Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus
Abstract
1. Introduction
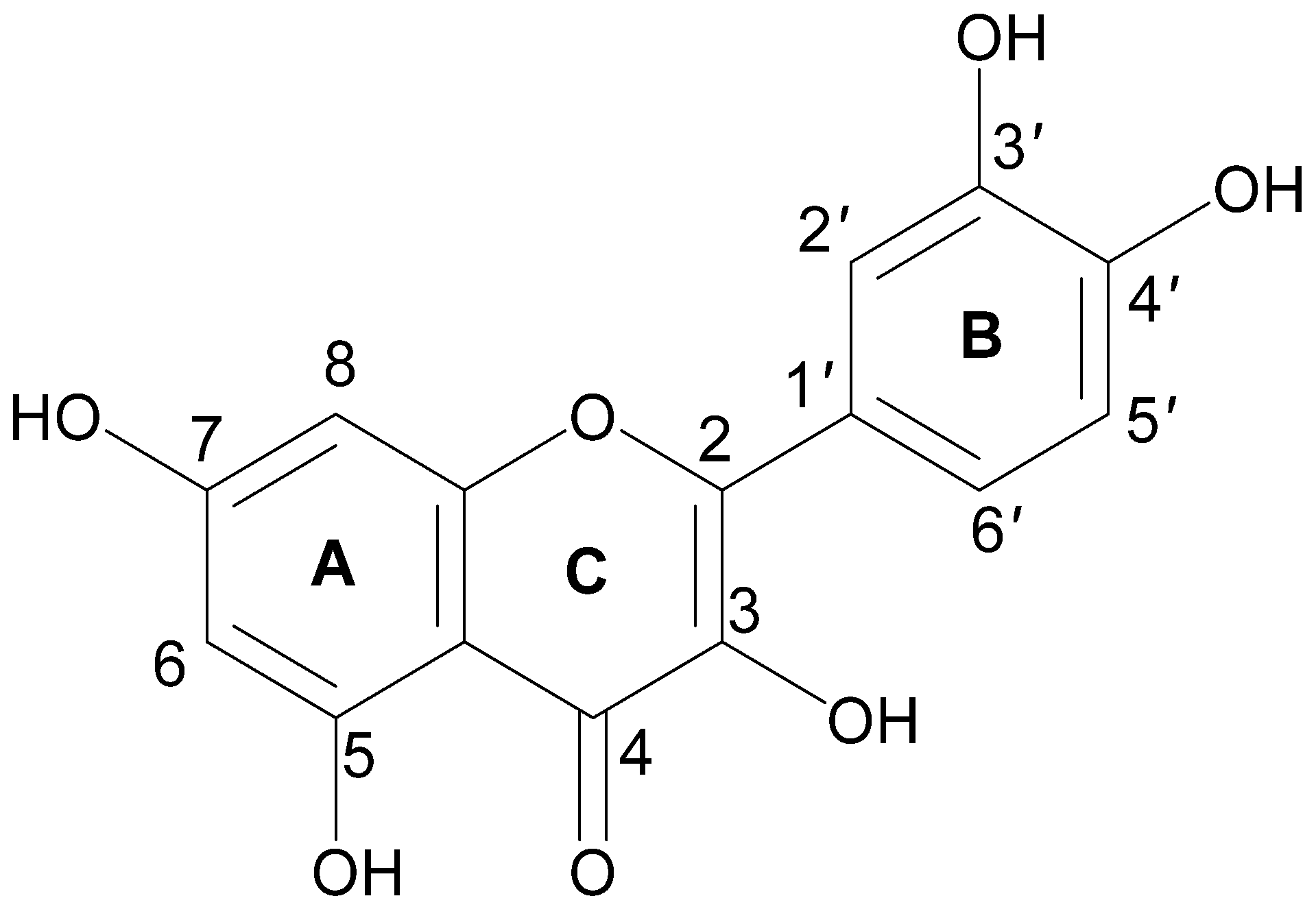
2. Chemistry of Quercetin
3. Pharmacological Actions of Quercetin in Diabetes and Associated Metabolic Disorders
4. Other Activities and Side Effects of Quercetin
| Plant Names | Plant Part(s) | Diabetic Model/s | Pharmacological Actions of Plants | Dose of Quercetin | Duration of Treatment | Pharmacological Actions of Quercetin | References |
|---|---|---|---|---|---|---|---|
| Acanthopanax senticosus | Root | Alloxan-induced diabetic rats | ↓ Blood glucose, total cholesterol, total bilirubin, creatinine, urea ↓ Oxidative stress | 50 mg/kg | 30 days | Inhibits α-glucosidase activity Reduces oxidative stress | [66,70,71] |
| Ginkgo biloba | Leaf | STZ-induced diabetic rats | ↑ β-cell mass and insulin secretion ↓ Amyloid-β neurotoxicity | 90 mg/kg | 10 weeks | Delays the progression of STZ-induced diabetic cataracts Reduces AGE products activity | [72,73] |
| Psidium guajava | Leaf | NA-STZ-induced diabetic rats | ↓ Oxidative stress ↓ Protein glycation ↓ Inflammation | 10- 50 mg/kg | 28 days | Reduces blood glucose levels Increases insulin secretion Improves T2DM-mediated cardiovascular disease | [74,75] |
| Momordica charantia | Fruit | HFF obese rats | ↓ Blood glucose, total cholesterol ↑ Insulin secretion | 50 mg/kg | 12 weeks | Reduces oxidation stress by inhibiting the release of chemokines and cytokines | [76,77] |
| Polygonum perfoliatum | Leaf | HFF obese rats | ↓ Blood glucose ↓ Inflammation | 60–240 mg/kg | 4 weeks | Inhibits α-glucosidase activity | [78] |
| Phyllanthus Emblica | Fruit | STZ-induced diabetic rats | ↓ Triglycerides, LDL, VLDL, total cholesterol ↑ HDL cholesterol | 25–75 mg/kg | 28 days | Decreases blood glucose Increases insulin secretion | [79] |
| Cuscuta chinensis | Seed | Alloxan-induced diabetic mice | ↓ Fasting blood glucose ↑ Insulin secretion Inhibits DPP-IV activity | 20 mg/kg | 3 weeks | Reduces fasting blood glucose level Enhances GLUT4 expression | [65,80] |
| Euphorbia helioscopia | Leaf, root | STZ-induced diabetic rats | ↑ Insulin secretion ↓ Blood glucose | 100 mg/kg | 7 weeks | Reduces blood glucose and blood glycated hemoglobin levels | [81,82] |
| Brassica rapa | Root | STZ-induced diabetic rats | ↓ Fasting blood glucose ↓ Inflammation ↓ Hypertension Inhibits DPP-IV activity | 15 mg/kg | 25 days | Decreases blood glucose levels Improves glucose tolerance | [83,84] |
| Crataegus pinnatifida | Leaf, fruit | STZ-induced diabetic rats | ↓ Fasting blood glucose ↓ VLDL and LDL cholesterol | 100 mg/kg | 14 days | Decreases blood glucose Increases plasma insulin | [85,86] |
| Sophora japonica | Bud, flower | STZ-induced diabetic rats | ↑ Insulin release Inhibits DPP-IV activity | 10–15 mg/kg | 10 days | Reduces blood glucose levels Improves glucose tolerance | [50,87] |
| Coriandrum sativum | Herb | STZ-induced diabetic rats | ↑ Insulin secretion ↓ Blood glucose ↓ Inflammation | 50 mg/kg | 8 weeks | Decreases fasting blood glucose Suppresses TNF-α, IL-1β, and production of AGEs | [88,89,90] |
| Cymbopogon citratus | Herb | STZ-induced diabetic rats | ↓ Fasting blood glucose ↓ Inflammation ↓ Hypertension ↑ Insulin secretion | 20–50 mg/kg | 6 weeks | Reduces blood glucose levels Decreases the production of reactive oxygen species (ROS) Improves T2DM-mediated testicular damage | [91,92,93] |
| Allium cepa | Bulb | STZ-induced diabetic rats | ↓ Blood glucose ↓ Triglycerides, LDL, VLDL, total cholesterol ↑ HDL cholesterol ↑ Insulin secretion | 100–200 mg/kg | 6 weeks | Lowers blood glucose Improves glucose tolerance | [94,95,96] |
| Prunus avium | Fruit | STZ-induced diabetic rats | ↓ Blood glucose ↑ Insulin secretion ↓ LDL and VLDL cholesterol | 50–80 mg/kg | 45 days | Reduces blood glucose levels Improves oxidative stress | [97,98,99] |
| Capparis spinosa | Fruit | Alloxan-induced diabetic mice | ↓ Fasting blood glucose ↑ Insulin secretion ↓ Liver damage | 50 mg/kg | 7 days | Decreases fasting blood glucose Reduces ALT and AST levels | [100,101,102] |
| Brassica oleracea var. Italica | Flower | STZ-induced diabetic rats | ↑ Insulin secretion ↓ Blood glucose | 10 mg/kg | 4 weeks | Decreases blood glucose levels Reduces creatinine and blood urea nitrogen levels | [103,104,105] |
| Lactuca sativa | Leaf | Alloxan-induced diabetic rats | ↓ Fasting blood glucose ↑ Insulin secretion ↓ Inflammation | 50 mg/kg | 4 weeks | Reduces blood glucose levels Decreases creatinine, ALT, AST, and cholesterol levels | [106,107,108] |
| Asparagus officinalis | Stem | STZ-induced diabetic rats | ↑ Insulin secretion ↓ Blood glucose ↓ Inflammation | 50 mg/kg | 12 weeks | Reduces fasting blood glucose Decreases the production of reactive oxygen species (ROS) Improves glucose tolerance | [109,110] |
| Acacia arabica | Bark | HFF-induced obese diabetic rats | ↑ Insulin secretion Inhibits DPP-IV activity ↓ Protein glycation | 30 mg/kg | 8 weeks | Reduces fasting blood glucose Decreases LDL and TG levels Increases HDL levels | [15,111] |
| Solanum lycopersicum | Fruit | STZ-induced diabetic rats | ↓ Blood glucose ↑ Insulin secretion | 10 mg/kg | 28 days | Decreases blood glucose levels Increases insulin secretion Inhibits apoptosis | [112,113] |
| Piper nigrum | Flower | Alloxan-induced diabetic mice | ↑ Insulin secretion ↓ Blood glucose ↓ Inflammation | 50 mg/kg | 7 days | Reduces blood glucose levels | [114,115] |
| Toona sinensis | HFF-induced obese diabetic rats | ↑ Insulin secretion ↓ Blood glucose ↓ Inflammation | 200 mg/kg | 4 weeks | Improves glucose tolerance Decreases TG and TC levels | [116] |
5. Mechanisms of Action of Quercetin
6. Effects of Quercetin on Diabetic Complications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | Diabetes Mellitus |
| T2DM | Type 2 Diabetes Mellitus |
| DPP-IV | Dipeptidyl peptidase 4 |
| GLP-1 | Glucagon-like peptide-1 |
| GIP | Glucose-dependent insulinotropic polypeptide |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| SGLT2 | Sodium-glucose cotransporter-2 |
| PPAR | Peroxisome proliferator-activated receptors |
| VEGF | Vascular endothelial growth factor |
| TG | Triglycerides |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| VLDL | Very low-density lipoprotein |
| HMG-CoA | 3-hydroxy-3-methylglutaryl coenzyme A |
| HFD | High fat fed |
| CTGF | Connective tissue growth factor |
| TGF-β1 | Transforming growth factor-β1 |
| CYP2E1 | Cytochrome P450 2E1 |
| BMI | Body mass index |
| NAFLD | Non-alcoholic fatty liver disease |
| NA | Nicotinamide |
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| STZ | Streptozotocin |
| AGE | Advanced Glycation End Products |
| IRS-1 | Insulin receptor substrate 1 |
| AMPK | AMP-activated protein kinase |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| G6Pase | Glucose 6 Phosphate |
| Akt | Protein kinase B |
| AMP | Adenosine monophosphate |
| ATP | Adenosine triphosphate |
| ROS | Reactive oxygen species |
| MMP-9 | Matrix metalloproteinase 9 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| JNK | c-Jun N-terminal kinase |
| NF-κB | Nuclear transcription factor kappa-B |
| PKC | Protein kinase C |
| Syk | Spleen tyrosine kinase |
| MAPK | p38 mitogen-activated protein kinase |
| COX-2 | Cyclooxygenase-2 |
| GPX4 | Glutathione peroxidase 4 |
| NPC1L1 | Niemann-Pick C1-Like 1 |
| RAAS | Renin-angiotensin-aldosterone system |
References
- Kharroubi, A.T.; Darwish, H.M. Diabetes Mellitus: The Epidemic of the Century. World J. Diabetes 2015, 6, 850. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 37, S81–S90. [Google Scholar] [CrossRef]
- Lamb, M.M.; Yin, X.; Zerbe, G.O.; Klingensmith, G.J.; Dabelea, D.; Fingerlin, T.E.; Rewers, M.; Norris, J.M. Height Growth Velocity, Islet Autoimmunity and Type 1 Diabetes Development: The Diabetes Autoimmunity Study in the Young. Diabetologia 2009, 52, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P.; Harmon, J.; Tran, P.O.; Tanaka, Y.; Takahashi, H. Glucose Toxicity in β-Cells: Type 2 Diabetes, Good Radicals Gone Bad, and the Glutathione Connection. Diabetes 2003, 52, 581–587. [Google Scholar] [CrossRef]
- Akhtar, S.; Nasir, J.A.; Sarwar, A.; Nasr, N.; Javed, A.; Majeed, R.; Salam, M.A.; Billah, B. Prevalence of Diabetes and Pre-Diabetes in Bangladesh: A Systematic Review and Meta-Analysis. BMJ Open 2020, 10, e036086. [Google Scholar] [CrossRef]
- Craig, M.E.; Hattersley, A.; Donaghue, K.C. Definition, Epidemiology and Classification of Diabetes in Children and Adolescents. Pediatr. Diabetes 2009, 10, 3–12. [Google Scholar] [CrossRef]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 Diabetes Mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef]
- Kilic, G.; Alvarez-Mercado, A.I.; Zarrouki, B.; Opland, D.; Liew, C.W.; Alonso, L.C.; Myers, M.G.; Jonas, J.-C.; Poitout, V.; Kulkarni, R.N.; et al. The Islet Estrogen Receptor-α Is Induced by Hyperglycaemia and Protects against Oxidative Stress-Induced Insulin-Deficient Diabetes. PLoS ONE 2014, 9, e87941. [Google Scholar] [CrossRef]
- Devendra, D.; Liu, E.; Eisenbarth, G.S. Type 1 Diabetes: Recent Developments. BMJ 2004, 328, 750–754. [Google Scholar] [CrossRef]
- Dabelea, D.; Mayer-Davis, E.J.; Saydah, S.; Imperatore, G.; Linder, B.; Divers, J.; Bell, R.; Badaru, A.; Talton, J.W.; Crume, T.; et al. Prevalence of Type 1 and Type 2 Diabetes among Children and Adolescents from 2001 to 2009. JAMA 2014, 311, 1778–1786. [Google Scholar] [CrossRef]
- Chiang, J.L.; Kirkman, M.S.; Laffel, L.M.B.; Peters, A.L. Type 1 Diabetes through the Life Span: A Position Statement of the American Diabetes Association. Diabetes Care 2014, 37, 2034–2054. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Insulin Secretory and Antidiabetic Actions of Heritiera Fomes Bark Together with Isolation of Active Phytomolecules. PLoS ONE 2022, 17, e0264632. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Insulinotropic and antidiabetic properties of Eucalyptus citriodora leaves and isolation of bioactive phytomolecules. J. Pharm. Pharmacol. 2021, 73, 1049–1061. [Google Scholar] [CrossRef]
- Parasuraman, S.; Anand David, A.; Arulmoli, R. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Identification of Multiple Pancreatic and Extra-Pancreatic Pathways Underlying the Glucose-Lowering Actions of Acacia Arabica Bark in Type-2 Diabetes and Isolation of Active Phytoconstituents. Plants 2021, 10, 1190. [Google Scholar] [CrossRef]
- Diamant, M.; Heine, R.J. Thiazolidinediones in Type 2 Diabetes Mellitus. Drugs 2003, 63, 1373–1405. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Liu, Y.-C.; Jheng, J.-R.; Tsai, H.-P.; Jan, J.-T.; Wong, W.-R.; Horng, J.-T. Anti-Enterovirus 71 Activity Screening of Chinese Herbs with Anti-Infection and Inflammation Activities. Am. J. Chin. Med. 2009, 37, 143–158. [Google Scholar] [CrossRef]
- Talirevic, E.; Sehovic, J. Quercetin in the Treatment of Dyslipidemia. Med. Res. Arch. 2012, 66, 87. [Google Scholar] [CrossRef]
- Ay, M.; Luo, J.; Langley, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Molecular Mechanisms Underlying Protective Effects of Quercetin against Mitochondrial Dysfunction and Progressive Dopaminergic Neurodegeneration in Cell Culture and MitoPark Transgenic Mouse Models of Parkinson’s Disease. J. Neurochem. 2017, 141, 766–782. [Google Scholar] [CrossRef]
- Halban, P.A.; Polonsky, K.S.; Bowden, D.W.; Hawkins, M.A.; Ling, C.; Mather, K.J.; Powers, A.C.; Rhodes, C.J.; Sussel, L.; Weir, G.C. β-Cell Failure in Type 2 Diabetes: Postulated Mechanisms and Prospects for Prevention and Treatment. J. Clin. Endocrinol. Metab. 2014, 99, 1983–1992. [Google Scholar] [CrossRef]
- Usman, B.; Sharma, N.; Satija, S.; Mehta, M.; Vyas, M.; Khatik, G.L.; Khurana, N.; Hansbro, P.M.; Williams, K.; Dua, K. Recent Developments in Alpha-Glucosidase Inhibitors for Management of Type-2 Diabetes: An Update. Curr. Pharm. Des. 2019, 25, 2510–2525. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Prakash, O. Enzymes Inhibition and Antidiabetic Effect of Isolated Constituents from Dillenia Indica. Biomed Res. Int. 2013, 2013, e382063. [Google Scholar] [CrossRef]
- Wang, G.S.; Hoyte, C. Review of Biguanide (Metformin) Toxicity. J. Intensive Care Med. 2018, 34, 863–876. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The Mechanisms of Action of Metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Lamos, E.M.; Levitt, D.L.; Munir, K.M. A Review of Dopamine Agonist Therapy in Type 2 Diabetes and Effects on Cardio-Metabolic Parameters. Prim. Care Diabetes 2016, 10, 60–65. [Google Scholar] [CrossRef]
- Andersen, I.B.; Andreassen, M.; Krogh, J. The Effect of Dopamine Agonists on Metabolic Variables in Adults with Type 2 Diabetes: A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomized Clinical Trials. Diabetes Obes. Metab. 2020, 23, 58–67. [Google Scholar] [CrossRef]
- Pathak, R.; Bridgeman, M.B. Dipeptidyl Peptidase-4 (DPP-4) Inhibitors in the Management of Diabetes. Pharmacol. Ther. 2010, 35, 509–513. [Google Scholar]
- Capuano, A.; Sportiello, L.; Maiorino, M.I.; Rossi, F.; Giugliano, D.; Esposito, K. Dipeptidyl Peptidase-4 Inhibitors in Type 2 Diabetes therapy–Focus on Alogliptin. Drug Des. Devel. Ther. 2013, 7, 989–1001. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Marchesini, G. Time for Glucagon like Peptide-1 Receptor Agonists Treatment for Patients with NAFLD? J. Hepatol. 2016, 64, 262–264. [Google Scholar] [CrossRef]
- Unger, J.R.; Parkin, C.G. Glucagon-like Peptide-1 (GLP-1) Receptor Agonists: Differentiating the New Medications. Diabetes Ther. 2011, 2, 29–39. [Google Scholar] [CrossRef]
- Black, C.; Donnelly, P.; McIntyre, L.; Royle, P.; Shepherd, J.J.; Thomas, S. Meglitinide Analogues for Type 2 Diabetes Mellitus. Cochrane Database Syst. Rev. 2007, 2010, CD004654. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake, P.; Hupfeld, C.; Mudaliar, S. Sodium-Glucose Cotransporter Type 2 (SGLT-2) Inhibitors and Ketogenesis: The Good and the Bad. Curr. Diab. Rep. 2020, 20, 74. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, R.A.; Baar, M.J.B.; Lytvyn, Y.; Bjornstad, P.; Nieuwdorp, M.; Cherney, D.Z.I.; Raalte, D.H. Sodium Glucose Cotransporter (SGLT)-2 Inhibitors: Do We Need Them for Glucose-Lowering, for Cardiorenal Protection or Both? Diabetes Obes. Metab. 2019, 21, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Sola, D.; Rossi, L.; Schianca, G.P.C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; Bartoli, E.; Derosa, G. State of the Art Paper Sulfonylureas and Their Use in Clinical Practice. Arch. Med. Sci. 2015, 4, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Aamir, A.H.; Raza, A.; Das, A.K.; Khan, A.A.; Shrestha, D.; Qureshi, M.F.; Fariduddin, M.; Pathan, M.F.; Jawad, F.; et al. Place of Sulfonylureas in the Management of Type 2 Diabetes Mellitus in South Asia: A Consensus Statement. Indian J. Endocrinol. Metab. 2015, 19, 577. [Google Scholar] [CrossRef]
- Rizos, C.V.; Kei, A.; Elisaf, M.S. The Current Role of Thiazolidinediones in Diabetes Management. Arch. Toxicol. 2016, 90, 1861–1881. [Google Scholar] [CrossRef]
- Shebeko, S.K.; Zupanets, I.A.; Popov, O.S.; Tarasenko, O.O.; Shalamay, A.S. Effects of Quercetin and Its Combinations on Health. In Polyphenols: Mechanisms of Action in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 373–394. [Google Scholar] [CrossRef]
- Williams, C.A.; Grayer, R.J. Anthocyanins and Other Flavonoids. Nat. Prod. Rep. 2004, 21, 539. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and Prooxidant Behavior of Flavonoids: Structure-Activity Relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Metodiewa, D.; Jaiswal, A.K.; Cenas, N.; Dickancaité, E.; Segura-Aguilar, J. Quercetin May Act as a Cytotoxic Prooxidant after Its Metabolic Activation to Semiquinone and Quinoidal Product. Free Radic. Biol. Med. 1999, 26, 107–116. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.; Ayeni, P.O.; Omojokun, O.S.; Bello, F.O. Comparative Effect of Quercetin and Rutin on α-Amylase, α-Glucosidase, and Some Pro-Oxidant-Induced Lipid Peroxidation in Rat Pancreas. Comp. Clin. Path. 2014, 24, 1103–1110. [Google Scholar] [CrossRef]
- Spínola, V.; Llorent-Martínez, E.J.; Castilho, P.C. Inhibition of α-Amylase, α-Glucosidase and Pancreatic Lipase by Phenolic Compounds of Rumex Maderensis (Madeira Sorrel). Influence of Simulated Gastrointestinal Digestion on Hyperglycaemia-Related Damage Linked with Aldose Reductase Activity and Protein Glycation. LWT 2020, 118, 108727. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-Amylase and α-Glucosidase: Potential Linkage for Whole Cereal Foods on Prevention of Hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Yang, D.K.; Kang, H.-S. Anti-Diabetic Effect of Cotreatment with Quercetin and Resveratrol in Streptozotocin-Induced Diabetic Rats. Biomol. Ther. 2018, 26, 130–138. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediators Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef]
- Adewole, S.; Caxton-Martins, E.; Ojewole, J. Protective Effect of Quercetin on the Morphology of Pancreatic β-Cells of Streptozotocin-Treated Diabetic Rats. Afr. J. Tradit. Complement Altern. Med. 2007, 4, 64–74. [Google Scholar] [CrossRef]
- Dhanya, R.; Arya, A.D.; Nisha, P.; Jayamurthy, P. Quercetin, a Lead Compound against Type 2 Diabetes Ameliorates Glucose Uptake via AMPK Pathway in Skeletal Muscle Cell Line. Front. Pharmacol. 2017, 8, 336. [Google Scholar] [CrossRef]
- Chen, P.; Chen, J.; Zheng, Q.; Chen, W.; Wang, Y.; Xu, X. Pioglitazone, Extract of Compound Danshen Dripping Pill, and Quercetin Ameliorate Diabetic Nephropathy in Diabetic Rats. J. Endocrinol. Investig. 2013, 36, 422–427. [Google Scholar] [CrossRef]
- Thomas, A.A.; Feng, B.; Chakrabarti, S. ANRIL: A Regulator of VEGF in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 470. [Google Scholar] [CrossRef]
- Vessal, M.; Hemmati, M.; Vasei, M. Antidiabetic Effects of Quercetin in Streptozocin-Induced Diabetic Rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 135, 357–364. [Google Scholar] [CrossRef]
- Shi, G.-J.; Li, Y.; Cao, Q.-H.; Wu, H.-X.; Tang, X.-Y.; Gao, X.-H.; Yu, J.-Q.; Chen, Z.; Yang, Y. In Vitro and in Vivo Evidence That Quercetin Protects against Diabetes and Its Complications: A Systematic Review of the Literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.; Zhao, A.; Wang, K.; Fan, Z.; Yang, H.; Liao, W.; Bao, S.; Zhao, L.; Zhang, Y.; et al. Transcriptomic and Metabonomic Profiling Reveal Synergistic Effects of Quercetin and Resveratrol Supplementation in High Fat Diet Fed Mice. J. Proteome Res. 2012, 11, 4961–4971. [Google Scholar] [CrossRef]
- Saisho, Y.; Kou, K.; Tanaka, K.; Abe, T.; Kurosawa, H.; Shimada, A.; Meguro, S.; Kawai, T.; Itoh, H. Postprandial Serum C-Peptide to Plasma Glucose Ratio as a Predictor of Subsequent Insulin Treatment in Patients with Type 2 Diabetes. Endocr. J. 2011, 58, 315–322. [Google Scholar] [CrossRef]
- Kulkarni, C.R.; Joglekar, M.M.; Patil, S.B.; Arvindekar, A.U. Antihyperglycemic and Antihyperlipidemic Effect OfSantalum Albumin Streptozotocin Induced Diabetic Rats. Pharm. Biol. 2011, 50, 360–365. [Google Scholar] [CrossRef]
- Yim, S.; Malhotra, A.; Veves, A. Antioxidants and CVD in Diabetes: Where Do We Stand Now? Curr. Diab. Rep. 2007, 7, 8–13. [Google Scholar] [CrossRef][Green Version]
- Spencer, J.P.E.; Vauzour, D.; Rendeiro, C. Flavonoids and Cognition: The Molecular Mechanisms Underlying Their Behavioural Effects. Arch. Biochem. Biophys. 2009, 492, 1–9. [Google Scholar] [CrossRef]
- Sikder, K.; Das, N.; Kesh, S.B.; Dey, S. Quercetin and Beta-Sitosterol Prevent High Fat Diet Induced Dyslipidemia and Hepatotoxicity in Swiss Albino Mice. Indian J. Exp. Biol. 2014, 52, 60–66. [Google Scholar]
- Mazloom, Z.; Abdollahzadeh, S.M.; Dabbaghmanesh, M.H.; Rezaianzadeh, A. The Effect of Quercetin Supplementation on Oxidative Stress, Glycemic Control, Lipid Profile, and Insulin Resistance in Type 2 Diabetes: A Randomized Clinical Trial. J. Health Sci. Surveill. Sys. 2014, 2, 8–14. [Google Scholar]
- Youl, E.; Bardy, G.; Magous, R.; Cros, G.; Sejalon, F.; Virsolvy, A.; Richard, S.; Quignard, J.; Gross, R.; Petit, P.; et al. Quercetin Potentiates Insulin Secretion and Protects INS-1 Pancreatic β-Cells against Oxidative Damage via the ERK1/2 Pathway. Br. J. Pharmacol. 2010, 161, 799–814. [Google Scholar] [CrossRef]
- Heřmánková, E.; Zatloukalová, M.; Biler, M.; Sokolová, R.; Bancířová, M.; Tzakos, A.G.; Křen, V.; Kuzma, M.; Trouillas, P.; Vacek, J. Redox Properties of Individual Quercetin Moieties. Free Radic. Biol. Med. 2019, 143, 240–251. [Google Scholar] [CrossRef]
- Sohn, E.-J.; Kim, J.M.; Kang, S.-H.; Kwon, J.; An, H.J.; Sung, J.-S.; Cho, K.A.; Jang, I.-S.; Choi, J.-S. Restoring Effects of Natural Antioxidant Quercetin on Cellular Senescent Human Dermal Fibroblasts. Am. J. Chin. Med. 2018, 46, 853–873. [Google Scholar] [CrossRef]
- Nekohashi, M.; Ogawa, M.; Ogihara, T.; Nakazawa, K.; Kato, H.; Misaka, T.; Abe, K.; Kobayashi, S. Luteolin and Quercetin Affect the Cholesterol Absorption Mediated by Epithelial Cholesterol Transporter Niemann–Pick C1-like 1 in Caco-2 Cells and Rats. PLoS ONE 2014, 9, e97901. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, M.S.; Nandini, C.D. Influence of Quercetin, Naringenin and Berberine on Glucose Transporters and Insulin Signalling Molecules in Brain of Streptozotocin-Induced Diabetic Rats. Biomed. Pharmacother. 2017, 94, 605–611. [Google Scholar] [CrossRef]
- Haddad, P.; Eid, H.; Nachar, A.; Thong, F.; Sweeney, G. The Molecular Basis of the Antidiabetic Action of Quercetin in Cultured Skeletal Muscle Cells and Hepatocytes. Pharmacogn. J. 2015, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Meerza, D.; Naseem, I. Protective Effect of Quercetin on Hyperglycaemia, Oxidative Stress and DNA Damage in Alloxan Induced Type 2 Diabetic Mice. Life Sci. 2014, 109, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Fu, J.; Yuan, J.; Zhang, N.; Gao, B.; Fu, G.; Tu, Y.; Zhang, Y. Anti-Diabetic Activities of Acanthopanax Senticosus Polysaccharide (ASP) in Combination with Metformin. Int. J. Biol. Macromol. 2012, 50, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Takahashi, S.; Yoshimori, A.; Yamamoto, T.; Sato, A.; Kamiya, T.; Abe, H.; Abe, T.; Tanuma, S. Discovery of a New Type of Scaffold for the Creation of Novel Tyrosinase Inhibitors. Bioorg. Med. Chem. 2016, 24, 4509–4515. [Google Scholar] [CrossRef]
- Yi, H.; Peng, H.; Wu, X.; Xu, X.; Kuang, T.; Zhang, J.; Du, L.; Fan, G. The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence. Oxid. Med. Cell. Longev. 2021, 2021, e6678662. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A Flavonol with Multifaceted Therapeutic Applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Zhou, H.; Xing, J.; Liu, S.; Song, F.; Cai, Z.; Pi, Z.; Liu, Z.; Liu, S. Screening and Determination for Potential α-Glucosidase Inhibitors from Leaves of Acanthopanax Senticosus Harms by Using UF-LC/MS and ESI-MSn. Phytochem. Anal. 2011, 23, 315–323. [Google Scholar] [CrossRef]
- Nabi, R.K.; Abdullah, M.A. Effect of Quercetin on Parenchymatous Organ of the Alloxan Induced Diabetes in Male Rats. IJRMS 2020, 8, 3809. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, G.-H.; Wang, H.-N.; Hu, Q.; Chen, L.-L.; Guan, X.-Q.; Li, H.-L.; Chen, H.-Z.; Tang, H.; Ge, G.-B. Discovery of Naturally Occurring Inhibitors against SARS-CoV-2 3Clpro from Ginkgo Biloba Leaves via Large-Scale Screening. Fitoterapia 2021, 152, 104909. [Google Scholar] [CrossRef]
- Lu, Q.; Hao, M.; Wu, W.; Zhang, N.; Isaac, A.T.; Yin, J.; Zhu, X.; Du, L.; Yin, X. Antidiabetic Cataract Effects of GbE, Rutin and Quercetin Are Mediated by the Inhibition of Oxidative Stress and Polyol Pathway. Acta Biochim. Pol. 2017, 65, 35–41. [Google Scholar] [CrossRef]
- Lin, C.-F.; Kuo, Y.-T.; Chen, T.-Y.; Chien, C.-T. Quercetin-Rich Guava (Psidium Guajava) Juice in Combination with Trehalose Reduces Autophagy, Apoptosis and Pyroptosis Formation in the Kidney and Pancreas of Type II Diabetic Rats. Molecules 2016, 21, 334. [Google Scholar] [CrossRef]
- Roslan, J.; Giribabu, N.; Karim, K.; Salleh, N. Quercetin Ameliorates Oxidative Stress, Inflammation and Apoptosis in the Heart of Streptozotocin-Nicotinamide-Induced Adult Male Diabetic Rats. Biomed. Pharmacother. 2017, 86, 570–582. [Google Scholar] [CrossRef]
- Ansari, P.; Hannon-Fletcher, M.P.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Effects of 22 Traditional Anti-Diabetic Medicinal Plants on DPP-IV Enzyme Activity and Glucose Homeostasis in High-Fat Fed Obese Diabetic Rats. Biosci. Rep. 2021, 41, BSR20203824. [Google Scholar] [CrossRef]
- Rasheed, R.A.; Elshikh, M.S.; Mohamed, M.O.; Darweesh, M.F.; Hussein, D.S.; Almutairi, S.M.; Embaby, A.S. Quercetin Mitigates the Adverse Effects of High Fat Diet on Pancreatic and Renal Tissues in Adult Male Albino Rats. J. King Saud Univ. Sci. 2022, 34, 101946. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Xu, C.; Wang, B.; Yu, J.; Kang, X.; Liu, X.; Zhou, L.; Qin, Y.; Liao, L.; et al. Effects of total flavonoids extracted from Polygonum perfoliatum L. on hypolipidemic and antioxidant in hyperlipidemia rats induced by high-fat diet. Int. J. Clin. Exp. Med. 2018, 11, 6758–6766. [Google Scholar]
- Srinivasan, P.; Vijayakumar, S.; Kothandaraman, S.; Palani, M. Anti-Diabetic Activity of Quercetin Extracted from Phyllanthus emblica, L. Fruit: In Silico and in Vivo Approaches. J. Pharm. Anal. 2018, 8, 109–118. [Google Scholar] [CrossRef]
- Al-Sultany, F.H.; Al-Hussaini, I.M.; Al- Saadi, A.H. Studying Hypoglycemic Activity of Cuscuta Chinesis Lam. On Type 1 Diabetes Mellitus in White Male Rats. J. Phys. Conf. Ser. 2019, 1294, 062020. [Google Scholar] [CrossRef]
- Mustafa, I.; Anwar, H.; Irfan, S.; Muzaffar, H.; Ijaz, M.U. Attenuation of Carbohydrate Metabolism and Lipid Profile by Methanolic Extract of Euphorbia Helioscopia and Improvement of Beta Cell Function in a Type 2 Diabetic Rat Model. BMC Complement Altern. Med. 2022, 22, 23. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kang, M.-J.; Choi, H.-N.; Jeong, S.-M.; Lee, Y.-M.; Kim, J.-I. Quercetin Attenuates Fasting and Postprandial Hyperglycemia in Animal Models of Diabetes Mellitus. Nutr. Res. Prac. 2011, 5, 107–111. [Google Scholar] [CrossRef]
- Hassanpour Fard, M.; Naseh, G.; Lotfi, N.; Hosseini, S.M.; Hosseini, M. Effects of Aqueous Extract of Turnip Leaf (Brassica Rapa) in Alloxan-Induced Diabetic Rats. Avicenna J. Med. 2015, 5, 148–156. [Google Scholar]
- Abdelmoaty, M.A.; Ibrahim, M.A.; Ahmed, N.S.; Abdelaziz, M.A. Confirmatory Studies on the Antioxidant and Antidiabetic Effect of Quercetin in Rats. Indian J. Clin. Biochem. 2010, 25, 188–192. [Google Scholar] [CrossRef]
- Dehghani, S.; Mehri, S.; Hosseinzadeh, H. The Effects of Crataegus Pinnatifida (Chinese Hawthorn) on Metabolic Syndrome: A Review. Iran. J. Basic Med. Sci. 2019, 22, 460. [Google Scholar] [CrossRef]
- Iskender, H.; Dokumacioglu, E.; Sen, T.M.; Ince, I.; Kanbay, Y.; Saral, S. The Effect of Hesperidin and Quercetin on Oxidative Stress, NF-ΚB and SIRT1 Levels in a STZ-Induced Experimental Diabetes Model. Biomed. Pharmacother. 2017, 90, 500–508. [Google Scholar] [CrossRef]
- Wang, T.; Miao, M.; Bai, M.; Li, Y.; Li, M.; Li, C.; Xu, Y. Effect of Sophora Japonica Total Flavonoids on Pancreas, Kidney Tissue Morphology of Streptozotocin-Induced Diabetic Mice Model. Saudi J. Biol. Sci. 2017, 24, 741–747. [Google Scholar] [CrossRef]
- Eidi, M.; Eidi, A.; Saeidi, A.; Molanaei, S.; Sadeghipour, A.; Bahar, M.; Bahar, K. Effect of Coriander Seed (Coriandrum Sativum L.) Ethanol Extract on Insulin Release from Pancreatic Beta Cells in Streptozotocin-Induced Diabetic Rats. Phytother. Res. 2009, 23, 404–406. [Google Scholar] [CrossRef]
- Tang, L.; Li, K.; Zhang, Y.; Li, H.; Li, A.; Xu, Y.; Wei, B. Quercetin Liposomes Ameliorate Streptozotocin-Induced Diabetic Nephropathy in Diabetic Rats. Sci. Rep. 2020, 10, 2440. [Google Scholar] [CrossRef]
- Das, S.; Chaware, S.; Narkar, N.; Tilak, A.V.; Raveendran, S.; Rane, P. Antidiabetic Activity of Coriandrum Sativum in Streptozotocin Induced Diabetic Rats. Int. J. Basic Clin. Pharmacol. 2019, 8, 925–929. [Google Scholar] [CrossRef]
- Elekofehinti, O.O.; Onunkun, A.T.; Olaleye, T.M. Cymbopogon Citratus (DC.) Stapf Mitigates ER-Stress Induced by Streptozotocin in Rats via Down-Regulation of GRP78 and Up-Regulation of Nrf2 Signaling. J. Ethnopharmacol. 2020, 262, 113130. [Google Scholar] [CrossRef]
- Ahmed, N.Z.; Ibrahim, S.R.; Ahmed-Farid, O.A. Quercetin and Apigenin of Cymbopogon Citratus Mediate Inhibition of HCT-116 and PC-3 Cell Cycle Progression and Ameliorate Doxorubicin-Induced Testicular Dysfunction in Male Rats. Biomed. Res. Ther. 2018, 5, 2466–2479. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Zhai, Q.; Zhu, Y.; Liu, B.; Xu, Y. Quercetin Ameliorates Testosterone Secretion Disorder by Inhibiting Endoplasmic Reticulum Stress through the MiR-1306-5p/HSD17B7 Axis in Diabetic Rats. Bosn. J. Basic Med. Sci. 2022, 22, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Ozougwu, C.J. Anti-Diabetic Effects of Allium Cepa (Onions) Aqueous Extracts on Alloxan-Induced Diabetic Rattus Novergicus. J. Med. Plant Res. 2011, 5, 1134–1139. [Google Scholar] [CrossRef]
- Khaki, A.; Fathiazad, F.; Ashtiani, H.R.; Rezazadeh, S.; Rastegar, H.; Imani, A.M. Compartments of Quercetin & Allium Cepa (Onion) on Blood Glucose in Diabetic Rats. J. Med. Plants 2010, 9, 107–112. [Google Scholar]
- Campos, K.E.; Diniz, Y.S.; Cataneo, A.C.; Faine, L.A.; Alves, M.J.Q.F.; Novelli, E.L.B. Hypoglycaemic and Antioxidant Effects of Onion, Allium Cepa: Dietary Onion Addition, Antioxidant Activity and Hypoglycaemic Effects on Diabetic Rats. Int. J. Food Sci. Nutr. 2003, 54, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Vinitha, E.; Singh, H.J.C.; Kakalij, R.M.; Kshirsagar, R.P.; Kumar, B.H.; Diwan, P.V. Neuroprotective Effect of Prunus Avium on Streptozotocin Induced Neurotoxicity in Mice. Biomed. Prev. Nutr. 2014, 4, 519–525. [Google Scholar] [CrossRef]
- Faienza, M.F.; Corbo, F.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Grano, M.; Wang, D.Q.; D’Amato, G.; Muraglia, M.; Franchini, C.; et al. Novel Insights in Health-Promoting Properties of Sweet Cherries. J. Funct. Foods 2020, 69, 103945. [Google Scholar] [CrossRef]
- Mahesh, T.; Menon, V.P. Quercetin Allievates Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Phytother. Res. 2004, 18, 123–127. [Google Scholar] [CrossRef]
- Huseini, H.F.; Hasani-Rnjbar, S.; Nayebi, N.; Heshmat, R.; Sigaroodi, F.K.; Ahvazi, M.; Alaei, B.A.; Kianbakht, S. Capparis spinosa L. (Caper) Fruit Extract in Treatment of Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Complement. Ther. Med. 2013, 21, 447–452. [Google Scholar] [CrossRef]
- Sirovina, D.; Oršolić, N.; Končić, M.Z.; Kovačević, G.; Benković, V.; Gregorović, G. Quercetin vs Chrysin. Hum. Exp. Toxicol. 2013, 32, 1058–1066. [Google Scholar] [CrossRef]
- Kalantari, H.; Foruozandeh, H.; Khodayar, M.J.; Siahpoosh, A.; Saki, N.; Kheradmand, P. Antioxidant and Hepatoprotective Effects of Capparis spinosa L. Fractions and Quercetin on Tert-Butyl Hydroperoxide- Induced Acute Liver Damage in Mice. J. Tradit. Complement. Med. 2018, 8, 120–127. [Google Scholar] [CrossRef]
- Gupta, S.; Burman, S.; Nair, A.B.; Chauhan, S.; Sircar, D.; Roy, P.; Dhanwat, M.; Lahiri, D.; Mehta, D.; Das, R.; et al. Brassica Oleracea Extracts Prevent Hyperglycaemia in Type 2 Diabetes Mellitus. Prev. Nutr. Food Sci. 2022, 27, 50–62. [Google Scholar] [CrossRef]
- Shah, M.A.; Sarker, M.M.R.; Gousuddin, M. Antidiabetic Potential of Brassica Oleracea Var. Italica in Type 2 Diabetic Sprague Dawley (sd) Rats. IJPPR 2016, 8, 462–469. [Google Scholar]
- Anjaneyulu, M.; Chopra, K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin. Exp. Pharmacol. 2004, 31, 244–248. [Google Scholar] [CrossRef]
- Chadchan, K.S.; Jargar, J.G.; Das, S.N. Anti-Diabetic Effects of Aqueous Prickly Lettuce (Lactuca Scariola Linn.) Leaves Extract in Alloxan-Induced Male Diabetic Rats Treated with Nickel (II). J. Basic Clin. Physiol. Pharmacol. 2016, 27, 49–56. [Google Scholar] [CrossRef]
- Ismail, H.; Gillespie, A.L.; Calderwood, D.; Iqbal, H.; Gallagher, C.; Chevallier, O.P.; Elliott, C.T.; Pan, X.; Mirza, B.; Green, B.D. The Health Promoting Bioactivities of Lactuca Sativa Can Be Enhanced by Genetic Modulation of Plant Secondary Metabolites. Metabolites 2019, 9, 97. [Google Scholar] [CrossRef]
- Nabi, R.K.; Abdullah, M.A. Effect of Quercetin on the Biochemical Parameters of the Alloxan Induced Diabetes in Male Rats. Bas. J. Vet. Res. 2019, 18, 158–170. [Google Scholar]
- Hafizur, R.M.; Kabir, N.; Chishti, S. Asparagus Officinalis Extract Controls Blood Glucose by Improving Insulin Secretion and β-Cell Function in Streptozotocin-Induced Type 2 Diabetic Rats. Brit. J. Nutr. 2012, 108, 1586–1595. [Google Scholar] [CrossRef]
- Xie, J.; Song, W.; Liang, X.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X. Protective Effect of Quercetin on Streptozotocin-Induced Diabetic Peripheral Neuropathy Rats through Modulating Gut Microbiota and Reactive Oxygen Species Level. Biomed. Pharmacother. 2020, 127, 110147. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, J.; Zhang, J.; Kang, X.; Qian, D. Quercetin Modulates AMPK/SIRT1/NF-ΚB Signaling to Inhibit Inflammatory/Oxidative Stress Responses in Diabetic High Fat Diet-Induced Atherosclerosis in the Rat Carotid Artery. Exp. Ther. Med. 2020, 20, 280. [Google Scholar] [CrossRef]
- Kermani, J.; Goodarzi, N.; Bakhtiari, M. An Experimental Study to Evaluate the Protective Effects of Solanum Lycopersicum Seed Essential Oil on Diabetes-Induced Testicular Injuries. Medicina 2019, 55, 499. [Google Scholar] [CrossRef]
- Ojo, O.O.; Olorunsogo, O.O. Quercetin and Vitamin E Attenuate Diabetes-Induced Testicular Anomaly in Wistar Rats via the Mitochondrial-Mediated Apoptotic Pathway. Andrologia 2021, 53, e14185. [Google Scholar] [CrossRef]
- Atal, S.; Atal, S.; Vyas, S.; Phadnis, P. Bio-Enhancing Effect of Piperine with Metformin on Lowering Blood Glucose Level in Alloxan Induced Diabetic Mice. Pharmacogn. Res. 2016, 8, 56. [Google Scholar] [CrossRef]
- Oršolić, N.; Gajski, G.; Garaj-Vrhovac, V.; Đikić, D.; Prskalo, Z.Š.; Sirovina, D. DNA-Protective Effects of Quercetin or Naringenin in Alloxan-Induced Diabetic Mice. Eur. J. Pharmacol. 2011, 656, 110–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, H.; Wang, M.; Zhang, J. Quercetin Isolated from Toona Sinensis Leaves Attenuates Hyperglycemia and Protects Hepatocytes in High-Carbohydrate/High-Fat Diet and Alloxan Induced Experimental Diabetic Mice. J. Diabetes Res. 2016, 2016, 8492780. [Google Scholar] [CrossRef]
- Fontana Pereira, D.; Cazarolli, L.H.; Lavado, C.; Mengatto, V.; Figueiredo, M.S.R.B.; Guedes, A.; Pizzolatti, M.G.; Silva, F.R.M.B. Effects of Flavonoids on α-Glucosidase Activity: Potential Targets for Glucose Homeostasis. Nutrition 2011, 27, 1161–1167. [Google Scholar] [CrossRef]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and Cancer: New Insights into Its Therapeutic Effects on Ovarian Cancer Cells. Cell Biosci. 2020, 10, 32. [Google Scholar] [CrossRef]
- Borghi, S.M.; Mizokami, S.S.; Pinho-Ribeiro, F.A.; Fattori, V.; Crespigio, J.; Clemente-Napimoga, J.T.; Napimoga, M.H.; Pitol, D.L.; Issa, J.P.M.; Fukada, S.Y.; et al. The Flavonoid Quercetin Inhibits Titanium Dioxide (TiO2)-Induced Chronic Arthritis in Mice. J. Nutr. Biochem. 2018, 53, 81–95. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative in Vivo and Its Antibacterial Mechanism in Vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Alam, F.; Islam, A.; Khalil, I.; Hua Gan, S. Metabolic Control of Type 2 Diabetes by Targeting the GLUT4 Glucose Transporter: Intervention Approaches. Curr. Pharm. Des. 2016, 22, 3034–3049. [Google Scholar] [CrossRef]
- Dhanya, R.; Arun, K.B.; Syama, H.P.; Nisha, P.; Sundaresan, A.; Santhosh Kumar, T.R.; Jayamurthy, P. Rutin and Quercetin Enhance Glucose Uptake in L6 Myotubes under Oxidative Stress Induced by Tertiary Butyl Hydrogen Peroxide. Food Chem. 2014, 158, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, J.M.; Shahab, U.; Tabrez, S.; Lee, E.J.; Choi, I.; Ahmad, S. Quercetin as a Finer Substitute to Aminoguanidine in the Inhibition of Glycation Products. Int. J. Biol. Macromol. 2015, 77, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R.; Kartha, C.C. Quercetin Improves Oxidative Stress-Induced Pancreatic Beta Cell Alterations via MTOR-Signaling. Mol. Cell. Biochem. 2021, 476, 3879–3887. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin Inhibits Advanced Glycation End Product Formation by Trapping Methylglyoxal and Glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158. [Google Scholar] [CrossRef]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin Reduces Blood Pressure in Hypertensive Subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Claycombe, K.J.; Moustaid-Moussa, N. (N-3) Fatty Acids Alleviate Adipose Tissue Inflammation and Insulin Resistance: Mechanistic Insights. Adv. Nutr. 2011, 2, 304–316. [Google Scholar] [CrossRef]
- Ferreira, P.E.B. Diabetic Neuropathy: An Evaluation of the Use of Quercetin in the Cecum of Rats. World J. Gastroenterol. 2013, 19, 6416. [Google Scholar] [CrossRef]
- Shoelson, S.E. Inflammation and Insulin Resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50. [Google Scholar] [CrossRef]
- Tziomalos, K.; Athyros, V.G. Diabetic Nephropathy: New Risk Factors and Improvements in Diagnosis. Rev. Diabet. Stud. 2015, 12, 110–118. [Google Scholar] [CrossRef]
- Cermak, R.; Landgraf, S.; Wolffram, S. Quercetin Glucosides Inhibit Glucose Uptake into Brush-Border-Membrane Vesicles of Porcine Jejunum. Br. J. Nutr. 2004, 91, 849–855. [Google Scholar] [CrossRef]
- Cao, Z.; Zeng, Z.; Wang, B.; Liu, C.; Liu, C.; Wang, Z.; Li, S. Identification of Potential Bioactive Compounds and Mechanisms of GegenQinlian Decoction on Improving Insulin Resistance in Adipose, Liver, and Muscle Tissue by Integrating System Pharmacology and Bioinformatics Analysis. J. Ethnopharmacol. 2021, 264, 113289. [Google Scholar] [CrossRef]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.; Kruhlak, M.; Levine, M. Inhibition of the Intestinal Glucose Transporter GLUT2 by Flavonoids. FASEB J. 2006, 21, 366–377. [Google Scholar] [CrossRef]
- Sha, W.; Hu, F.; Xi, Y.; Chu, Y.; Bu, S. Mechanism of Ferroptosis and Its Role in Type 2 Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 9999612. [Google Scholar] [CrossRef]
- Li, D.; Jiang, C.; Mei, G.; Zhao, Y.; Chen, L.; Liu, J.; Tang, Y.; Gao, C.; Yao, P. Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients 2020, 12, 2954. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, Inflammation, and Insulin Resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Altmann, C.; Schmidt, M.H.H. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 110. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, S.K.; Srinivasan, B.P.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A. Hesperetin Rescues Retinal Oxidative Stress, Neuroinflammation and Apoptosis in Diabetic Rats. Microvasc. Res. 2013, 87, 65–74. [Google Scholar] [CrossRef]
- Sonawane, R.D.; Vishwakarma, S.L.; Lakshmi, S.; Rajani, M.; Padh, H.; Goyal, R.K. Amelioration of STZ-Induced Type 1 Diabetic Nephropathy by Aqueous Extract of Enicostemma Littorale Blume and Swertiamarin in Rats. Mol. Cell. Biochem. 2010, 340, 1–6. [Google Scholar] [CrossRef]
- Peeyush, K.T.; Gireesh, G.; Jobin, M.; Paulose, C.S. Neuroprotective Role of Curcumin in the Cerebellum of Streptozotocin-Induced Diabetic Rats. Life Sci. 2009, 85, 704–710. [Google Scholar] [CrossRef]
- Li, X.-H.; Xin, X.; Wang, Y.; Wu, J.; Jin, Z.; Ma, L.; Nie, C.; Xiao, X.; Hu, Y.; Jin, M. Pentamethylquercetin Protects against Diabetes-Related Cognitive Deficits in Diabetic Goto-Kakizaki Rats. J. Alzheimer’s Dis. 2013, 34, 755–767. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Zheng, H.; Cai, F. GW28-E0635 Quercetin Retards Progression of Diabetic Cardiomyopathy through Modulations of SIRT1 and AMP-Activated Protein Kinase. J. Am. Coll. Cardiol. 2017, 70, C63–C64. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Hu, J.; Lin, J.; Wang, T.; Duan, Y.; Man, W.; Feng, J.; Sun, L.; Jia, H.; et al. MST1 Coordinately Regulates Autophagy and Apoptosis in Diabetic Cardiomyopathy in Mice. Diabetologia 2016, 59, 2435–2447. [Google Scholar] [CrossRef]
- Bostancıeri, N.; Elbe, H.; Eşrefoğlu, M.; Vardı, N. Cardioprotective Potential of Melatonin, Quercetin and Resveratrol in an Experimental Model of Diabetes. Biotech. Histochem. 2021, 97, 152–157. [Google Scholar] [CrossRef]
- Zahedi, M.; Ghiasvand, R.; Feizi, A.; Asgari, G.; Darvish, L. Does Quercetin Improve Cardiovascular Risk Factors and Inflammatory Biomarkers in Women with Type 2 Diabetes: A Double-Blind Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2013, 4, 777–785. [Google Scholar]
- Liu, J.; Li, Q.; Yang, Y.; Ma, L. Iron Metabolism and Type 2 Diabetes Mellitus: A Meta-Analysis and Systematic Review. J. Diabetes Investig. 2020, 11, 946–955. [Google Scholar] [CrossRef]


| Type 2 Antidiabetic Agents | Pharmacological Actions | Side Effects | References |
|---|---|---|---|
| α-glucosidase inhibitors (Acarbose, miglitol) | Inhibit the intestinal absorption of carbohydrates | Flatulence, bloating, diarrhoea | [20,21] |
| Biguanides (Metformin) | Inhibit hepatic gluconeogenesis, Reduce the liver and intestinal absorption of sugar Increase insulin sensitivity and glucose uptake | Kidney complications, upset stomach, tiredness, and dizziness | [22,23] |
| Dopamine agonists (Bromocriptine, cabergoline, apomorphine) | Regulate plasma glucose, free fatty acids, and triglyceride levels in insulin-resistant patients | Visual hallucinations and confusion, edema | [24,25] |
| Dipeptidyl peptidase-4 (DPP-4) inhibitors (Sitagliptin, saxagliptin, linagliptin) | Increase the half-life of GLP-1 and GIP | Gastrointestinal problems, flu-like symptoms (headache, runny nose, sore throat) | [26,27] |
| GLP-1 agonists (Dulaglutide, exenatide, albiglutide) | Enhance insulin release Reduce glucagon release | Gastrointestinal problems and nausea | [28,29] |
| Meglitinides (Nateglinide, repaglinide) | Stimulate the release of insulin | Weight gain, hypoglycaemia, excessive sweating | [30,31] |
| Sodium-glucose Co-transporter-2 (SGLT-2) inhibitors (Dapagliflozin, canagliflozin, empagliflozin) | Inhibit glucose reabsorption in the renal tubule | Urinary tract infection and increased urination, upper respiratory tract infections, joint pain, nausea, and thirst | [32,33] |
| Sulfonylureas (Tolbutamide, tolazamide, chlorpropamide) | Inhibit ATP-sensitive potassium (KATP) channel in pancreatic β-cells | Hypoglycaemia, upset stomach, skin rash, and itching | [34] |
| Thiazolidinediones (Rosiglitazone, pioglitazone) | Bind with the peroxisome proliferator-activated receptor (PPAR)-γ receptor resulting in the activation of several genes that regulate glucose metabolism in the liver | Anaemia risk, weight gain, edema, heart failure | [35,36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, P.; Choudhury, S.T.; Seidel, V.; Rahman, A.B.; Aziz, M.A.; Richi, A.E.; Rahman, A.; Jafrin, U.H.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life 2022, 12, 1146. https://doi.org/10.3390/life12081146
Ansari P, Choudhury ST, Seidel V, Rahman AB, Aziz MA, Richi AE, Rahman A, Jafrin UH, Hannan JMA, Abdel-Wahab YHA. Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life. 2022; 12(8):1146. https://doi.org/10.3390/life12081146
Chicago/Turabian StyleAnsari, Prawej, Samara T. Choudhury, Veronique Seidel, Akib Bin Rahman, Md. Abdul Aziz, Anika E. Richi, Ayesha Rahman, Umme H. Jafrin, J. M. A. Hannan, and Yasser H. A. Abdel-Wahab. 2022. "Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus" Life 12, no. 8: 1146. https://doi.org/10.3390/life12081146
APA StyleAnsari, P., Choudhury, S. T., Seidel, V., Rahman, A. B., Aziz, M. A., Richi, A. E., Rahman, A., Jafrin, U. H., Hannan, J. M. A., & Abdel-Wahab, Y. H. A. (2022). Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life, 12(8), 1146. https://doi.org/10.3390/life12081146












