Prognostic Factors Analysis for Intracranial Cavernous Malformations Treated with Linear Accelerator Stereotactic Radiosurgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Design
2.2. Patient Population
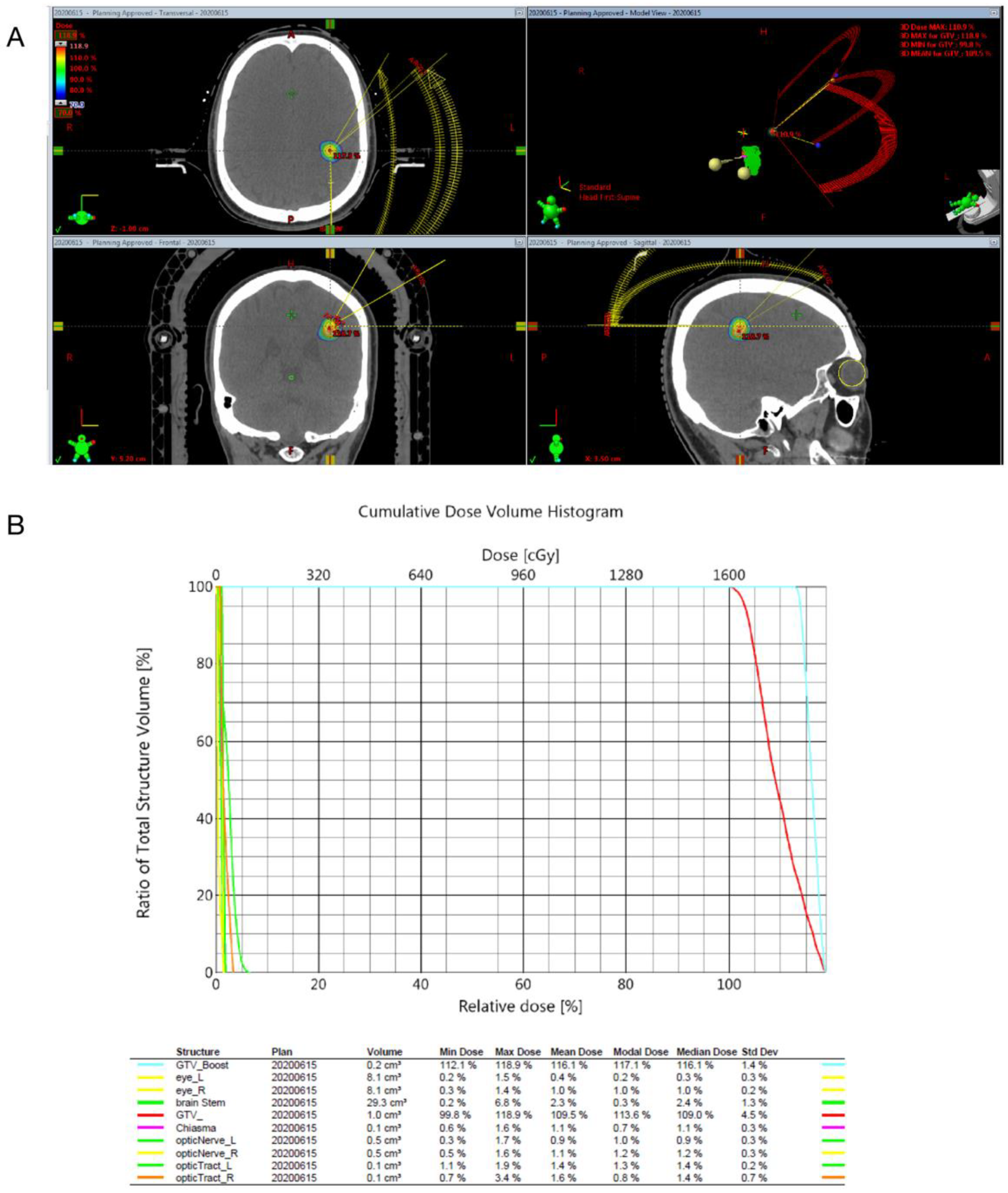
2.3. Radiosurgical Techniques
2.4. Clinical and Image Follow-Up
2.5. Outcome Assessment
2.6. Statistical Analysis
3. Results
3.1. Patient Selection and Characteristics
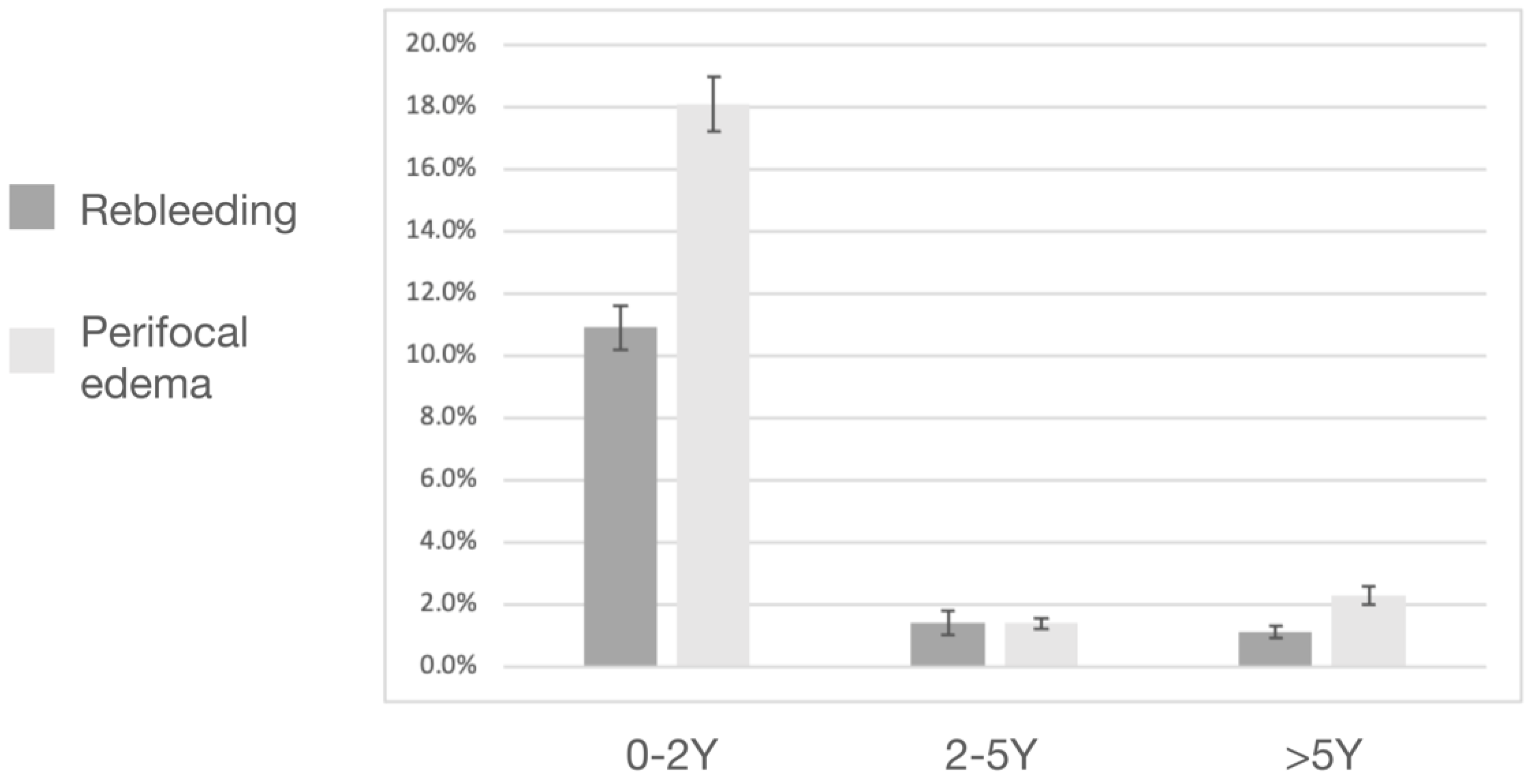
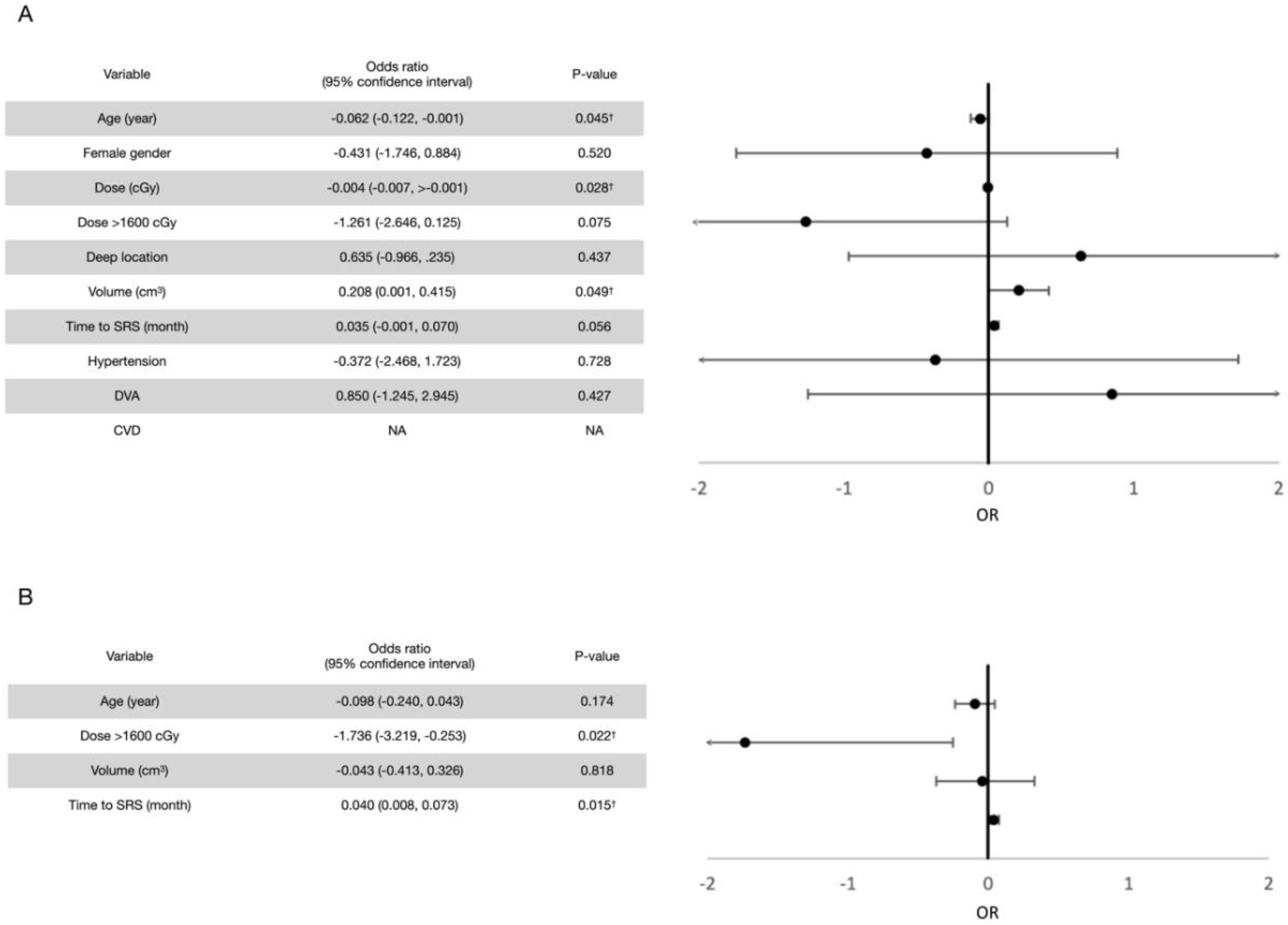
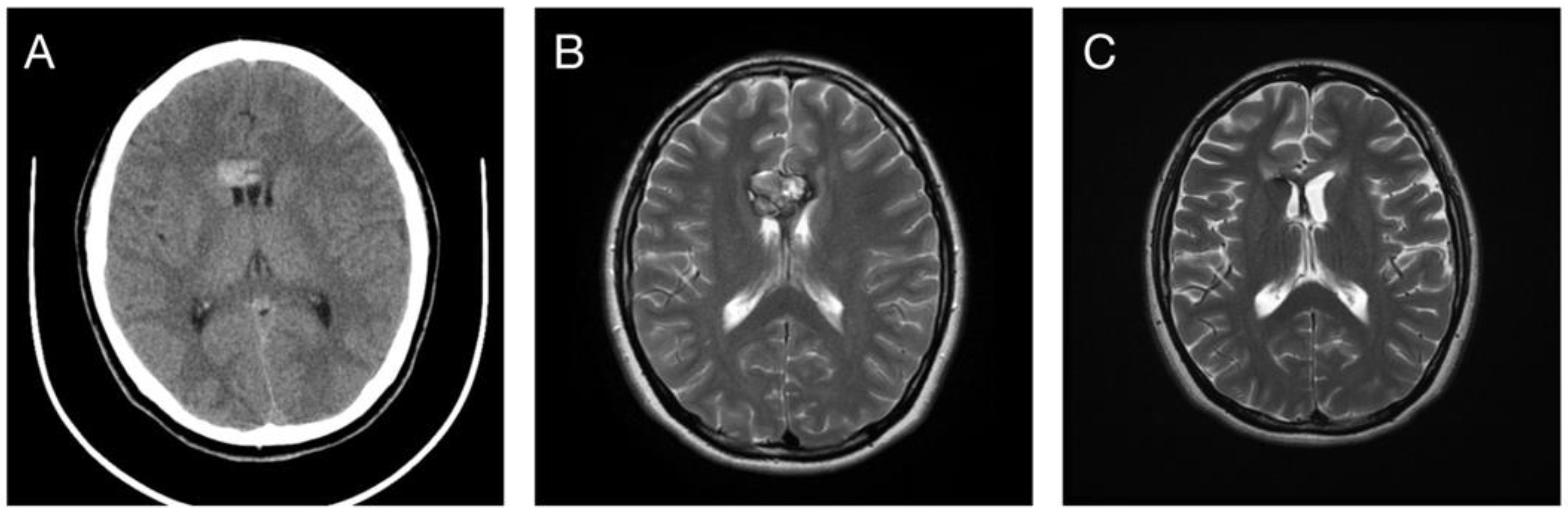
3.2. Post-Radiosurgical Rebleeding Analysis
3.3. Response in Volume Control
3.4. Post-Radiosurgical Perifocal Brain Edema
4. Discussion
4.1. Evidence of SRS in Bleeding Control for CCMs
4.2. Post-Radiosurgical Response in Volume Change
4.3. Post-Radiosurgical Adverse Radiation Effects
4.4. Optimal Marginal Dose for CCMs
4.5. Optimal Timing of SRS Treatment
4.6. LINAC SRS versus GKRS
4.7. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldstein, H.E.; Solomon, R.A. Epidemiology of cavernous malformations. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 143, pp. 241–247. [Google Scholar]
- Washington, C.W.; McCoy, K.E.; Zipfel, G.J. Update on the natural history of cavernous malformations and factors predicting aggressive clinical presentation. Neurosurg. Focus 2010, 29, E7. [Google Scholar] [CrossRef] [PubMed]
- Dalyai, R.T.; Ghobrial, G.; Awad, I.; Tjoumakaris, S.; Gonzalez, L.F.; Dumont, A.S.; Chalouhi, N.; Randazzo, C.; Rosenwasser, R.; Jabbour, P. Management of incidental cavernous malformations: A review. Neurosurg. Focus 2011, 31, E5. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.W.; Detwiler, P.W.; Spetzler, R.F. Surgical technique for resection of cavernous malformations of the brain stem. Oper. Technol. Neurosurg. 2000, 3, 124–130. [Google Scholar] [CrossRef]
- Gross, B.A.; Batjer, H.H.; Awad, I.A.; Bendok, B.R. Brainstem cavernous malformations. Neurosurgery 2009, 64, E805–E818. [Google Scholar] [CrossRef]
- Horne, M.A.; Flemming, K.D.; Su, I.-C.; Stapf, C.; Jeon, J.P.; Li, D.; Maxwell, S.S.; White, P.; Christianson, T.J.; Agid, R. Clinical course of untreated cerebral cavernous malformations: A meta-analysis of individual patient data. Lancet Neurol. 2016, 15, 166–173. [Google Scholar] [CrossRef]
- Gross, B.A.; Du, R. Hemorrhage from cerebral cavernous malformations: A systematic pooled analysis. J. Neurosurg. 2017, 126, 1079–1087. [Google Scholar] [CrossRef]
- Taslimi, S.; Modabbernia, A.; Amin-Hanjani, S.; Barker, F.G.; Macdonald, R.L. Natural history of cavernous malformation: Systematic review and meta-analysis of 25 studies. Neurology 2016, 86, 1984–1991. [Google Scholar] [CrossRef]
- Barker, F.G.; Amin-Hanjani, S.; Butler, W.E.; Lyons, S.; Ojemann, R.G.; Chapman, P.H.; Ogilvy, C.S. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery 2001, 49, 15–25. [Google Scholar]
- Stapleton, C.J.; Barker, F.G. Cranial cavernous malformations: Natural history and treatment. Stroke 2018, 49, 1029–1035. [Google Scholar] [CrossRef]
- Akers, A.; Al-Shahi Salman, R.; Awad, I.A.; Dahlem, K.; Flemming, K.; Hart, B.; Kim, H.; Jusue-Torres, I.; Kondziolka, D.; Lee, C. Synopsis of guidelines for the clinical management of cerebral cavernous malformations: Consensus recommendations based on systematic literature review by the angioma alliance scientific advisory board clinical experts panel. Neurosurgery 2017, 80, 665–680. [Google Scholar] [CrossRef]
- Zabramski, J.M.; Wascher, T.M.; Spetzler, R.F.; Johnson, B.; Golfinos, J.; Drayer, B.P.; Brown, B.; Rigamonti, D.; Brown, G. The natural history of familial cavernous malformations: Results of an ongoing study. J. Neurosurg. 1994, 80, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Quadri, S.A.; Farooqui, M.; Ikram, A.; Robinson, M.; Hart, B.L.; Mabray, M.C.; Vigil, C.; Tang, A.T.; Kahn, M.L. Familial cerebral cavernous malformations. Stroke 2019, 50, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y.; Hasegawa, T.; Iwai, Y.; Shuto, T.; Satoh, M.; Kondoh, T.; Hayashi, M. Radiosurgery for symptomatic cavernous malformations: A multi-institutional retrospective study in Japan. Surg. Neurol. Int. 2015, 6, S249. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Lunsford, L.D.; Kestle, J.R. The natural history of cerebral cavernous malformations. J. Neurosurg. 1995, 83, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Kupersmith, M.J.; Kalish, H.; Epstein, F.; Yu, G.; Berenstein, A.; Woo, H.; Jafar, J.; Mandel, G.; De Lara, F. Natural history of brainstem cavernous malformations. Neurosurgery 2001, 48, 47–54. [Google Scholar] [PubMed]
- Nikoubashman, O.; Di Rocco, F.; Davagnanam, I.; Mankad, K.; Zerah, M.; Wiesmann, M. Prospective hemorrhage rates of cerebral cavernous malformations in children and adolescents based on MRI appearance. Am. J. Neuroradiol. 2015, 36, 2177–2183. [Google Scholar] [CrossRef]
- Poorthuis, M.H.; Rinkel, L.A.; Lammy, S.; Salman, R.A.-S. Stereotactic radiosurgery for cerebral cavernous malformations: A systematic review. Neurology 2019, 93, e1971–e1979. [Google Scholar] [CrossRef]
- Karaaslan, B.; Gülsuna, B.; Erol, G.; Dağli, Ö.; Emmez, H.; Kurt, G.; Çeltikçi, E.; Börcek, A.Ö. Stereotactic radiosurgery for cerebral cavernous malformation: Comparison of hemorrhage rates before and after stereotactic radiosurgery. J. Neurosurg. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- Niranjan, A.; Lunsford, L.D. Stereotactic radiosurgery guidelines for the management of patients with intracranial cavernous malformations. Gamma Knife Radiosurg. Brain Vasc. Malform. 2013, 27, 166–175. [Google Scholar]
- Clatterbuck, R.E.; Moriarity, J.L.; Elmaci, I.; Lee, R.R.; Breiter, S.N.; Rigamonti, D. Dynamic nature of cavernous malformations: A prospective magnetic resonance imaging study with volumetric analysis. J. Neurosurg. 2000, 93, 981–986. [Google Scholar] [CrossRef]
- Wen, R.; Shi, Y.; Gao, Y.; Xu, Y.; Xiong, B.; Li, D.; Gong, F.; Wang, W. The efficacy of gamma knife radiosurgery for cavernous malformations: A meta-analysis and review. World Neurosurg. 2019, 123, 371–377. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, K.H.; Lee, M.H.; Lee, J.-I. Stereotactic radiosurgery for brainstem cavernous malformations: An updated systematic review and meta-analysis. World Neurosurg. 2019, 130, e648–e659. [Google Scholar] [CrossRef] [PubMed]
- Turnquist, C.; Harris, B.T.; Harris, C.C. Radiation-induced brain injury: Current concepts and therapeutic strategies targeting neuroinflammation. Neuro-Oncol. Adv. 2020, 2, vdaa057. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Chen, P.; Haimovitz-Friedman, A.; Reilly, R.M.; Wong, C.S. Endothelial apoptosis initiates acute blood–brain barrier disruption after ionizing radiation. Cancer Res. 2003, 63, 5950–5956. [Google Scholar] [PubMed]
- Baker, D.G.; Krochak, R.J. The response of the microvascular system to radiation: A review. Cancer Investig. 1989, 7, 287–294. [Google Scholar] [CrossRef]
- Raber, J.; Rola, R.; LeFevour, A.; Morhardt, D.; Curley, J.; Mizumatsu, S.; VandenBerg, S.R.; Fike, J.R. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat. Res. 2004, 162, 39–47. [Google Scholar] [CrossRef]
- Acharya, M.M.; Christie, L.-A.; Lan, M.L.; Giedzinski, E.; Fike, J.R.; Rosi, S.; Limoli, C.L. Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. 2011, 71, 4834–4845. [Google Scholar] [CrossRef]
- Gondi, V.; Hermann, B.P.; Mehta, M.P.; Tomé, W.A. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e487–e493. [Google Scholar] [CrossRef]
- Khan, S.Y.; Awad, E.M.; Oszwald, A.; Mayr, M.; Yin, X.; Waltenberger, B.; Stuppner, H.; Lipovac, M.; Uhrin, P.; Breuss, J.M. Premature senescence of endothelial cells upon chronic exposure to TNFα can be prevented by N-acetyl cysteine and plumericin. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Rochfort, K.D.; Cummins, P.M. The blood–brain barrier endothelium: A target for pro-inflammatory cytokines. Biochem. Soc. Trans. 2015, 43, 702–706. [Google Scholar] [CrossRef]
- Chang, J.; Chang, J.; Choi, J.; Park, Y.; Chung, S. Complications after gamma knife radiosurgery for benign meningiomas. J. Neurol. Neurosurg. Psychiatry 2003, 74, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Kalapurakal, J.A.; Silverman, C.L.; Akhtar, N.; Laske, D.W.; Braitman, L.E.; Boyko, O.B.; Thomas, P. Intracranial meningiomas: Factors that influence the development of cerebral edema after stereotactic radiosurgery and radiation therapy. Radiology 1997, 204, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Harat, M.; Lebioda, A.; Lasota, J.; Makarewicz, R. Evaluation of brain edema formation defined by MRI after LINAC-based stereotactic radiosurgery. Radiol. Oncol. 2017, 51, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Razak, A.; Rowe, J.G.; Hodgson, T.J.; Coley, S.C.; Radatz, M.W.; Patel, U.J.; Kemeny, A.A. Stereotactic radiosurgery for deep-seated cavernous malformations: A move toward more active, early intervention. J. Neurosurg. 2010, 113, 691–699. [Google Scholar] [CrossRef]
- Idiculla, P.S.; Gurala, D.; Philipose, J.; Rajdev, K.; Patibandla, P. Cerebral cavernous malformations, developmental venous anomaly, and its coexistence: A review. Eur. Neurol. 2020, 83, 360–368. [Google Scholar] [CrossRef]
- Mooney, M.A.; Zabramski, J.M. Developmental venous anomalies. Handb. Clin. Neurol. 2017, 143, 279–282. [Google Scholar]
- Tu, J.; Stoodley, M.; Morgan, M.; Storer, K.; Smee, R. Different responses of cavernous malformations and arteriovenous malformations to radiosurgery. J. Clin. Neurosci. 2009, 16, 945–949. [Google Scholar] [CrossRef]
- Nyáry, I.; Major, O.; Hanzély, Z.; Szeifert, G.T. Pathological considerations to irradiation of cavernous malformations. In Radiosurgery and Pathological Fundamentals; Karger Publishers: Basel, Switzerland, 2007; Volume 20, pp. 231–234. [Google Scholar]
- Hasegawa, H.; Yamamoto, M.; Shin, M.; Barfod, B.E. Gamma knife radiosurgery for brain vascular malformations: Current evidence and future tasks. Ther. Clin. Risk Manag. 2019, 15, 1351. [Google Scholar] [CrossRef]
- Hasegawa, T.; McInerney, J.; Kondziolka, D.; Lee, J.Y.; Flickinger, J.C.; Lunsford, L.D. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery 2002, 50, 1190–1198. [Google Scholar]
- Park, K.; Kim, J.W.; Chung, H.-T.; Paek, S.H.; Kim, D.G. Long-term outcome of gamma knife radiosurgery for symptomatic brainstem cavernous malformation. World Neurosurg. 2018, 116, e1054–e1059. [Google Scholar] [CrossRef]
- Liu, H.B.; Wang, Y.; Yang, S.; Gong, F.L.; Xu, Y.Y.; Wang, W. Gamma knife radiosurgery for brainstem cavernous malformations. Clin. Neurol. Neurosurg. 2016, 151, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, H.J.; Shin, H.S.; Choi, S.K.; Oh, I.H.; Lim, Y.J. Gamma knife radiosurgery for brainstem cavernous malformations: Should a patient wait for the rebleed? Acta Neurochir. 2014, 156, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Pan, D.H.-C.; Chung, W.-Y.; Liu, K.-D.; Yang, H.-C.; Wu, H.-M.; Guo, W.-Y.; Shih, Y.-H. Brainstem cavernous malformations: The role of Gamma Knife surgery. J. Neurosurg. 2012, 117, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Lunsford, L.D.; Khan, A.A.; Niranjan, A.; Kano, H.; Flickinger, J.C.; Kondziolka, D. Stereotactic radiosurgery for symptomatic solitary cerebral cavernous malformations considered high risk for resection. J. Neurosurg. 2010, 113, 23–29. [Google Scholar] [CrossRef]
- Kida, Y. Radiosurgery for cavernous malformations in basal ganglia, thalamus and brainstem. In Japanese Experience with Gamma Knife Radiosurgery; Karger Publishers: Basel, Switzerland, 2009; Volume 22, pp. 31–37. [Google Scholar]
- Monaco, E.A.; Khan, A.A.; Niranjan, A.; Kano, H.; Grandhi, R.; Kondziolka, D.; Flickinger, J.C.; Lunsford, L.D. Stereotactic radiosurgery for the treatment of symptomatic brainstem cavernous malformations. Neurosurg. Focus 2010, 29, E11. [Google Scholar] [CrossRef]
- Jacobs, R.; Kano, H.; Gross, B.A.; Niranjan, A.; Monaco III, E.A.; Lunsford, L.D. Defining long-term clinical outcomes and risks of stereotactic radiosurgery for brainstem cavernous malformations. World Neurosurg. 2019, 124, e58–e64. [Google Scholar] [CrossRef]
- Sheen, J.J.; Lee, D.H.; Lee, D.H.; Song, Y.; Kwon, D.H. Long-term outcome of gamma knife radiosurgery for brain cavernoma: Factors associated with subsequent De Novo Cavernoma formation. World Neurosurg. 2018, 120, e17–e23. [Google Scholar] [CrossRef]
- Kim, D.; Choe, W.; Paek, S.; Chung, H.; Kim, I.; Han, D. Radiosurgery of intracranial cavernous malformations. Acta Neurochir. 2002, 144, 869–878. [Google Scholar] [CrossRef]


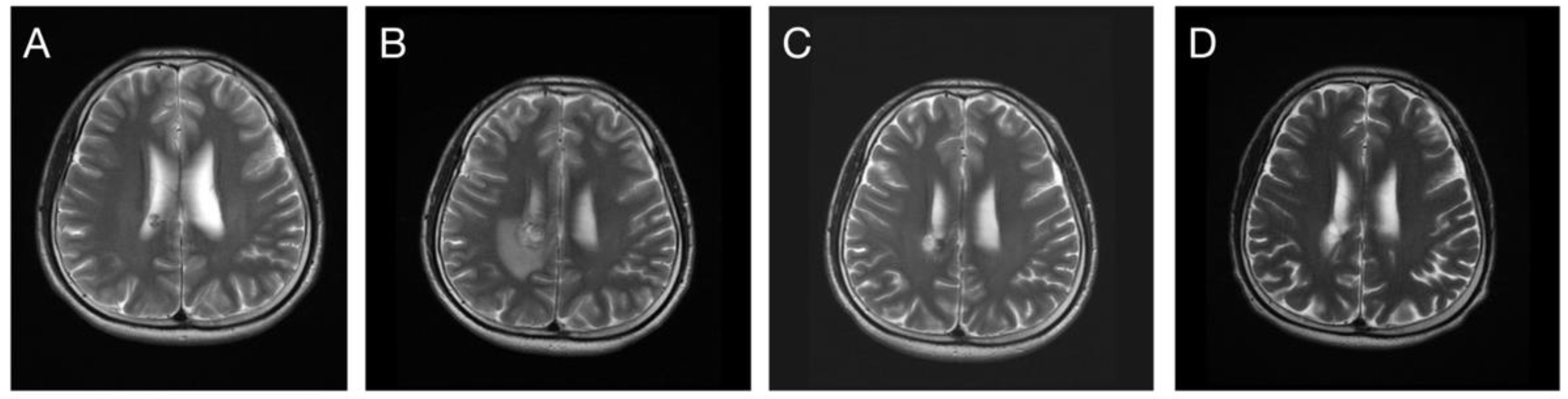
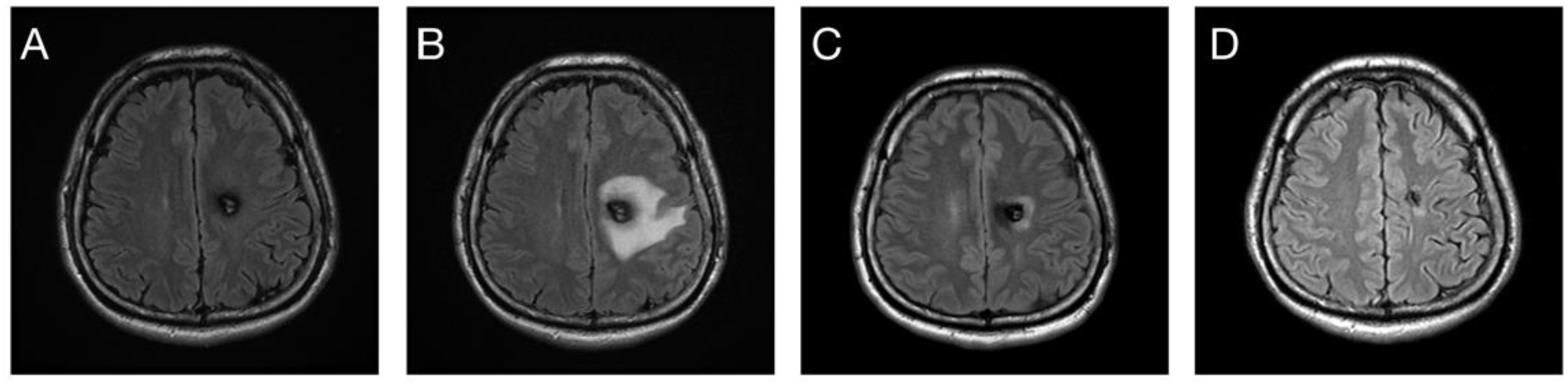
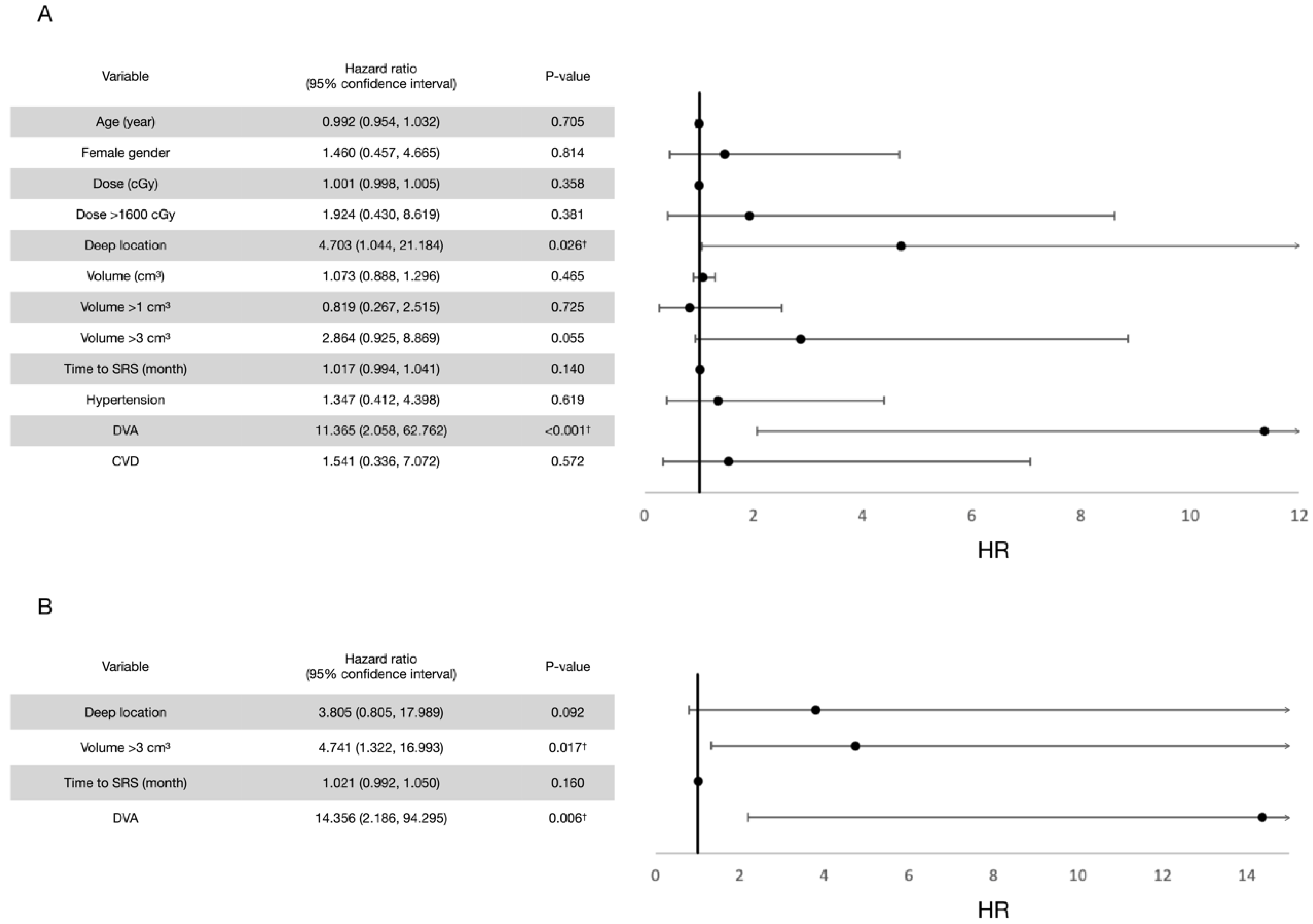
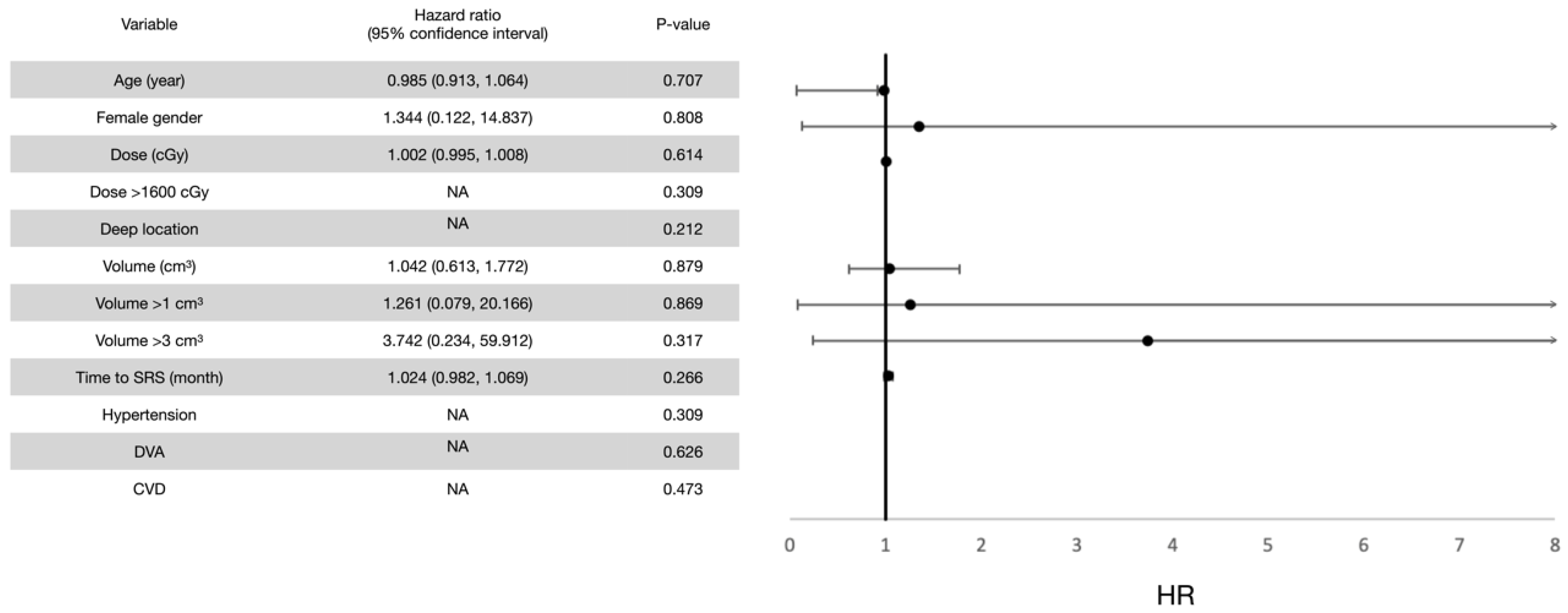
| Number (%) or Median (Standard Deviation) | |
|---|---|
| Gender | |
| Male | 12 (40%) |
| Female | 18 (60%) |
| Age (year) | 43 (15.2) |
| Initial Zabramski classification | |
| Class I | 4 (13.3%) |
| Class II | 25 (83.3%) |
| Class III | 1 (3.3%) |
| Class IV | 0 (0%) |
| Location | |
| Non-eloquent WGJ | 9 (30%) |
| Eloquent WGJ | 1 (3.3%) |
| Subcortical nucleus | 4 (13.3%) |
| White matter | 15 (50%) |
| Cerebellum | 1 (3.3%) |
| Initial symptoms | |
| Motor deficit | 11 (36.7%) |
| Headache | 8 (26.7%) |
| Seizure | 8 (26.7%) |
| Dizziness | 6 (20%) |
| Cranial nerve deficit | 5 (16.7%) |
| Sensory deficit | 1 (3.3%) |
| Time to treatment (months) | 11 (20.8) |
| Marginal dose (cGy) | 2000 (201.9) |
| Initial volume (cm3) | 0.97 (5.67) |
| Follow-up period (months) | |
| Total | 61 (63.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, M.-W.; Chuang, C.-C.; Wang, C.-C.; Chen, H.-C.; Hsu, P.-W. Prognostic Factors Analysis for Intracranial Cavernous Malformations Treated with Linear Accelerator Stereotactic Radiosurgery. Life 2022, 12, 1363. https://doi.org/10.3390/life12091363
Chung M-W, Chuang C-C, Wang C-C, Chen H-C, Hsu P-W. Prognostic Factors Analysis for Intracranial Cavernous Malformations Treated with Linear Accelerator Stereotactic Radiosurgery. Life. 2022; 12(9):1363. https://doi.org/10.3390/life12091363
Chicago/Turabian StyleChung, Meng-Wu, Chi-Cheng Chuang, Chun-Chieh Wang, Hsien-Chih Chen, and Peng-Wei Hsu. 2022. "Prognostic Factors Analysis for Intracranial Cavernous Malformations Treated with Linear Accelerator Stereotactic Radiosurgery" Life 12, no. 9: 1363. https://doi.org/10.3390/life12091363







