Diabetes Study (UKPDS): Clinical and therapeutic implications for type 2 diabetes. Br. J. Clin. Pharmacol. 1999, 48, 643–648. [Google Scholar] [CrossRef]
- Mathur, N.; Pedersen, B.K. Exercise as a Mean to Control Low-Grade Systemic Inflammation. Mediat. Inflamm. 2008, 2008, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, A.; Hahn, D.; Kempná, P.; Hofer, G.; Nuoffer, J.-M.; Mullis, P.E.; Flück, C.E. Metformin Inhibits Human Androgen Production by Regulating Steroidogenic Enzymes HSD3B2 and CYP17A1 and Complex I Activity of the Respiratory Chain. Endocrinology 2012, 153, 4354–4366. [Google Scholar] [CrossRef] [Green Version]
- Hoppeler, H.; Baum, O.; Mueller, M.; Lurman, G. Molekulare Mechanismen Der Anpassungsfähigkeit Der Skelettmuskulatur. Schweiz. Z. Sportmed. Sporttraumatol. 2011, 59, 6–13. [Google Scholar]
- Martin-Montalvo, A.; Mercken, E.M.; Mitchell, S.J.; Palacios, H.H.; Mote, P.L.; Scheibye-Knudsen, M.; Gomes, A.P.; Ward, T.M.; Minor, R.K.; Blouin, M.-J.; et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013, 4, 2192. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Evidence for prescribing exercise as therapy in chronic disease. Scand. J. Med. Sci. Sports 2006, 16, 3–63. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Nader, A.G. Molecular effects of exercise in patients with inflammatory rheumatic disease. Nat. Clin. Pr. Rheumatol. 2008, 4, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Han, B.; Fan, M.; Wang, N.; Wang, H.; Zhu, H.; Cheng, T.; Zhao, S.; Song, H.; Qiao, J. Oxidative stress increases the 17,20-lyase-catalyzing activity of adrenal P450c17 through p38α in the development of hyperandrogenism. Mol. Cell. Endocrinol. 2019, 484, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Pangrazzi, L.; Balasco, L.; Bozzi, Y. Natural Antioxidants: A Novel Therapeutic Approach to Autism Spectrum Disorders? Antioxidants 2020, 9, 1186. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front. Physiol. 2014, 5, 150. [Google Scholar] [CrossRef] [Green Version]
- Gasser, B.A.; Buerki, S.F.; Kurz, J.; Mohaupt, M.G. Hyperandrogenism? Increased 17, 20-Lyase Activity? A Metanalysis and Systematic Review of Altered Androgens in Boys and Girls with Autism. Int. J. Mol. Sci. 2021, 22, 12324. [Google Scholar] [CrossRef] [PubMed]
- Gasser, B.A.; Kurz, J.; Dick, B.; Mohaupt, M.G. Steroid Metabolites Support Evidence of Autism as a Spectrum. Behav. Sci. 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasser, B.A.; Kurz, J.; Dick, B.; Mohaupt, M.G. A reply to ‘Alteration of steroidogenesis in boys with autism spectrum disorders’. Transl. Psychiatry 2021, 11, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Gasser, B.A.; Kurz, J.; Dick, B.; Mohaupt, M.G. Are Steroid Hormones Dysregulated in Autistic Girls? Diseases 2020, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Gasser, B.A.; Kurz, J.; Senn, W.; Escher, G.; Mohaupt, M.G. Stress-induced alterations of social behavior are reversible by antagonism of steroid hormones in C57/BL6 mice. Naunyn-Schmied. Arch. Exp. Pathol. Pharmakol. 2020, 394, 127–135. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, B.; Shen, Y.; Yan, R.-N.; Li, F.-F.; Sun, R.; Jing, T.; Lee, K.-O.; Ma, J.-H. Rapid Changes in Serum Testosterone in Men With Newly Diagnosed Type 2 Diabetes With Intensive Insulin and Metformin. Diabetes Care 2021, 44, 1059–1061. [Google Scholar] [CrossRef]
- Safiah, M.; Hyassat, D.; Khader, Y.; Farahid, O.; Batieha, A.; El-Khateeb, M.; Ajlouni, K. Effect of Metformin on Anthropometric Measurements and Hormonal and Biochemical Profile in Patients with Prediabetes. J. Diabetes Res. 2021, 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Ma, Y.; Tong, X.; Yang, W.; Dai, Y.; Pan, Y.; Ren, P.; Liu, L.; Fan, H.-Y.; Zhang, Y.; et al. Metformin inhibits testosterone-induced endoplasmic reticulum stress in ovarian granulosa cells via inactivation of p38 MAPK. Hum. Reprod. 2020, 35, 1145–1158, Erratum in Hum Reprod. 2020, 35, 1947–1948. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. Monitoring the kynurenine system: Concentrations, ratios or what else? Adv. Clin. Exp. Med. 2021, 30, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A.M. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol. Autism 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Cai, Y.; Fan, X. Metformin Administration During Early Postnatal Life Rescues Autistic-Like Behaviors in the BTBR T+ Itpr3tf/J Mouse Model of Autism. Front. Behav. Neurosci. 2018, 12, 290. [Google Scholar] [CrossRef]
- Casto, K.V.; Edwards, D.A. Testosterone, cortisol, and human competition. Horm. Behav. 2016, 82, 21–37. [Google Scholar] [CrossRef]
- Doi, H.; Tsumura, N.; Kanai, C.; Masui, K.; Mitsuhashi, R.; Nagasawa, T. Automatic Classification of Adult Males With and Without Autism Spectrum Disorder by Non-contact Measurement of Autonomic Nervous System Activation. Front. Psychiatry 2021, 12, 978. [Google Scholar] [CrossRef]
- Taylor, J.L.; Corbett, B.A. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology 2014, 49, 207–228. [Google Scholar] [CrossRef] [Green Version]
- Garfunkel, D.; Anagnostou, E.A.; Aman, M.G.; Handen, B.L.; Sanders, K.B.; Macklin, E.A.; Chan, J.; Veenstra-VanderWeele, J. Pharmacogenetics of Metformin for Medication-Induced Weight Gain in Autism Spectrum Disorder. J. Child Adolesc. Psychopharmacol. 2019, 29, 448–455. [Google Scholar] [CrossRef]
- Kuhla, A.; Brichmann, E.; Rühlmann, C.; Thiele, R.; Meuth, L.; Vollmar, B. Metformin Therapy Aggravates Neurodegenerative Processes in ApoE–/– Mice. J. Alzheimer’s Dis. 2019, 68, 1415–1427. [Google Scholar] [CrossRef]
- Gantois, I.; Popic, J.; Khoutorsky, A.; Sonenberg, N. Metformin for Treatment of Fragile X Syndrome and Other Neurological Disorders. Annu. Rev. Med. 2019, 70, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Biag, H.M.B.; Potter, L.A.; Wilkins, V.; Afzal, S.; Rosvall, A.; Salcedo-Arellano, M.J.; Rajaratnam, A.; Manzano-Nunez, R.; Schneider, A.; Tassone, F.; et al. Metformin treatment in young children with fragile X syndrome. Mol. Genet. Genom. Med. 2019, 7, e956. [Google Scholar] [CrossRef] [PubMed]
- Protic, D.; Aydin, E.Y.; Tassone, F.; Tan, M.M.; Hagerman, R.J.; Schneider, A. Cognitive and behavioral improvement in adults with fragile X syndrome treated with metformin-two cases. Mol. Genet. Genom. Med. 2019, 7, e745. [Google Scholar] [CrossRef] [Green Version]
- Zemdegs, J.; Martin, H.; Pintana, H.; Bullich, S.; Manta, S.; Marqués, M.A.; Moro, C.; Layé, S.; Ducrocq, F.; Chattipakorn, N.; et al. Metformin Promotes Anxiolytic and Antidepressant-Like Responses in Insulin-Resistant Mice by Decreasing Circulating Branched-Chain Amino Acids. J. Neurosci. 2019, 39, 5935–5948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellena, G.; Battaglia, S.; Làdavas, E. The spatial effect of fearful faces in the autonomic response. Exp. Brain Res. 2020, 238, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R. Androgen excess is the key element in polycystic ovary syndrome. Fertil. Steril. 2003, 80, 252–254. [Google Scholar] [CrossRef]
- Misugi, T.; Ozaki, K.; El Beltagy, K.; Tokuyama, O.; Honda, K.-I.; Ishiko, O. Insulin-Lowering Agents Inhibit Synthesis of Testosterone in Ovaries of DHEA-Induced PCOS Rats. Gynecol. Obstet. Investig. 2006, 61, 208–215. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.; Huang, Q. Comparison of the effect between pioglitazone and metformin in treating patients with PCOS:a meta-analysis. Arch. Gynecol. Obstet. 2017, 296, 661–677. [Google Scholar] [CrossRef] [Green Version]
- Chang, R.J.; Nakamura, R.M.; Judd, H.L.; Kaplan, S.A. Insulin Resistance in Nonobese Patients with Polycystic Ovarian Disease. J. Clin. Endocrinol. Metab. 1983, 57, 356–359. [Google Scholar] [CrossRef]
- Kempná, P.; Marti, N.; Udhane, S.; Flück, C.E. Regulation of androgen biosynthesis—A short review and preliminary results from the hyperandrogenic starvation NCI-H295R cell model. Mol. Cell. Endocrinol. 2015, 408, 124–132. [Google Scholar] [CrossRef]
- Nestler, J.E.; Jakubowicz, D.J. Lean Women with Polycystic Ovary Syndrome Respond to Insulin Reduction with Decreases in Ovarian P450c17? Activity and Serum Androgens 1. J. Clin. Endocrinol. Metab. 1997, 82, 4075–4079. [Google Scholar] [CrossRef] [PubMed]
- Baillargeon, J.-P.; Jakubowicz, D.J.; Iuorno, M.J.; Nestler, J.E. Effects of metformin and rosiglitazone, alone and in combination, in nonobese women with polycystic ovary syndrome and normal indices of insulin sensitivity. Fertil. Steril. 2004, 82, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Ito-Yamaguchi, A.; Suganuma, R.; Kumagami, A.; Hashimoto, S.; Yoshida-Komiya, H.; Fujimori, K. Effects of metformin on endocrine, metabolic milieus and endometrial expression of androgen receptor in patients with polycystic ovary syndrome. Gynecol. Endocrinol. 2015, 31, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, S.; Chang, Y.; Fang, C.; Liu, H.; Zhang, X.; Wang, Y. Effect of metformin treatment during pregnancy on women with PCOS: A systematic review and meta-analysis. Clin. Investig. Med. 2016, 39, 120–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campagnoli, C.; Abbà, C.; Ambroggio, S.; Brucato, T.; Pasanisi, P. Life-style and metformin for the prevention of endometrial pathology in postmenopausal women. Gynecol. Endocrinol. 2012, 29, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.H.; Corleta, H.V.E.; Capp, E.; Spritzer, P.M. Clinical, Metabolic and Endocrine Parameters in Response to Metformin in Obese Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind and Placebo-Controlled Trial. Horm. Metab. Res. 2003, 35, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Fanta, M.; Vrbikova, J.; Stanicka, S.; Dvorakova, K.; Hill, M.; Skrha, J.; Živný, J.; Skřenková, J. The effect of combination therapy with metformin and combined oral contraceptives (COC) versus COC alone on insulin sensitivity, hyperandrogenaemia, SHBG and lipids in PCOS patients. Hum. Reprod. 2005, 20, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Gambineri, A.; Pelusi, C.; Genghini, S.; Morselli/labate, A.M.; Cacciari, M.; Pagotto, U.; Pasquali, R. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin. Endocrinol. 2004, 60, 241–249. [Google Scholar] [CrossRef]
- Ibáñez, L.; Valls, C.; Marcos, M.V.; Ong, K.; Dunger, D.; De Zegher, F. Insulin Sensitization for Girls with Precocious Pubarche and with Risk for Polycystic Ovary Syndrome: Effects of Prepubertal Initiation and Postpubertal Discontinuation of Metformin Treatment. J. Clin. Endocrinol. Metab. 2004, 89, 4331–4337. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Glanville, J.; Hayden, C.J.; White, D.; Barth, J.H.; Balen, A.H. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo-controlled, double-blind multicentre study. Hum. Reprod. 2006, 21, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Popper, K.R. Logik der Forschung; Akademie: Wien, Austria, 1969. [Google Scholar]
- Bishop, D.V.; Maybery, M.; Maley, A.; Wong, D.; Hill, W.; Hallmayer, J. Using self-report to identify the broad phenotype in parents of children with autistic spectrum disorders: A study using the Autism-Spectrum Quotient. J. Child Psychol. Psychiatry 2004, 45, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Wheelwright, S.; Skinner, R.; Martin, J.; Clubley, E. The Autism-Spectrum Quotient (AQ): Evidence from Asperger Syndrome/High-Functioning Autism, Males and Females, Scientists and Mathematicians. J. Autism Dev. Disord. 2001, 31, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Woodbury-Smith, M.R.; Robinson, J.; Wheelwright, S.; Baron-Cohen, S. Screening Adults for Asperger Syndrome Using the AQ: A Preliminary Study of its Diagnostic Validity in Clinical Practice. J. Autism Dev. Disord. 2005, 35, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Auyeung, B.; Norgaardpedersen, B.; Hougaard, D.M.; Abdallah, M.W.; Melgaard, L.; Cohen, A.S.; Chakrabarti, B.; Ruta, L.; Lombardo, M. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry 2014, 20, 369–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Burghen, G.A.; Givens, J.R.; Kitabchi, A.E. Correlation of Hyperandrogenism with Hyperinsulinism in Polycystic Ovarian Disease*. J. Clin. Endocrinol. Metab. 1980, 50, 113–116. [Google Scholar] [CrossRef]
- Shackleton, C. Profiling steroid hormones and urinary steroids. J. Chromatogr. B Biomed. Sci. Appl. 1986, 379, 91–156. [Google Scholar] [CrossRef]
- Shackleton, C.H.L. Role of a Disordered Steroid Metabolome in the Elucidation of Sterol and Steroid Biosynthesis. Lipids 2012, 47, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Vogt, B.; Dick, B.; N’Gankam, V.; Frey, F.J.; Frey, B.M. Reduced 11B-hydroxysteroid dehydrogenase activity in patients with the nephrotic syndrome. J. Clin. Endocrinol. Metab. 1999, 84, 811–814. [Google Scholar]
- Morin-Papunen, L.; Vauhkonen, I.; Koivunen, R.; Ruokonen, A.; Martikainen, H.; Tapanainen, J.S. Metformin Versus Ethinyl Estradiol-Cyproterone Acetate in the Treatment of Nonobese Women with Polycystic Ovary Syndrome: A Randomized Study. J. Clin. Endocrinol. Metab. 2003, 88, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Georgiev, G.Z. Sample Size Calculator. Available online: https://www.gigacalculator.com/calculators/power-sample-size-calculator.php (accessed on 12 April 2021).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, Revision edition; Academic Press: New York, NY, USA, 1977; ISBN 9780121790608. [Google Scholar]
- Uanhoro JO Effect Size Calculators. Available online: https://github.com/stonegold546/cohens_d_calculators/blob/master/README.md (accessed on 12 April 2021).
- Lachenbruch, P.A. Statistical Power Analysis for the Behavioral Sciences (2nd Ed.). J. Am. Stat. Assoc. 1989, 84, 1096. [Google Scholar] [CrossRef]
- Hall, J.F. Review of Pallant J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using SPSS for Windows (Version 10 and 11); Open University Press: Berkshire, UK, 2001. [Google Scholar]
- Ackermann, D.; Groessl, M.; Pruijm, M.; Ponte, B.; Escher, G.; D’Uscio, C.H.; Guessous, I.; Ehret, G.; Pechère-Bertschi, A.; Martin, P.-Y.; et al. Reference intervals for the urinary steroid metabolome: The impact of sex, age, day and night time on human adult steroidogenesis. PLoS ONE 2019, 14, e0214549, Erratum in PLoS ONE 2021, 16, e0253678. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Jasuja, R. Selective androgen receptor modulators as function promoting therapies. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, E.; Guest, P.; Rahmoune, H.; Wang, L.; Levin, Y.; Ingudomnukul, E.; Ruta, L.; Kent, L.; Spain, M.; Baron-Cohen, S.; et al. Sex-specific serum biomarker patterns in adults with Asperger’s syndrome. Mol. Psychiatry 2010, 16, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M. The Influence of Social Hierarchy on Primate Health. Science 2005, 308, 648–652. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Mol. Psychiatry 2022, 27, 784–786. [Google Scholar] [CrossRef]
- EMA-European Medicines Agency. ICH Topic E 8 General Considerations for Clinical Trials; EMA-European Medicines Agency: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Die Bundesversammlung der Schweizerischen Eidgenossenschaft. Bundesgesetz Über Die Forschung Am Menschen (Humanforschungsgesetz, HFG); Die Bundesversammlung der Schweizerischen Eidgenossenschaft: Bern, Switzerland, 2011. [Google Scholar]
- Der Schweizerische Bundesrat. Verordnung Über Die Humanforschung Mit Ausnahme Der Klinischen Versuche (Humanforschungsverordnung, HFV); Der Schweizerische Bundesrat: Bern, Switzerland, 2013. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasser, B.; Kurz, J.; Buerki, S.; Mohaupt, M. Metformin-Treatment Option for Social Impairment? An Open Clinical Trial to Elucidate the Effects of Metformin Treatment on Steroid Hormones and Social Behavior. Life 2022, 12, 998. https://doi.org/10.3390/life12070998
Gasser B, Kurz J, Buerki S, Mohaupt M. Metformin-Treatment Option for Social Impairment? An Open Clinical Trial to Elucidate the Effects of Metformin Treatment on Steroid Hormones and Social Behavior. Life. 2022; 12(7):998. https://doi.org/10.3390/life12070998
Chicago/Turabian StyleGasser, Benedikt, Johann Kurz, Samuel Buerki, and Markus Mohaupt. 2022. "Metformin-Treatment Option for Social Impairment? An Open Clinical Trial to Elucidate the Effects of Metformin Treatment on Steroid Hormones and Social Behavior" Life 12, no. 7: 998. https://doi.org/10.3390/life12070998
APA StyleGasser, B., Kurz, J., Buerki, S., & Mohaupt, M. (2022). Metformin-Treatment Option for Social Impairment? An Open Clinical Trial to Elucidate the Effects of Metformin Treatment on Steroid Hormones and Social Behavior. Life, 12(7), 998. https://doi.org/10.3390/life12070998





