Cryopreservation of Pig Semen Using a Quercetin-Supplemented Freezing Extender
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Semen Collection and Handling
2.3. Ethical Approval
2.4. Safety and Toxicity of Dimethyl Sulfoxide (DMSO) for Sperms
2.5. Semen Cryopreservation
2.6. Post-Thaw Semen Analysis
2.6.1. Assessment of Sperm Plasma Membrane Functional Integrity
2.6.2. Mitochondrial Activity Assay and Acrosome Integrity
2.6.3. Mucus Penetration Test
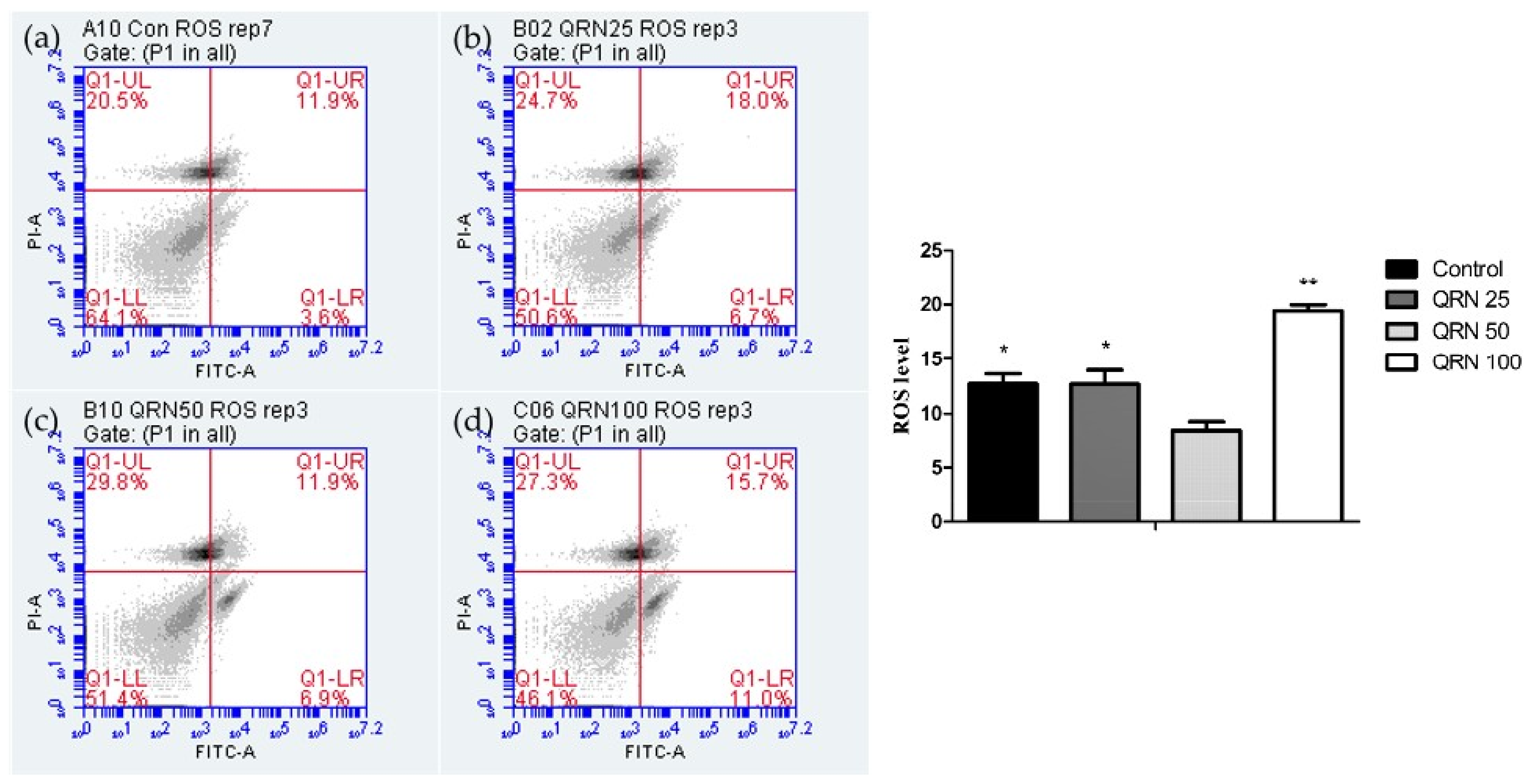
2.6.4. Assessment of Reactive Oxygen Species (ROS) Level
2.6.5. Assessment of Viability and Apoptotic Status using Flow Cytometry
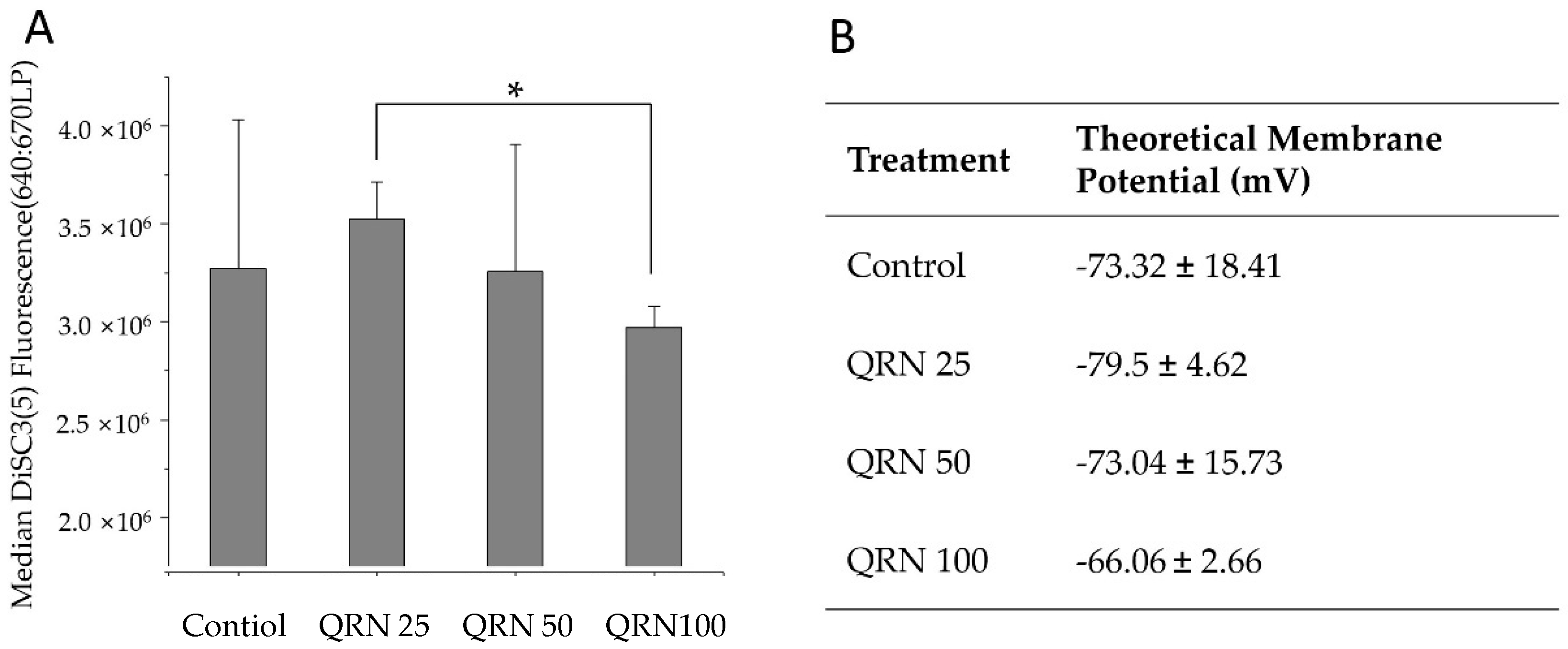
2.6.6. Measurement of Sperm Absolute Membrane Potential
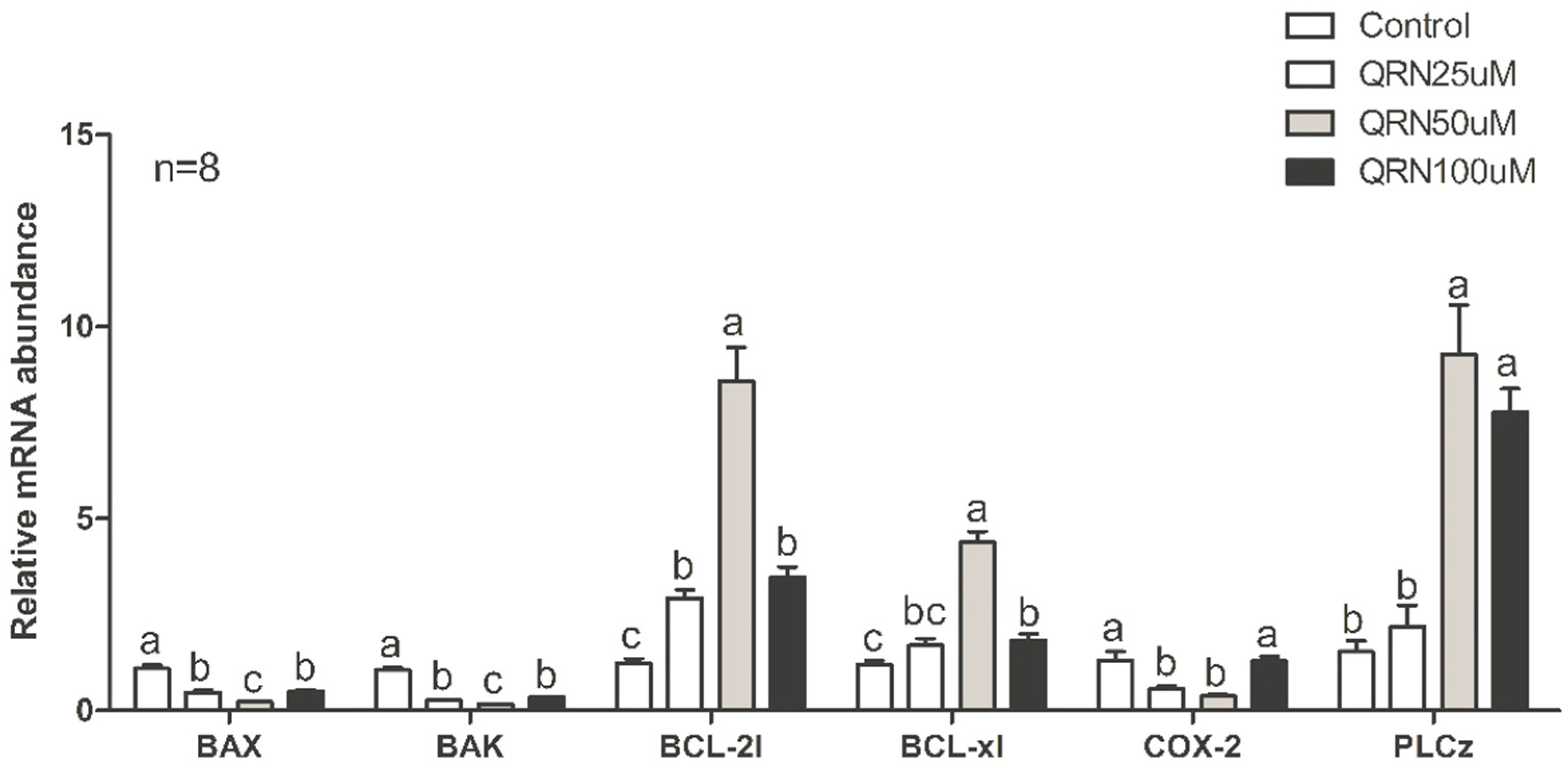
2.7. Gene Expression through Real-Time PCR Analysis
2.8. Statistical Analysis
| Gene | Primer Sequence (5′–3′) | Accession No. |
|---|---|---|
| GAPDH | F: AGAAGGTGGTGAAGCAGG R: AGCTTGACGAAGTGGTCG | XM_003126531 |
| BAX | F: AAGCGCATTGGAGATGAACT R: CTGGACTTCCTTCGAGATCG | AJ606301 |
| BAK | F: ACCGACCCAGAGATGGTCAC R: CAGTTGATGCCACTCTCGAA | AJ001204 |
| BCL-2l | F: GAAACCCCTAGTGCCATCAA R: GGGACGTCAGGTCACTGAAT | NM_214285 |
| BCL-xl | F: CTGAATCAGAAGCGGAAACC R: GGGACGTCAGGTCACTGAA | AF216205 |
| COX-2 | F: CAACGCCTCTACCAGTCTGC R: TTCGGGTGCAGTCACACTTA | ss319605207 |
| PLCz | F: CATGAGATAGACTGCCCTCTGA R: CTGAATTCCCAGCAGACATTC | ss319605203 |
3. Results
3.1. Effect of Quercetin on Sperm Motility after Freezing/Thawing
3.2. Integrity of Plasma Membrane and Acrosome
3.3. Impacts of Quercetin on Oxidative Stress and Apoptosis of Frozen/Thawed Boar Sperm
3.4. Effect of Quercetin on Sperm Membrane Potential after Freezing/Thawing
3.5. Effects of Quercetin on Sperm Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waberski, D.; Riesenbeck, A.; Schulze, M.; Weitze, K.F.; Johnson, L. Application of preserved boar semen for artificial insemination: Past, present and future challenges. Theriogenology 2019, 137, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Jovičić, M.; Chmelíková, E.; Sedmíková, M. Cryopreservation of boar semen. Czech J. Anim. Sci. 2020, 65, 115–123. [Google Scholar] [CrossRef]
- Watson, P. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reprod. Fertil. Dev. 1995, 7, 871–891. [Google Scholar] [CrossRef]
- Agarwal, A.; Makker, K.; Sharma, R. REVIEW ARTICLE: Clinical Relevance of Oxidative Stress in Male Factor Infertility: An Update. Am. J. Reprod. Immunol. 2007, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Prabakaran, S.A.; Said, T.M. Prevention of Oxidative Stress Injury to Sperm. J. Androl. 2005, 26, 654–660. [Google Scholar] [CrossRef]
- Satorre, M.M.; Breininger, E.; Beconi, M.T.; Beorlegui, N.B. Alpha-tocopherol modifies tyrosine phosphorylation and capacitation-like state of cryopreserved porcine sperm. Theriogenology 2007, 68, 958–965. [Google Scholar] [CrossRef]
- Breininger, E.; Beorlegui, N.B.; O’Flaherty, C.M.; Beconi, M.T. Alpha-tocopherol improves biochemical and dynamic parameters in cryopreserved boar semen. Theriogenology 2005, 63, 2126–2135. [Google Scholar] [CrossRef]
- Gadea, J.; Sellés, E.; Marco, M.A.; Coy, P.; Matás, C.; Romar, R.; Ruiz, S. Decrease in glutathione content in boar sperm after cryopreservation: Effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology 2004, 62, 690–701. [Google Scholar] [CrossRef]
- Funahashi, H.; Sano, T. Select antioxidants improve the function of extended boar semen stored at 10 °C. Theriogenology 2005, 63, 1605–1616. [Google Scholar] [CrossRef]
- Varo-Ghiuru, F.; Miclea, I.; Hettig, A.; Ladoşi, I.; Miclea, V.; Egerszegi, I.; Zăhan, M. Lutein, Trolox, ascorbic acid and combination of Trolox with ascorbic acid can improve boar semen quality during cryopreservation. Cryo Lett. 2015, 36, 1–7. [Google Scholar]
- Mazzi, L.; Geminiani, M.; Collodel, G.; Iacoponi, F.; Martini, S.; Bonechi, C.; Rossi, C.; Moretti, E. Quercetin and rutin: Effects of two flavonoids on induced oxidative stress in human ejaculated sperm. J. Siena Acad. Sci. 2011, 3, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Moretti, E.; Mazzi, L.; Terzuoli, G.; Bonechi, C.; Iacoponi, F.; Martini, S.; Rossi, C.; Collodel, G. Effect of quercetin, rutin, naringenin and epicatechin on lipid peroxidation induced in human sperm. Reprod. Toxicol. 2012, 34, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Seifi-Jamadi, A.; Kohram, H.; Shahneh, A.Z.; Ansari, M.; Macías-García, B. Quercetin Ameliorate Motility in Frozen-Thawed Turkmen Stallions Sperm. J. Equine Veter Sci. 2016, 45, 73–77. [Google Scholar] [CrossRef]
- Zribi, N.; Chakroun, N.F.; Ben Abdallah, F.; Elleuch, H.; Sellami, A.; Gargouri, J.; Rebai, T.; Fakhfakh, F.; Keskes, L.A. Effect of freezing–thawing process and quercetin on human sperm survival and DNA integrity. Cryobiology 2012, 65, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, V.; Shahverdi, A.H.; Moghadasian, M.H.; Alizadeh, A.R. Dietary fatty acids affect semen quality: A review. Andrology 2015, 3, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Cerolini, S.; Maldjian, A.; Surai, P.; Noble, R. Viability, susceptibility to peroxidation and fatty acid composition of boar semen during liquid storage. Anim. Reprod. Sci. 2000, 58, 99–111. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Liu, F.; Pan, Y.; Miao, L.; Zhu, Q.; Tan, S. The Feasibility of Antioxidants Avoiding Oxidative Damages from Reactive Oxygen Species in Cryopreservation. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Cilio, S.; Rienzo, M.; Villano, G.; Mirto, B.F.; Giampaglia, G.; Capone, F.; Ferretti, G.; Di Zazzo, E.; Crocetto, F. Beneficial Effects of Antioxidants in Male Infertility Management: A Narrative Review. Oxygen 2022, 2, 1–11. [Google Scholar] [CrossRef]
- Malo, C.; Gil, L.; Gonzalez, N.; Martínez, F.; Cano, R.; de Blas, I.; Espinosa, E. Anti-oxidant supplementation improves boar sperm characteristics and fertility after cryopreservation: Comparison between cysteine and rosemary (Rosmarinus officinalis). Cryobiology 2010, 61, 142–147. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Kim, M.-K.; Song, H.-J.; Kang, E.-J.; Ock, S.-A.; Kumar, B.M.; Balasubramanian, S.; Rho, G.-J. Effect of α-tocopherol supplementation during boar semen cryopreservation on sperm characteristics and expression of apoptosis related genes. Cryobiology 2009, 58, 181–189. [Google Scholar] [CrossRef]
- Partyka, A.; Niżański, W. Supplementation of Avian Semen Extenders with Antioxidants to Improve Semen Quality—Is It an Effective Strategy? Antioxidants 2021, 10, 1927. [Google Scholar] [CrossRef] [PubMed]
- Len, J.S.; Koh, W.S.D.; Tan, S.-X. The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nekoonam, S.; Nashtaei, M.S.; Naji, M.; Zangi, B.M.; Amidi, F. Effect of Trolox on sperm quality in normozospermia and oligozospermia during cryopreservation. Cryobiology 2016, 72, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Mirsafaei, L.; Reiner, Z.; Shafabakhsh, R.; Asemi, Z. Molecular and Biological Functions of Quercetin as a Natural Solution for Cardiovascular Disease Prevention and Treatment. Plant Foods Hum. Nutr. 2020, 75, 307–315. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Moon, Y.J.; Morris, M.E. Pharmacokinetics and Bioavailability of the Bioflavonoid Biochanin A: Effects of Quercetin and EGCG on Biochanin a Disposition in Rats. Mol. Pharm. 2007, 4, 865–872. [Google Scholar] [CrossRef]
- El-Khawagah, A.R.M.; Kandiel, M.M.M.; Samir, H. Effect of Quercetin Supplementation in Extender on Sperm Kinematics, Extracellular Enzymes Release, and Oxidative Stress of Egyptian Buffalo Bulls Frozen–Thawed Semen. Front. Veter. Sci. 2020, 7, 604460. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Khalil, W.A.; Alharbi, M.G.; Lee, S.H. The Current Trends in Using Nanoparticles, Liposomes, and Exosomes for Semen Cryopreservation. Animals 2020, 10, 2281. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Kia, H.D.; Mehdipour, M.; Hamishehkar, H.; Álvarez-Rodríguez, M. Effect of quercetin loaded liposomes or nanostructured lipid carrier (NLC) on post-thawed sperm quality and fertility of rooster sperm. Theriogenology 2020, 152, 122–128. [Google Scholar] [CrossRef]
- Bang, S.; Qamar, A.Y.; Tanga, B.M.; Fang, X.; Seong, G.; Nabeel, A.H.T.; Yu, I.-J.; Saadeldin, I.M.; Cho, J. Quercetin improves the apoptotic index and oxidative stress in post-thaw dog sperm. Environ. Sci. Pollut. Res. 2021, 29, 21925–21934. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.M.A.; Ali, H.A.; Saadeldin, I.M.; Ahmed, M.M. Querectin Alleviates Zinc Oxide Nanoreprotoxicity in Male Albino Rats. J. Biochem. Mol. Toxicol. 2016, 30, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A review on anti-cancer properties of Quercetin in breast cancer. Life Sci. 2020, 248, 117463. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xu, T.; Fang, Q.; Zhang, H.; Yue, L.; Hu, G.; Sun, L. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021, 44, 102010. [Google Scholar] [CrossRef]
- Moodi, Z.; Bagherzade, G.; Peters, J. Quercetin as a Precursor for the Synthesis of Novel Nanoscale Cu (II) Complex as a Catalyst for Alcohol Oxidation with High Antibacterial Activity. Bioinorg. Chem. Appl. 2021, 2021, 8818452. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Hwangbo, Y.; Cheong, H.-T.; Park, H.-T.C.A.C.-K. Effects of Discontinuous Percoll Gradient Containing Alpha-linolenic Acid on Characteristics of Frozen-thawed Boar Spermatozoa. J. Anim. Reprod. Biotechnol. 2020, 35, 58–64. [Google Scholar] [CrossRef]
- Sellés, E.; Gadea, J.; Romar, R.; Matás, C.; Ruiz, S. Analysis of In vitro Fertilizing Capacity to Evaluate the Freezing Procedures of Boar Semen and to Predict the Subsequent Fertility. Reprod. Domest. Anim. 2003, 38, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Gączarzewicz, D.; Piasecka, M.; Udała, J.; Błaszczyk, B.; Stankiewicz, T.; Laszczyńska, M. Plasma membrane changes during the liquid storage of boar spermatozoa: A comparison of methods. Acta Veter. Hung. 2010, 58, 105–116. [Google Scholar] [CrossRef]
- Fraser, L.; Lecewicz, M.; Strzezek, J. Fluorometric assessments of viability and mitochondrial status of boar spermatozoa following liquid storage. Pol. J. Veter. Sci. 2002, 5, 85–92. [Google Scholar]
- Rajabi-Toustani, R.; Akter, Q.S.; Almadaly, E.A.; Hoshino, Y.; Adachi, H.; Mukoujima, K.; Murase, T. Methodological improvement of fluorescein isothiocyanate peanut agglutinin (FITC-PNA) acrosomal integrity staining for frozen-thawed Japanese Black bull spermatozoa. J. Veter. Med Sci. 2019, 81, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Yogev, L.; Gamzu, R.; Paz, G.; Kleiman, S.; Botchan, A.; Hauser, R.; Lessing, J.; Yavetz, H. Improvement in the cervical mucus penetration test by using standard sperm control. Arch. Androl. 1999, 43, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, H.D.; Welch, G.R. Use of fluorescence-activated flow cytometry to determine membrane lipid peroxidation during hypothermic liquid storage and freeze-thawing of viable boar sperm loaded with 4, 4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid1. J. Anim. Sci. 2007, 85, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Oparka, M.; Walczak, J.; Malinska, D.; van Oppen, L.M.; Szczepanowska, J.; Koopman, W.J.; Wieckowski, M.R. Quantifying ROS levels using CM-H 2 DCFDA and HyPer. Methods 2016, 109, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Peña, F. Assessment of fresh and frozen–thawed boar semen using an Annexin-V assay: A new method of evaluating sperm membrane integrity. Theriogenology 2003, 60, 677–689. [Google Scholar] [CrossRef]
- Matamoros-Volante, A.; Castillo-Viveros, V.; Torres-Rodríguez, P.; Treviño, M.B.; Treviño, C.L. Time-Lapse Flow Cytometry: A Robust Tool to Assess Physiological Parameters Related to the Fertilizing Capability of Human Sperm. Int. J. Mol. Sci. 2020, 22, 93. [Google Scholar] [CrossRef]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef]
- Kaneto, M.; Harayama, H.; Miyake, M.; Kato, S. Capacitation-like alterations in cooled boar spermatozoa: Assessment by the chlortetracycline staining assay and immunodetection of tyrosine-phosphorylated sperm proteins. Anim. Reprod. Sci. 2002, 73, 197–209. [Google Scholar] [CrossRef]
- Santi, C.; Orta, G.; Salkoff, L.; Visconti, P.; Darszon, A.; Treviño, C. K+ and Cl− Channels and Transporters in Sperm Function. Curr. Top. Dev. Biol. 2012, 102, 385–421. [Google Scholar] [CrossRef] [Green Version]
- Noto, F.; Recuero, S.; Valencia, J.; Saporito, B.; Robbe, D.; Bonet, S.; Carluccio, A.; Yeste, M. Inhibition of Potassium Channels Affects the Ability of Pig Spermatozoa to Elicit Capacitation and Trigger the Acrosome Exocytosis Induced by Progesterone. Int. J. Mol. Sci. 2021, 22, 1992. [Google Scholar] [CrossRef]
- Ko, J.; Myeong, J.; Kwak, M.; Jeon, J.-H.; So, I. Identification of phospholipase C β downstream effect on transient receptor potential canonical 1/4, transient receptor potential canonical 1/5 channels. Korean J. Physiol. Pharmacol. 2019, 23, 357–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafa, T.; Rashed, L.; Nabil, N.; Amin, R. Seminal BAX and BCL2 Gene and Protein Expressions in Infertile Men with Varicocele. Urology 2014, 84, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Vardiyan, R.; Ezati, D.; Anvari, M.; Ghasemi, N.; Talebi, A. Effect of L-carnitine on the expression of the apoptotic genes Bcl-2 and Bax. Clin. Exp. Reprod. Med. 2020, 47, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, I.; Santoro, M.; Guido, C.; Avena, P.; Tripepi, S.; De Amicis, F.; Gervasi, M.C.; Aquila, S. Expression of cyclooxygenase-1 (COX-1) and COX-2 in human male gametes from normal patients, and those with varicocele and diabetes: A potential molecular marker for diagnosing male infertility disorders. J. Anat. 2012, 221, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Kaewmala, K.; Uddin, M.; Cinar, M.; Große-Brinkhaus, C.; Jonas, E.; Tesfaye, D.; Phatsara, C.; Tholen, E.; Looft, C.; Schellander, K. Investigation into Association and Expression of PLCz and COX-2 as Candidate Genes for Boar Sperm Quality and Fertility. Reprod. Domest. Anim. 2011, 47, 213–223. [Google Scholar] [CrossRef]
- Pan, Q.; Ju, Z.; Huang, J.; Zhang, Y.; Qi, C.; Gao, Q.; Zhou, L.; Li, Q.; Wang, L.; Zhong, J.; et al. PLCz Functional Haplotypes Modulating Promoter Transcriptional Activity Are Associated with Semen Quality Traits in Chinese Holstein Bulls. PLoS ONE 2013, 8, e58795. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.; Kashir, J.; Thanassoulas, A.; Safieh-Garabedian, B.; Lai, F.A.; Nomikos, M. Essential Role of Sperm-Specific PLC-Zeta in Egg Activation and Male Factor Infertility: An Update. Front. Cell Dev. Biol. 2020, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Tvrdá, E.; Debacker, M.; Ďuračka, M.; Kováč, J.; Bučko, O. Quercetin and Naringenin Provide Functional and Antioxidant Protection to Stored Boar Semen. Animals 2020, 10, 1930. [Google Scholar] [CrossRef] [PubMed]
- Winn, E.; Whitaker, B.D. Quercetin supplementation to the thawing and incubation media of boar sperm improves post-thaw sperm characteristics and the in vitro production of pig embryos. Reprod. Biol. 2020, 20, 315–320. [Google Scholar] [CrossRef]
- Kim, T.-H.; Yuh, I.-S.; Park, I.-C.; Cheong, H.-T.; Kim, J.-T.; Park, C.-K.; Yang, B.-K. Effects of Quercetin and Genistein on Boar Sperm Characteristics and Porcine IVF Embyo Developments. J. Anim. Reprod. Biotechnol. 2014, 29, 141–148. [Google Scholar] [CrossRef]
- Desroches, N.; McNiven, M.; Foote, K.; Richardson, G. The Effect of Blueberry Extracts and Quercetin on Capacitation Status of Stored Boar Sperm. Cell Preserv. Technol. 2005, 3, 165–168. [Google Scholar] [CrossRef]
- Majzoub, A.; Agarwal, A. Antioxidants in Sperm Cryopreservation. In Male Infertility: Contemporary Clinical Approaches, Andrology, ART and Antioxidants; Parekattil, S.J., Esteves, S.C., Agarwal, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 671–678. [Google Scholar] [CrossRef]
- Zou, H.; Ye, H.; Kamaraj, R.; Zhang, T.; Zhang, J.; Pavek, P. A review on pharmacological activities and synergistic effect of quercetin with small molecule agents. Phytomedicine 2021, 92, 153736. [Google Scholar] [CrossRef] [PubMed]
- Eisvand, F.; Tajbakhsh, A.; Seidel, V.; Zirak, M.R.; Tabeshpour, J.; Shakeri, A. Quercetin and its role in modulating endoplasmic reticulum stress: A review. Phytotherapy Res. 2021, 36, 73–84. [Google Scholar] [CrossRef] [PubMed]

| DMSO (%) | Motility (%) | Progress Motility (%) | VCL (μm/s) | VAP (μm/s) | VSL (μm/s) | Straightness | Linearity (%) | ALH (μm) |
|---|---|---|---|---|---|---|---|---|
| 0 | 37.66 ± 5.55 a | 7.78 ± 2.76 | 31.00 ± 1.34 a | 18.72 ± 1.05 a | 10.06 ± 1.25 a | 58.43 ± 4.43 a | 38.87 ± 5.15 a | 1.15 ± 0.02 a |
| 0.5 | 38.13 ± 0.32 a | 6.91 ± 0.24 | 23.25 ± 1.76 b | 13.66 ± 1.28 ab | 4.68 ± 0.89 b | 38.72 ± 0.67 b | 19.70 ± 3.40 b | 0.87 ± 0.08 b |
| 1.0 | 37.11 ± 0.91a | 8.26 ± 2.26 | 21.94 ± 2.78 b | 10.04 ± 1.48b | 4.13 ± 1.37 b | 40.11 ± 2.19 b | 15.85 ± 2.95 b | 0.83 ± 0.75 b |
| 1.5 | 33.96 ± 1.96 ab | 6.06 ± 1.24 | 25.91 ± 3.44 b | 14.09 ± 3.03 ab | 5.51 ± 1.82 b | 45.96 ± 5.45 b | 20.42 ± 5.45 b | 0.93 ± 0.78 b |
| 2.0 | 27.24 ± 1.71 b | 3.86 ± 0.48 | 20.83 ± 2.15 b | 11.81 ± 1.18 b | 5.55 ± 1.10 b | 47.42 ± 11.48 b | 26.23 ± 6.95 a | 0.88 ± 0.43 b |
| Groups | Motility (%) | Progress Motility (%) | VCL (µm/s) | VAP (µm/s) | VSL (µm/s) | Straightness (%) | Linearity (%) | ALH (µm) |
|---|---|---|---|---|---|---|---|---|
| Control | 29.13 ± 0.92 b | 14.60 ± 2.73 | 74.29 ± 5.04 a | 37.36 ± 2.90 a | 15.76 ± 2.15 | 41.11 ± 2.37 | 21.26 ± 1.67 a | 2.15 ± 0.10 a |
| 25 µM QRN | 30.89 ± 1.15 ab | 10.81 ± 1.35 | 45.73 ± 13.00 b | 21.85 ± 2.91 b | 8.18 ± 1.60 | 36.25 ± 4.22 | 17.24 ± 3.52 a | 1.44 ± 0.13 b |
| 50 µM QRN | 33.73 ± 0.85 a | 16.16 ± 3.25 | 64.74 ± 10.36 a | 30.17 ± 6.64 a | 13.40 ± 4.73 | 44.19 ± 3.56 | 17.97 ± 2.78 a | 1.82 ± 0.21 a |
| 100 µM QRN | 28.57 ± 1.02 b | 10.89 ± 1.90 | 62.07 ± 11.30 a | 27.80 ± 5.63 a | 9.63 ± 1.96 | 36.48 ± 2.11 | 13.11 ± 1.26 b | 1.84 ± 0.28 a |
| Groups | HOS (%) | Mitochondrial Activity (%) | Acrosome Integrity (%) |
|---|---|---|---|
| Control | 43.1 ± 1.5 b | 39.1 ± 0.3 c | 66.3 ± 0.8 b |
| 25 µM QRN | 45.3 ± 0.6 b | 41.9 ± 0.3 b | 66.7 ± 1.3 b |
| 50 µM QRN | 47.5 ± 0.5 ab | 43.0 ± 0.3 b | 73.6 ± 1.2 a |
| 100 µM QRN | 44.1 ± 0.5 b | 43.2 ± 0.6 ab | 68.3 ± 1.0 b |
| Groups | Number of Sperm Penetrating Mucus | |
|---|---|---|
| 1 cm | 3 cm | |
| Control | 55.0 ± 2.2 c | 16.2 ± 1.0 b |
| 25 µM QRN | 57.4 ± 1.8 b | 18.9 ± 1.1 b |
| 50 µM QRN | 66.7 ± 1.9 a | 21.9 ± 1.1 a |
| 100 µM QRN | 54.3 ± 2.2 b | 18.7 ± 0.7 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bang, S.; Tanga, B.M.; Fang, X.; Seong, G.; Saadeldin, I.M.; Qamar, A.Y.; Lee, S.; Kim, K.-J.; Park, Y.-J.; Nabeel, A.H.T.; et al. Cryopreservation of Pig Semen Using a Quercetin-Supplemented Freezing Extender. Life 2022, 12, 1155. https://doi.org/10.3390/life12081155
Bang S, Tanga BM, Fang X, Seong G, Saadeldin IM, Qamar AY, Lee S, Kim K-J, Park Y-J, Nabeel AHT, et al. Cryopreservation of Pig Semen Using a Quercetin-Supplemented Freezing Extender. Life. 2022; 12(8):1155. https://doi.org/10.3390/life12081155
Chicago/Turabian StyleBang, Seonggyu, Bereket Molla Tanga, Xun Fang, Gyeonghwan Seong, Islam M. Saadeldin, Ahmad Yar Qamar, Sanghoon Lee, Keun-Jung Kim, Yun-Jae Park, Abdelbagi Hamad Talha Nabeel, and et al. 2022. "Cryopreservation of Pig Semen Using a Quercetin-Supplemented Freezing Extender" Life 12, no. 8: 1155. https://doi.org/10.3390/life12081155
APA StyleBang, S., Tanga, B. M., Fang, X., Seong, G., Saadeldin, I. M., Qamar, A. Y., Lee, S., Kim, K.-J., Park, Y.-J., Nabeel, A. H. T., Yu, I.-j., Cooray, A., Lee, K. P., & Cho, J. (2022). Cryopreservation of Pig Semen Using a Quercetin-Supplemented Freezing Extender. Life, 12(8), 1155. https://doi.org/10.3390/life12081155










