Gamma Knife Radiosurgery for Indirect Dural Carotid–Cavernous Fistula: Long-Term Ophthalmological Outcome
Abstract
:1. Introduction
2. Materials and Methods
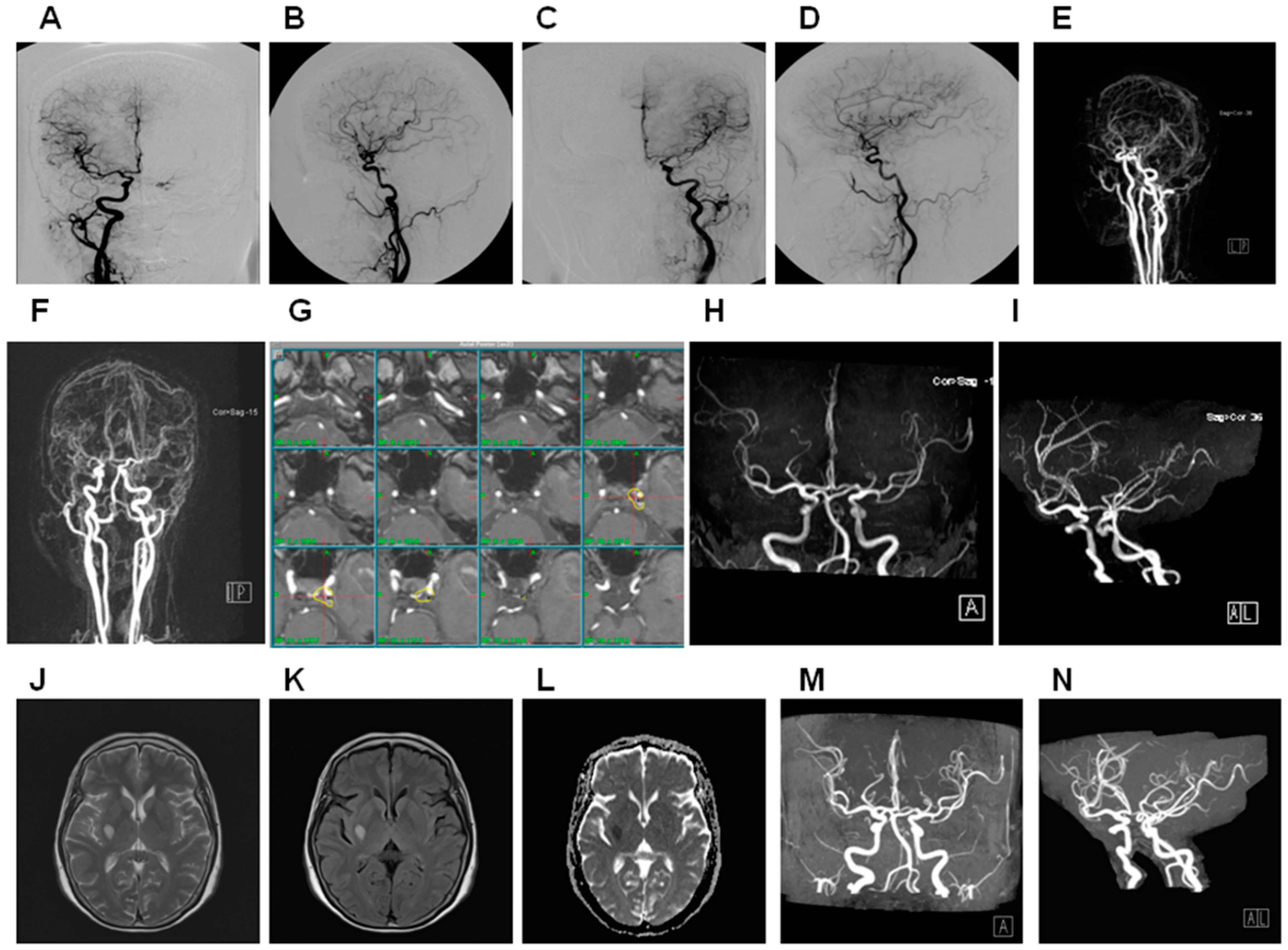
3. Results
3.1. General Data
3.2. Treatment Parameters
3.3. Treatment Outcome
3.4. Risk Factor Analysis Contributing to Adverse Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrow, D.L.; Spector, R.H.; Braun, I.F.; Landman, J.A.; Tindall, S.C.; Tindall, G.T. Classification and Treatment of Spontaneous Carotid-Cavernous Sinus Fistulas. J. Neurosurg. 1985, 62, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Cognard, C.; Gobin, Y.P.; Pierot, L.; Bailly, A.L.; Houdart, E.; Casasco, A.; Chiras, J.; Merland, J.J. Cerebral Dural Arteriovenous Fistulas: Clinical and Angiographic Correlation with a Revised Classification of Venous Drainage. Radiology 1995, 194, 671–680. [Google Scholar] [CrossRef]
- Gemmete, J.J.; Ansari, S.A.; Gandhi, D.M. Endovascular Techniques for Treatment of Carotid-Cavernous Fistula. J. Neuro. Ophthalmol. Off. J. N. Am. Neuro.Ophthalmol. Soc. 2009, 29, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Sun, M.H.; Chen, W.H.; Ting, C.C.; Sheehan, J. Minimally Invasive Approaches to Treating Chemosis of the Eyes from Unusual Dural Arteriovenous Fistulae. Minim. Invasive Neurosurg. 2009, 52, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Sun, M.H.; Sheehan, J.; Sheu, M.L.; Chen, C.C.; Lee, H.T.; Chiu, W.T.; Yang, D.Y. Radiosurgery for Dural Carotid-Cavernous Sinus Fistulas: Gamma Knife Compared with Xknife Radiosurgery. J. Neurosurg. 2010, 113 (Suppl), 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirsch, M.; Henkes, H.; Liebig, T.; Weber, W.; Esser, J.; Golik, S.; Kühne, D. Endovascular Management of Dural Carotid-Cavernous Sinus Fistulas in 141 Patients. Neuroradiology 2006, 48, 486–490. [Google Scholar] [CrossRef]
- Théaudin, M.; Saint-Maurice, J.P.; Chapot, R.; Vahedi, K.; Mazighi, M.; Vignal, C.; Saliou, G.; Stapf, C.; Bousser, M.G.; Houdart, E. Diagnosis and Treatment of Dural Carotid-Cavernous Fistulas: A Consecutive Series of 27 Patients. J. Neurol. Neurosurg. Psychiatry 2007, 78, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.M.; Chan, C.M.; Cheung, Y.L. Transvenous Embolisation of Dural Carotid-Cavernous Fistulas by Multiple Venous Routes: A Series of 27 Cases. Acta Neurochir. 2003, 145, 17–29. [Google Scholar] [CrossRef]
- Jung, K.H.; Kwon, B.J.; Chu, K.; Noh, Y.; Lee, S.T.; Cho, Y.D.; Han, M.H.; Roh, J.K. Clinical and Angiographic Factors Related to the Prognosis of Cavernous Sinus Dural Arteriovenous Fistula. Neuroradiology 2011, 53, 983–992. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, D.I.; Suh, S.H.; Kim, J.; Lee, S.K.; Kim, E.Y.; Chung, T.S. Results of Transvenous Embolization of Cavernous Dural Arteriovenous Fistula: A Single-Center Experience with Emphasis on Complications and Management. AJNR Am. J. Neuroradiol. 2006, 27, 2078–2082. [Google Scholar]
- Meyers, P.M.; Halbach, V.V.; Dowd, C.F.; Lempert, T.E.; Malek, A.M.; Phatouros, C.C.; Lefler, J.E.; Higashida, R.T. Dural Carotid Cavernous Fistula: Definitive Endovascular Management and Long-Term Follow-Up. Am. J. Ophthalmol. 2002, 134, 85–92. [Google Scholar] [CrossRef]
- Nishimuta, Y.; Awa, R.; Sugata, S.; Nagayama, T.; Makiuchi, T.; Tomosugi, T.; Hanaya, R.; Tokimura, H.; Hirano, H.; Moinuddin, F.M.; et al. Long-Term Outcome After Endovascular Treatment of Cavernous Sinus Dural Arteriovenous Fistula and a Literature Review. Acta Neurochir. 2017, 159, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Ito, Y.; Hasegawa, H.; Kikuchi, B.; Shimbo, J.; Kitazawa, K.; Fujii, Y. Cranial Nerve Palsy Following Transvenous Embolization for a Cavernous Sinus Dural Arteriovenous Fistula: Association with the Volume and Location of Detachable Coils. J. Neurosurg. 2008, 109, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.J.; Chua, M.; Fusco, M.; Ogilvy, C.S.; Tubbs, R.S.; Harrigan, M.R.; Griessenauer, C.J. Proposal of Venous Drainage-Based Classification System for Carotid Cavernous Fistulae With Validity Assessment in a Multicenter Cohort. Neurosurgery 2015, 77, 380–385; discussion 385. [Google Scholar] [CrossRef] [PubMed]
- Wenderoth, J. Novel Approaches to Access and Treatment of Cavernous Sinus Dural Arteriovenous Fistula (Cs-Davf): Case Series and Review of the Literature. J. Neurointerv. Surg. 2017, 9, 290–296. [Google Scholar] [CrossRef]
- Yoshida, K.; Melake, M.; Oishi, H.; Yamamoto, M.; Arai, H. Transvenous Embolization of Dural Carotid Cavernous Fistulas: A Series of 44 Consecutive Patients. AJNR Am. J. Neuroradiol. 2010, 31, 651–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.Y.; Pan, D.H.; Wu, H.M.; Chung, W.Y.; Shiau, C.Y.; Wang, L.W.; Chiou, H.J.; Yen, M.Y.; Teng, M.M. Radiosurgery as a Treatment Alternative for Dural Arteriovenous Fistulas of the Cavernous Sinus. AJNR Am. J. Neuroradiol. 1998, 19, 1081–1087. [Google Scholar]
- Koebbe, C.J.; Singhal, D.; Sheehan, J.; Flickinger, J.C.; Horowitz, M.; Kondziolka, D.; Lunsford, L.D. Radiosurgery for Dural Arteriovenous Fistulas. Surg. Neurol. 2005, 64, 392–398; discussion 398–399. [Google Scholar] [CrossRef] [PubMed]
- Onizuka, M.; Mori, K.; Takahashi, N.; Kawahara, I.; Hiu, T.; Toda, K.; Baba, H.; Yonekura, M. Gamma Knife Surgery for the Treatment of Spontaneous Dural Carotid-Cavernous Fistulas. Neurol. Med. Chir. 2003, 43, 477–482; discussion–482–483. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.C.; Sun, M.H.; Yang, D.Y.; Wang, Y.C.; Lee, S.D.; Chen, W.H.; Chen, C.C. Multidisciplinary Treatment of Cavernous Sinus Dural Arteriovenous Fistulae with Radiosurgery and Embolization. J. Clin. Neurosci. 2005, 12, 744–749. [Google Scholar] [CrossRef]
- Chen, J.C.; Tsuruda, J.S.; Halbach, V.V. Suspected Dural Arteriovenous Fistula: Results with Screening Mr Angiography in Seven Patients. Radiology 1992, 183, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Korogi, Y.; Hamatake, S.; Ikushima, I.; Sugahara, T.; Sigematsu, Y.; Higashida, Y.; Takahashi, M. Three-Dimensional Fisp Imaging in the Evaluation of Carotid Cavernous Fistula: Comparison with Contrast-Enhanced Ct and Spin-Echo Mr. AJNR Am. J. Neuroradiol. 1998, 19, 253–259. [Google Scholar] [PubMed]
- Hiramatsu, K.; Utsumi, S.; Kyoi, K.; Sakaki, T.; Tada, T.; Iwasaki, S.; Kichikawa, K. Intracerebral Hemorrhage in Carotid-Cavernous Fistula. Neuroradiology 1991, 33, 67–69. [Google Scholar] [CrossRef]
- Ouanounou, S.; Tomsick, T.A.; Heitsman, C.; Holland, C.K. Cavernous Sinus and Inferior Petrosal Sinus Flow Signal on Three-Dimensional Time-of-Flight Mr Angiography. AJNR Am. J. Neuroradiol. 1999, 20, 1476–1481. [Google Scholar] [PubMed]
- Mehta, M.P.; Kinsella, T.J. Cavernous Sinus Cranial Neuropathies: Is There a Dose-Response Relationship Following Radiosurgery? Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 477–480. [Google Scholar] [CrossRef]
- Cruciani, F.; Lorenzatti, M.; Nazzarro, V.; Abdolrahimzadeh, S. Bilateral Acute Angle Closure Glaucoma and Myopia Induced by Topiramate. Clin. Ter 2009, 160, 215–216. [Google Scholar] [PubMed]
- Jørgensen, J.S.; Guthoff, R. The Role of Episcleral Venous Pressure in the Development of Secondary Glaucomas. Klin. Monbl. Augenheilkd. 1988, 193, 471–475. [Google Scholar] [CrossRef]
- Gigliotti, C.R.; Modorati, G.; Di Nicola, M.; Fiorino, C.; Perna, L.A.; Miserocchi, E.; Franzin, A.; Picozzi, P.; Bolognesi, A.; Mortini, P.; et al. Predictors of Radio-Induced Visual Impairment after Radiosurgery for Uveal Melanoma. Br. J. Ophthalmol. 2018, 102, 833–839. [Google Scholar] [CrossRef]
- Petrovich, Z.; McDonnell, J.M.; Palmer, D.; Langholz, B.M.; Liggett, P.E. Histopathologic Changes Following Irradiation for Uveal Tract Melanoma. Am. J. Clin. Oncol. 1994, 17, 298–306. [Google Scholar] [CrossRef]
- Ito, H.; Onodera, H.; Sase, T.; Uchida, M.; Morishima, H.; Oshio, K.; Shuto, T.; Tanaka, Y. Percutaneous Transluminal Angioplasty in a Patient with Internal Carotid Artery Stenosis Following Gamma Knife Radiosurgery for Recurrent Pituitary Adenoma. Surg. Neurol. Int. 2015, 6, S279–S283. [Google Scholar] [CrossRef]
- Lim, Y.J.; Leem, W.; Park, J.T.; Kim, T.S.; Rhee, B.A.; Kim, G.K. Cerebral Infarction with Ica Occlusion after Gamma Knife Radiosurgery for Pituitary Adenoma: A Case Report. Stereotact. Funct. Neurosurg. 1999, 72 (Suppl. S1), 132–139. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.E.; Nippoldt, T.B.; Stafford, S.L.; Foote, R.L.; Abboud, C.F. Results of Stereotactic Radiosurgery in Patients with Hormone-Producing Pituitary Adenomas: Factors Associated with Endocrine Normalization. J. Neurosurg. 2002, 97, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Barami, K.; Grow, A.; Brem, S.; Dagnew, E.; Sloan, A.E. Vascular Complications after Radiosurgery for Meningiomas. Neurosurg. Focus 2007, 22, E9. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Kurita, H.; Sasaki, T.; Tago, M.; Morita, A.; Ueki, K.; Kirino, T. Stereotactic Radiosurgery for Pituitary Adenoma Invading the Cavernous Sinus. J. Neurosurg. 2000, 93 (Suppl. S3), 2–5. [Google Scholar] [CrossRef] [PubMed]
- Spatola, G.; Frosio, L.; Losa, M.; Del Vecchio, A.; Piloni, M.; Mortini, P. Asymptomatic Internal Carotid Artery Occlusion after Gamma Knife Radiosurgery for Pituitary Adenoma: Report of Two Cases and Review of the Literature. Rep. Pract. Oncol. Radiother. 2016, 21, 555–559. [Google Scholar] [CrossRef] [Green Version]
- Abeloos, L.; Levivier, M.; Devriendt, D.; Massager, N. Internal Carotid Occlusion Following Gamma Knife Radiosurgery for Cavernous Sinus Meningioma. Stereotact. Funct. Neurosurg. 2007, 85, 303–306. [Google Scholar] [CrossRef]
- Szeifert, G.T.; Levivier, M.; Lorenzoni, J.; Nyáry, I.; Major, O.; Kemeny, A.A. Morphological Observations in Brain Arteriovenous Malformations after Gamma Knife Radiosurgery. Prog. Neurol. Surg. 2013, 27, 119–129. [Google Scholar]
- Brady, L.W.; Shields, J.; Augusburger, J.; Markoe, A.; Karlsson, U.L. Complications from Radiation Therapy to the Eye. Front. Radiat. Ther. Oncol. 1989, 23, 238–250; discussion 251–254. [Google Scholar] [PubMed]
- Parsons, J.T.; Bova, F.J.; Fitzgerald, C.R.; Mendenhall, W.M.; Million, R.R. Severe Dry-Eye Syndrome Following External Beam Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1994, 30, 775–780. [Google Scholar] [CrossRef]
- Horwath-Winter, J.; Schneider, M.R.; Wackernagel, W.; Rabensteiner, D.; Boldin, I.; Haller-Schober, E.M.; Langmann, G. Influence of Single-Fraction Gamma-Knife Radiosurgery on Ocular Surface and Tear Function in Choroidal Melanoma Patients. Br. J. Ophthalmol. 2013, 97, 466–470. [Google Scholar] [CrossRef]
- Goebbels, M. Tear Secretion and Tear Film Function in Insulin Dependent Diabetics. Br. J. Ophthalmol. 2000, 84, 19–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, S.E.; Klein, R.; Klein, B.E. Prevalence of and Risk Factors for Dry Eye Syndrome. Arch. Ophthalmol. 2000, 118, 1264–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, S.E.; Klein, R.; Klein, B.E. Incidence of Dry Eye in an Older Population. Arch. Ophthalmol. 2004, 122, 369–373. [Google Scholar] [CrossRef] [Green Version]
- Kennerdell, J.S.; Flores, N.E.; Hartsock, R.J. Low-Dose Radiotherapy for Lymphoid Lesions of the Orbit and Ocular Adnexa. Ophthalmic Plast. Reconstr. Surg. 1999, 15, 129–133. [Google Scholar] [CrossRef]
- Nuzzi, R.; Trossarello, M.; Bartoncini, S.; Marolo, P.; Franco, P.; Mantovani, C.; Ricardi, U. Ocular Complications After Radiation Therapy: An Observational Study. Clin. Ophthalmol. 2020, 14, 3153–3166. [Google Scholar] [CrossRef] [PubMed]
- Ainsbury, E.A.; Barnard, S.; Bright, S.; Dalke, C.; Jarrin, M.; Kunze, S.; Tanner, R.; Dynlacht, J.R.; Quinlan, R.A.; Graw, J.; et al. Ionizing Radiation Induced Cataracts: Recent Biological and Mechanistic Developments and Perspectives for Future Research. Mutat. Res. Rev. Mutat. Res. 2016, 770, 238–261. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.A.; Akleyev, A.V.; Hauer-Jensen, M.; Hendry, J.H.; Kleiman, N.J.; Macvittie, T.J.; Aleman, B.M.; Edgar, A.B.; Mabuchi, K.; Muirhead, C.R.; et al. ICRP Publication 118: ICRP Statement on Tissue Reactions and Early and Late Effects of Radiation in Normal Tissues and Organs—Threshold Doses for Tissue Reactions in a Radiation Protection Context. Ann. ICRP 2012, 41, 1–322. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.L.; Liliang, P.C.; Chen, T.B.; Hsu, H.C.; Chuang, F.C.; Wang, K.W.; Wang, H.K.; Yang, S.N.; Chen, H.J. The Risk of Cataractogenesis after Gamma Knife Radiosurgery: A Nationwide Population Based Case-Control Study. BMC Ophthalmol. 2017, 17, 40. [Google Scholar] [CrossRef] [Green Version]


| Mean ± SD | ||
|---|---|---|
| Age | 60.8 ± 14.9 | |
| Sex | M | 22 (33.8%) |
| F | 43 (66.2%) | |
| WBC/ul | 7160 ± 1294 | |
| N/L (ratio) | 2.68 ± 0.56 | |
| History of embolization | 7 (10.8%) | |
| Follow-up periods (months) | 97.7 ± 52.9 | |
| Onset to treatment (months) | 4.77 ± 6.31 | |
| Symptom/signs | Red eye | 60 (92.3%) |
| Increases IOP | 26 (40%) | |
| Cranial nerve palsy | 21 (32.7%) | |
| Location of symptom | Right | 17 (26.2%) |
| Left | 37 (56.9%) | |
| Bilateral | 11 (16.9%) | |
| Location of fistula | Right | 23 (35.4%) |
| left | 26 (40%) | |
| bilateral | 16 (24.6%) | |
| Barrow classification | B | 4 (6.1%) |
| C | 2 (3.1%) | |
| D | 59 (90.8%) | |
| Target volume (cc) | 2 ± 1.43 | |
| Margin dose (Gy) | 18 ± 0.79 | |
| Maximum dose (Gy) | 32.75 ± 3.65 | |
| Maximum error in angiography (mm) | 0.12 ± 0.04 | |
| Isodose line (%) | 55 ± 5.5 | |
| Conformity index | 1.28 ± 0.08 | |
| Number of isocenters | 5.4 ± 3.6 | |
| Mean ± SD | |
|---|---|
| Right optic nerve (Gy) | 3.84 ± 1.75 |
| Left optic nerve (Gy) | 3.90 ± 1.8 |
| Chiasma (Gy) | 2.6 ± 0.96 |
| Right lens (Gy) | 0.43 ± 0.33 |
| Left lens (Gy) | 0.44 ± 0.28 |
| Right lacrimal gland (Gy) | 0.45 ± 0.31 |
| Left lacrimal gland (Gy) | 0.46 ± 0.21 |
| Brain stem (Gy) | 6.6 ± 2.7 |
| Pituitary stalk (Gy) | 1.94 ± 0.84 |
| Right lateral cavernous sinus wall (Gy) | 9.51 ± 5.51 |
| Left lateral cavernous sinus wall (Gy) | 11.74 ± 5.62 |
| Right ICA volume (cc) | 0.46 ± 0.03 |
| Left ICA volume (cc) | 0.45 ± 0.02 |
| % of right ICA > 20 Gy | 14.92 ± 14.58 |
| % of left ICA > 20 Gy | 20.59 ± 13.14 |
| IOP (mmHg) | 17 ± 7.61 |
| Schirmer’s test (mm) | 6.97 ± 0.88 |
| Mean ± SD | ||
|---|---|---|
| Duration of S/S alleviated (months) | 3.71 ± 7.68 | |
| MRA outcome | Obliteration | 64 (98.4%) |
| Preservation of cavernous sinus | 65 (100%) | |
| ICA stenosis | 0 | |
| Residual symptom | Red eye | 2 (3.1%) |
| Cranial nerve palsy | 4 (6.2%) | |
| Glaucoma | 0 | |
| Dry eyes | 0 | |
| Last opththmalogical test | Schirmer’s test (mm) | 6.89 ± 0.99 |
| IOP (mmHg) | 13.08 ± 1.4 | |
| Complication post GKRS | Infarction | 2 (3.1%) |
| Cataract | 2 (3.1%) | |
| Transient optic nerve neuropathy | 1 (1.5%) | |
| Univariate | |||
|---|---|---|---|
| HR | 95%CI | p Value | |
| N/L (Pre-GKRS) | 2.18 | (0.14–35.22) | 0.583 |
| S/S (Yes vs. No) | |||
| Red eye | 0.06 | (0.00–0.97) | 0.047 * |
| Increase IOP | 1.38 | (0.08–22.84) | 0.823 |
| Cranial nerve palsy | 1.57 | (0.10–25.73) | 0.752 |
| Tumor volume (cc) | 0.76 | (0.19–3.04) | 0.701 |
| Number of isocenters | 0.97 | (0.66–1.42) | 0.880 |
| Maximum dose (Gy) | 0.79 | (0.56–1.12) | 0.188 |
| Peripheral isodose line (%) | 1.15 | (0.94–1.40) | 0.170 |
| Margin dose (Gy) | 0.93 | (0.19–4.52) | 0.927 |
| Rt optic nerve dosage (Gy) | 1.55 | (0.69–3.51) | 0.289 |
| Lt optic nerve dosage (Gy) | 1.55 | (0.68–3.50) | 0.296 |
| Chiasma dosage (Gy) | 1.25 | (0.34–4.60) | 0.742 |
| Pituitary stalk dosage (Gy) | 2.40 | (0.52–11.09) | 0.261 |
| Brain stem dosage (Gy) | 0.46 | (0.18–1.18) | 0.106 |
| Rt lens dosage (Gy) | 0.00 | (0.00–746.84) | 0.366 |
| Lt lens dosage (Gy) | 0.40 | (0.00–103.35) | 0.747 |
| Rt Lacrimal gland dosage (Gy) | 0.00 | (0.00–746.84) | 0.366 |
| Lt Lacrimal gland dosage (Gy) | 0.40 | (0.00–103.35) | 0.747 |
| Rt lateral cavernous sinus wall dosage (Gy) | 1.14 | (0.83–1.57) | 0.414 |
| Lt lateral cavernous sinus wall dosage (Gy) | 1.00 | (0.76–1.32) | 0.987 |
| IOP at GKRS (mmHg) | 1.04 | (0.89–1.22) | 0.612 |
| Schirmer’s test at GK (mm) | 1.36 | (0.23–7.89) | 0.733 |
| Percentage of Rt ICA > 20 Gy (%) | 1.03 | (0.94–1.13) | 0.541 |
| Percentage of Rt ICA (>20 Gy vs. ≤20 Gy) | 1.74 | (0.11–28.27) | 0.696 |
| Percentage of Lt ICA > 20 Gy (%) | 0.96 | (0.85–1.08) | 0.526 |
| Percentage of Lt ICA (>20 Gy vs. ≤20 Gy) | 0.74 | (0.05–11.89) | 0.832 |
| Univariate | |||
|---|---|---|---|
| HR | 95%CI | p Value | |
| age | 1.20 | (1.03–1.40) | 0.022 * |
| Sex | |||
| Male | ref. | ||
| Female | 0.56 | (0.05–6.42) | 0.643 |
| WBC (Pre GKRS)/μL | 1.00 | (1.00–1.00) | 0.061 |
| Tumor volume (cc) | 2.09 | (1.04–4.19) | 0.038 * |
| Number of isocenter | 1.17 | (0.90–1.51) | 0.235 |
| Maximum dose (Gy) | 1.07 | (0.81–1.42) | 0.632 |
| Peripheral isodose line (%) | 0.94 | (0.77–1.16) | 0.561 |
| Margin dose (Gy) | 0.79 | (0.23–2.73) | 0.704 |
| Rt optic nerve dosage (Gy) | 1.53 | (0.84–2.79) | 0.167 |
| Lt optic nerve dosage (Gy) | 1.18 | (0.65–2.17) | 0.585 |
| Chiasma dosage (Gy) | 1.40 | (0.56–3.48) | 0.470 |
| Pituitary stalk dosage (Gy) | 1.90 | (0.62–5.84) | 0.262 |
| Brain stem dosage (Gy) | 1.18 | (0.82–1.71) | 0.378 |
| Rt lateral cavernous sinus wall dosage (Gy) | 1.43 | (0.99–2.07) | 0.060 |
| Lt lateral cavernous sinus wall dosage (Gy) | 1.02 | (0.82–1.27) | 0.888 |
| IOP at GK (mmHg) | 1.30 | (0.87–1.94) | 0.205 |
| Schirmer’s test at GKRS (mm) | 0.53 | (0.15–1.88) | 0.328 |
| Percentage of Rt ICA > 20 Gy (%) | 1.05 | (0.98–1.12) | 0.140 |
| Percentage of Lt ICA > 20 Gy (%) | 1.04 | (0.96–1.13) | 0.339 |
| Percentage of Lt ICA (>20 Gy vs. ≤20 Gy) | 0.70 | (0.10–5.16) | 0.730 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.-C.; Tsuei, Y.-S.; Yang, M.-Y.; You, W.-C.; Sun, M.-H.; Sheu, M.-L.; Pan, L.-Y.; Sheehan, J.; Pan, H.-C. Gamma Knife Radiosurgery for Indirect Dural Carotid–Cavernous Fistula: Long-Term Ophthalmological Outcome. Life 2022, 12, 1175. https://doi.org/10.3390/life12081175
Shen C-C, Tsuei Y-S, Yang M-Y, You W-C, Sun M-H, Sheu M-L, Pan L-Y, Sheehan J, Pan H-C. Gamma Knife Radiosurgery for Indirect Dural Carotid–Cavernous Fistula: Long-Term Ophthalmological Outcome. Life. 2022; 12(8):1175. https://doi.org/10.3390/life12081175
Chicago/Turabian StyleShen, Chiung-Chyi, Yuang-Seng Tsuei, Meng-Yin Yang, Weir-Chiang You, Ming-His Sun, Meei-Ling Sheu, Liang-Yi Pan, Jason Sheehan, and Hung-Chuan Pan. 2022. "Gamma Knife Radiosurgery for Indirect Dural Carotid–Cavernous Fistula: Long-Term Ophthalmological Outcome" Life 12, no. 8: 1175. https://doi.org/10.3390/life12081175
APA StyleShen, C.-C., Tsuei, Y.-S., Yang, M.-Y., You, W.-C., Sun, M.-H., Sheu, M.-L., Pan, L.-Y., Sheehan, J., & Pan, H.-C. (2022). Gamma Knife Radiosurgery for Indirect Dural Carotid–Cavernous Fistula: Long-Term Ophthalmological Outcome. Life, 12(8), 1175. https://doi.org/10.3390/life12081175







