Identification and Current Palaeobiological Understanding of “Keratosa”-Type Nonspicular Demosponge Fossils in Carbonates: With a New Example from the Lowermost Triassic, Armenia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Preservation of “Keratosa”-Type Demosponges in Carbonates
3.2. Recognition Criteria of “Keratosa”-Type Demosponges in Carbonates
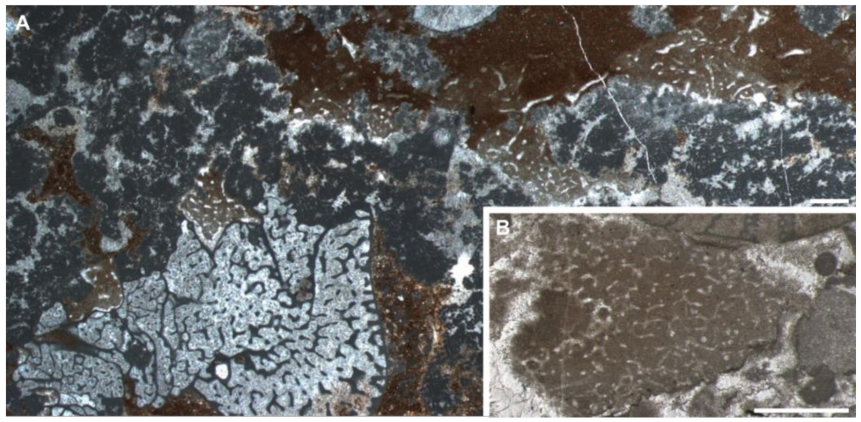
- Fibrous skeletons are preserved as microspar-cemented moulds in homogeneous automicrites.
- The skeletal fibres form an anastomosing network extending three-dimensionally in the micritic aggregation with a generally uniform density.
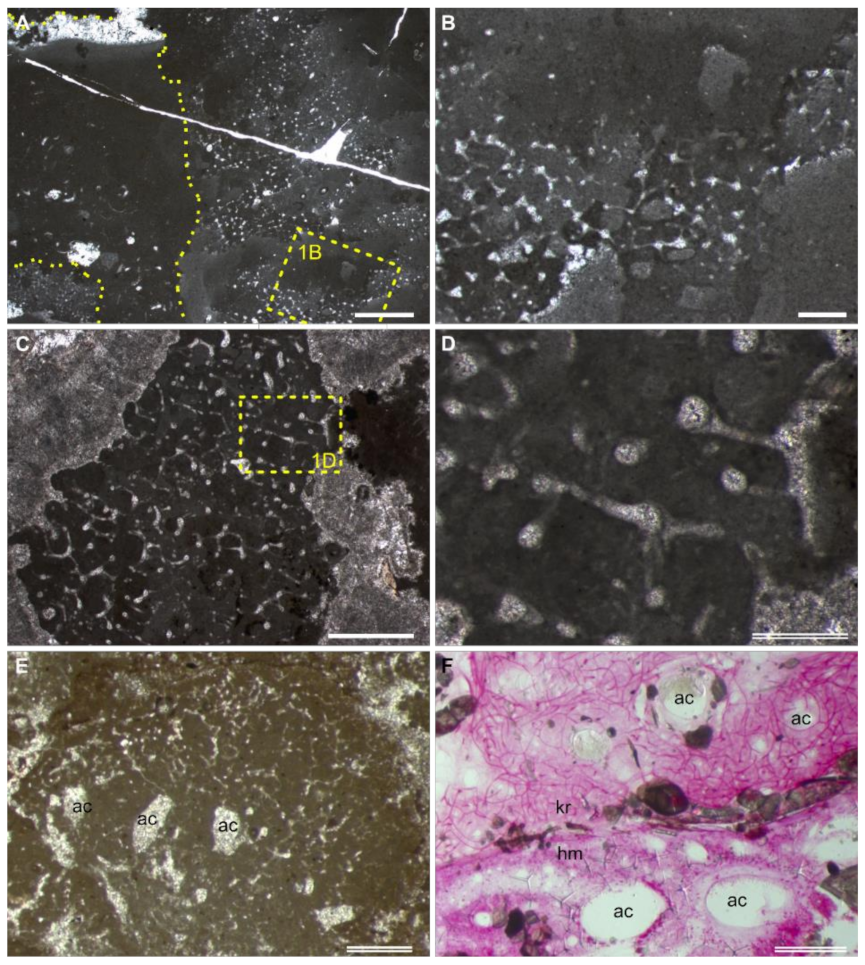
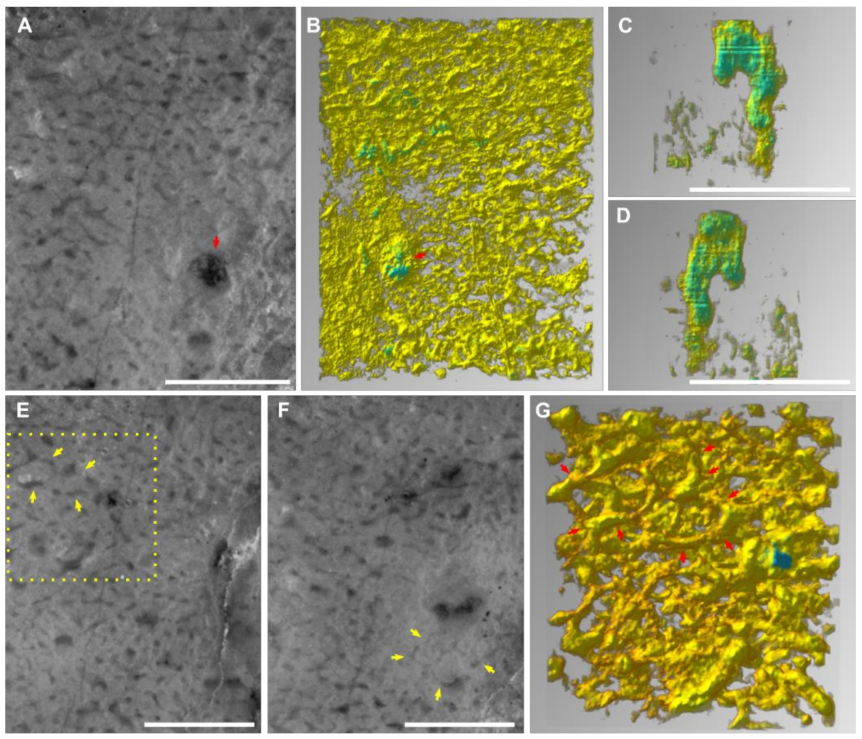
- The skeleton persists a uniform thickness along each fibre. In the whole skeletal frame, the fibre thicknesses either change gradually or exhibit regular orders or hierarchies. For reference, the diameters of skeletal fibres in living nonspicular demosponges vary from a few to hundreds of micrometres, with many being around tens of micrometres thick (Figure 2; Supplementary File S2).
- The fibrous network is constrained in the micritic aggregation and exhibits fibres lining the border of the aggregation, such as between the sponge body and the hard substrates and wrapped particles.
- Water canals of the sponge aquiferous system are sometimes preserved (Figure 1E,F; more discussion in Section 4.1). If present, they add credits to the sponge interpretation.
3.3. Observation of the Chanakhchi Fossils
4. Discussion
4.1. Differentiating “Keratosa”-Type Demosponge Fossils from Other Similar Structures Based on Proposed Criteria
4.2. Identifying Chanakhchi Fossils as Nonspicular Demosponges
4.3. “Keratosa”-Type Demosponge in the Fossil Record: Known and Unknown
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reitner, J.; Hühne, C.; Thiel, V. Porifera-rich mud mounds and microbialites as indicators of environmental changes within the Devonian/Lower Carboniferous critical interval. Terra Nostra 2001, 4, 60–65. [Google Scholar]
- Luo, C.; Reitner, J. First report of fossil “keratose” demosponges in Phanerozoic carbonates: Preservation and 3-D reconstruction. Naturwissenschaften 2014, 101, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Luo, C. “Keratose” Sponge Fossils and Microbialites: A Geobiological Contribution to the Understanding of Metazoan Origin. Doctoral Dissertation, University of Göttingen, Göttingen, Germany, 2015. [Google Scholar]
- Lee, J.-H.; Riding, R. Keratolite-stromatolite consortia mimic domical and branched columnar stromatolites. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 571, 110288. [Google Scholar] [CrossRef]
- Lee, J.-H.; Chen, J.-T.; Choh, S.-J.; Lee, D.-J.; Han, Z.-Z.; Chough, S.K. Furongian (Late Cambrian) sponge–microbial maze-like reefs in the North China Platform. Palaios 2014, 29, 27–37. [Google Scholar] [CrossRef]
- Luo, C.; Reitner, J. “Stromatolites” built by sponges and microbes—A new type of Phanerozoic bioconstruction. Lethaia 2016, 49, 555–570. [Google Scholar] [CrossRef]
- Lee, J.-H.; Riding, R. The “classic stromatolite” Cryptozoon is a keratose sponge-microbial consortium. Geobiology 2021, 19, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Chen, J.; Chough, S.K. The middle-late Cambrian reef transition and related geological events: A review and new view. Earth-Sci. Rev. 2015, 145, 66–84. [Google Scholar] [CrossRef]
- Friesenbichler, E.; Richoz, S.; Baud, A.; Krystyn, L.; Sahakyan, L.; Vardanyan, S.; Peckmann, J.; Reitner, J.; Heindel, K. Sponge-microbial build-ups from the lowermost Triassic Chanakhchi section in southern Armenia: Microfacies and stable carbon isotopes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 490, 653–672. [Google Scholar] [CrossRef]
- Heindel, K.; Foster, W.J.; Richoz, S.; Birgel, D.; Roden, V.J.; Baud, A.; Brandner, R.; Krystyn, L.; Mohtat, T.; Kosun, E.; et al. The formation of microbial-metazoan bioherms and biostromes following the latest Permian mass extinction. Gondwana Res. 2018, 61, 187–202. [Google Scholar] [CrossRef]
- Foster, W.J.; Heindel, K.; Richoz, S.; Gliwa, J.; Lehrmann, D.J.; Baud, A.; Kolar-Jurkovšek, T.; Aljinović, D.; Jurkovšek, B.; Korn, D.; et al. Suppressed competitive exclusion enabled the proliferation of Permian/Triassic boundary microbialites. Depositional. Rec. 2020, 6, 62–74. [Google Scholar] [CrossRef]
- Baud, A.; Richoz, S.; Brandner, R.; Krystyn, L.; Heindel, K.; Mohtat, T.; Mohtat-Aghai, P.; Horacek, M. Sponge takeover from end-Permian mass extinction to early Induan time: Records in central Iran microbial buildups. Front. Earth Sci. 2021, 9, 586210. [Google Scholar] [CrossRef]
- Pei, Y.; Duda, J.-P.; Schönig, J.; Luo, C.; Reitner, J. Late Anisian microbe-metazoan build-ups in the Germanic Basin: Aftermath of the Permian–Triassic crisis. Lethaia 2021, 54, 823–844. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Z.-Q.; Su, C.; Fang, Y.; Yang, H. Keratose sponge fabrics from the lowermost Triassic microbialites in South China: Geobiologic features and Phanerozoic evolution. Glob. Planet. Chang. 2022, 211, 103787. [Google Scholar] [CrossRef]
- Turner, E.C. Possible poriferan body fossils in early Neoproterozoic microbial reefs. Nature 2021, 596, 87–91. [Google Scholar] [CrossRef]
- Erwin, D.H. The origin of animal body plans: A view from fossil evidence and the regulatory genome. Development 2020, 147, dev182899. [Google Scholar] [CrossRef]
- Coulson, K.P.; Brand, L.R. Lithistid sponge-microbial reef-building communities construct laminated, Upper Cambrian (Furongian) “stromatolites”. Palaios 2016, 31, 358–370. [Google Scholar] [CrossRef]
- Larmagnat, S.; Neuweiler, F. Taphonomic filtering in Ordovician bryozoan carbonate mounds, Trenton Group, Montmorency Falls, Quebec, Canada. Palaios 2015, 30, 169–180. [Google Scholar] [CrossRef]
- Mei, M.; Latif, K.; Meng, Q.; Hu, Y. Cambrian bioherms dominated by microbial carbonate within the oolitic grainstone bank, Zhangxia Formation of the Miaolingian, Zhucaoying section in Qinhuangdao City of Hebei Province. Acta Geol. Sin. 2019, 93, 227–251. [Google Scholar]
- Kershaw, S.; Li, Q.; Li, Y. Addressing a Phanerozoic carbonate facies conundrum—sponges or clotted micrite? Evidence from Early Silurian reefs, South China Block. Sed. Rec. 2021, 19, 3–10. [Google Scholar] [CrossRef]
- Kris, A.; McMenamin, M. Putative Proterozoic sponge spicules reinterpreted as microburrows. Acad. Lett. 2021, 3800. [Google Scholar] [CrossRef]
- Minchin, E.A. Chapter III. Sponges. In A Treatise on Zoology. Part II. The Porifera and Coelenterata; Lankester, E.R., Ed.; Adam & Charles Black: London, UK, 1900; pp. 1–178. [Google Scholar]
- Erpenbeck, D.; Sutcliffe, P.; de Cook, S.C.; Dietzel, A.; Maldonado, M.; van Soest, R.W.M.; Hooper, J.N.A.; Wörheide, G. Horny sponges and their affairs: On the phylogenetic relationships of keratose sponges. Mol. Phylogenetics Evol. 2012, 63, 809–816. [Google Scholar] [CrossRef]
- Wörheide, G.; Dohrmann, M.; Erpenbeck, D.; Larroux, C.; Maldonado, M.; Voigt, O.; Borchiellini, C.; Lavrov, D.V. Chapter one—Deep phylogeny and evolution of sponges (phylum Porifera). In Advances in Marine Biology; Becerro, M.A., Uriz, M.J., Manuel Maldonado, X.T., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 61, pp. 1–78. ISBN 0065-2881. [Google Scholar]
- Morrow, C.; Cárdenas, P. Proposal for a revised classification of the Demospongiae (Porifera). Front. Zool. 2015, 12, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigby, J.K. Sponges of the Burgess Shale (Middle Cambrian), British Columbia; Palaeontographica Canadiana; University of Toronto Press: Toronto, ON, Canada, 1986; Volume 2, pp. 1–105. [Google Scholar]
- Ehrlich, H.; Rigby, J.K.; Botting, J.P.; Tsurkan, M.V.; Werner, C.; Schwille, P.; Petrášek, Z.; Pisera, A.; Simon, P.; Sivkov, V.N.; et al. Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta. Sci. Rep. 2013, 3, 3497. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, Y.D.; Biakov, A.S.; Baud, A.; Kozur, H.W. Significance of Caucasian sections for working out carbon-isotope standard for Upper Permian and Lower Triassic (Induan) and their correlation with the Permian of north-eastern Russia. J. China Univ. Geosci. 2005, 16, 141–151. [Google Scholar]
- Baud, A.; Magaritz, M.; Holser, W.T. Permian-Triassic of the Tethys: Carbon isotope studies. Geol. Rundsch. 1989, 78, 649–677. [Google Scholar] [CrossRef]
- Gross, J.; Sokal, Z.; Rougvie, M. Structural and chemical studies on the connective tissue of marine sponges. J. Histochem. Cytochem. 1956, 4, 227–246. [Google Scholar] [CrossRef]
- Junqua, S.; Robert, L.; Garrone, R.; De Ceccatty, M.P.; Vacelet, J. Biochemical and morphological studies on collagens of horny sponges. Ircinia filaments compared to spongines. Connect. Tissue Res. 1974, 2, 193–203. [Google Scholar] [CrossRef]
- Ehrlich, H.; Maldonado, M.; Spindler, K.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (Demospongia: Porifera). J. Exp. Zool. Part B Mol. Dev. Evol. 2007, 308, 347–356. [Google Scholar] [CrossRef]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef]
- Brachert, T.C. Environmental control on fossilization of siliceous sponge assemblages: A proposal. In Fossil and Recent Sponges; Reitner, J., Keupp, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 543–553. ISBN 978-3-642-75658-0. [Google Scholar]
- Reitner, J. Mikrofazielle, palökologische und paläogeographische Analyse ausgewählter Vorkommen flachmariner Karbonate im Basko-Kantabrischen Strike Slip Fault-Becken-System (Nordspanien) an der Wende von der Unterkreide zur Oberkreide. Doc. Nat. 1987, 40, 1–239. [Google Scholar]
- Fritz, G.K. Schwammstotzen, Tuberoide und Schuttbreccien im Weißen Jura der Schwäbischen Alb. Arb. Des Geol.-Paläontologischen Inst. TH Stuttg. NF 1958, 13, 1–118. [Google Scholar]
- Reitner, J. Modern cryptic microbialite/metazoan facies from Lizard Island (Great Barrier Reef, Australia): Formation and concepts. Facies 1993, 29, 3–39. [Google Scholar] [CrossRef]
- Warnke, K. Calcification processes of siliceous sponges in Viséan Limestones (Counties Sligo and Leitrim, Northwestern Ireland). Facies 1995, 33, 215–227. [Google Scholar] [CrossRef]
- Neuweiler, F.; Burdige, D.J. The modern calcifying sponge Spheciospongia vesparium (Lamarck, 1815), Great Bahama Bank: Implications for ancient sponge mud-mounds. Sediment. Geol. 2005, 175, 89–98. [Google Scholar] [CrossRef]
- Reitner, J.; Gautret, P.; Marin, F.; Neuweiler, F. Automicrites in a modern marine microbialite: Formation model via organic matrices (Lizard Island, Great Barrier Reef, Australia). Bull. De L’institut Oćeanographique Monaco 1995, 14, 237–303. [Google Scholar]
- Reitner, J.; Schumann-Kindel, G. Pyrite in mineralized sponge tissue—product of sulfate reducing sponge-related bacteria? Facies 1997, 36, 272–276. [Google Scholar]
- Neuweiler, F.; Daoust, I.; Bourque, P.-A.; Burdige, D.J. Degradative calcification of a modern siliceous sponge from the Great Bahama Bank, The Bahamas: A guide for interpretation of ancient sponge-bearing limestones. J. Sediment. Res. 2007, 77, 552–563. [Google Scholar] [CrossRef]
- Costa, G.; Betti, F.; Nepote, E.; Cattaneo-Vietti, R.; Pansini, M.; Bavestrello, G.; Bertolino, M. Sponge community variations within two semi-submerged caves of the Ligurian Sea (Mediterranean Sea) over a half-century time span. Eur. Zool. J. 2018, 85, 381–391. [Google Scholar] [CrossRef]
- Schönberg, C.H.L. No taxonomy needed: Sponge functional morphologies inform about environmental conditions. Ecol. Indic. 2021, 129, 107806. [Google Scholar] [CrossRef]
- Bergquist, P.R.; Cook, S.D.C. Family Pseudoceratinidae Carter, 1885. In Systema Porifera: A Guide to the Classification of Sponges; Hooper, J.N.A., Van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; pp. 1086–1088. [Google Scholar]
- Cook, S.D.C.; Bergquist, P.R. Order Dictyoceratida Minchin, 1900. In Systema Porifera: A Guide to the Classification of Sponges; Hooper, J.N.A., Van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; p. 1021. [Google Scholar]
- Bavestrello, G.; Burlando, B.; Sara’, M. The architecture of the canal systems of Petrosia ficiformis and Chondrosia reniformis studied by corrosion casts (Porifera, Demospongiae). Zoomorphology 1988, 108, 161–166. [Google Scholar] [CrossRef]
- Riding, R. Calcified Cyanobacteria. In Calcareous Algae and Stromatolites; Riding, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 55–87. ISBN 978-3-642-52337-3. [Google Scholar]
- Ivarsson, M.; Bengtson, S.; Belivanova, V.; Stampanoni, M.; Marone, F.; Tehler, A. Fossilized fungi in subseafloor Eocene basalts. Geology 2012, 40, 163–166. [Google Scholar] [CrossRef]
- Jackson, F.D.; Jin, X.; Schmitt, J. Fungi in a Lower Cretaceous turtle egg from China: Evidence of ecological interactions. Palaios 2009, 24, 840–845. [Google Scholar] [CrossRef]
- Taylor, T.N.; Taylor, E.L.; Krings, M. Chapter 3 Fungi, bacteria, and lichens. In Paleobotany: The Biology and Evolution of Fossil Plants; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA; Heidelberg, Germany; London, UK; New York, UK; Oxford, UK; Paris, France; San Diego, CA, USA; San Francisco, CA, USA; Singapore, Sydney, Australia; Tokyo, Japan, 2009; pp. 71–122. [Google Scholar]
- Jans, M.M.E. Microbial bioerosion of bone—A review. In Current Development in Bioerosion; Wisshak, M., Tapanila, L., Eds.; Erlangen Earth Conference Series; Springer: Berlin/Heidelberg, Germany, 2008; pp. 397–413. ISBN 978-3-540-77597-3. [Google Scholar]
- Schlagintweit, F.; Bover-Arnal, T.; Salas, R. New insights into Lithocodium aggregatum Elliott 1956 and Bacinella irregularis Radoičić 1959 (Late Jurassic–Lower Cretaceous): Two ulvophycean green algae (Order Ulotrichales) with a heteromorphic life cycle (epilithic/euendolithic). Facies 2010, 56, 509–547. [Google Scholar] [CrossRef]
- Cherchi, A.; Schroeder, R. Revision of the holotype of Lithocodium aggregatum Elliott, 1956 (Lower Cretaceous, Iraq): New interpretation as sponge–calcimicrobe consortium. Facies 2012, 59, 49–57. [Google Scholar] [CrossRef]
- Uriz, M.-J. Mineral skeletogenesis in sponges. Can. J. Zool. 2006, 84, 322–356. [Google Scholar] [CrossRef]
- Pisera, A. Fossil “Lithistids”: An overview. In Systema Porifera: A Guide to the Classification of Sponges; Hooper, J.N.A., Van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; pp. 388–402. [Google Scholar]
- Desqueyroux-Faúndez, R.; Valentine, C. Family Callyspongiidae de Laubenfels, 1936. In Systema Porifera: A Guide to the Classification of Sponges; Hooper, J.N.A., Van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; pp. 835–851. [Google Scholar]
- Wood, R.; Zhuravlev, A.Y.; Anaaz, C.T. The ecology of Lower Cambrian buildups from Zuune Arts, Mongolia: Implications for early metazoan reef evolution. Sedimentology 1993, 40, 829–858. [Google Scholar] [CrossRef]
- McMenamin, M.A.S. Early Cambrian Microburrow Nests and the Origin of Parenting Skills. In Proceedings of the Geological Society of America Abstracts with Programs, Charlotte, NC, USA, 4–7 November 2012; Volume 44, p. 500. [Google Scholar]
- Lee, J.-H.; Kim, B.-J.; Liang, K.; Park, T.-Y.S.; Choh, S.-J.; Lee, D.-J.; Woo, J. Cambrian reefs in the western North China Platform, Wuhai, Inner Mongolia. Acta Geol. Sin. Engl. Ed. 2016, 90, 1946–1954. [Google Scholar] [CrossRef]
- Parry, L.A.; Boggiani, P.C.; Condon, D.J.; Garwood, R.J.; Leme, J.D.M.; McIlroy, D.; Brasier, M.D.; Trindade, R.; Campanha, G.A.C.; Pacheco, M.L.A.F.; et al. Ichnological evidence for meiofaunal bilaterians from the terminal Ediacaran and earliest Cambrian of Brazil. Nat. Ecol. Evol. 2017, 1, 1455–1464. [Google Scholar] [CrossRef]
- Uchman, A. Trends in diversity, frequency and complexity of graphoglyptid trace fossils: Evolutionary and palaeoenvironmental aspects. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 192, 123–142. [Google Scholar] [CrossRef]
- Seilacher, A. Trace Fossil Analysis; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007; pp. 1–226. [Google Scholar]
- Wilson, R.D.; Schieber, J.; Stewart, C.J. The discovery of widespread agrichnia traces in Devonian black shales of North America: Another chapter in the evolving understanding of a “not so anoxic” ancient sea. PalZ 2021, 95, 661–681. [Google Scholar] [CrossRef]
- Flügel, E. Microfacies of Carbonate Rocks: Analysis, Interpretation and Application, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–984. [Google Scholar]
- Scholle, P.A.; Ulmer-Scholle, D.S. A Color Guide to the Petrography of Carbonate Rocks: Grains, Textures, Porosity, Diagenesis; AAPG Memoir; The American Association of Petroleum Geologists: Tulsa, OK, USA, 2003; pp. 1–459. [Google Scholar]
- Baron-Szabo, R.C.; Schafhauser, A.; Götz, S.; Stinnesbeck, W. Scleractinian corals from the Cardenas Formation (Maastrichtian), San Luis Potosí, Mexico. J. Paleontol. 2006, 80, 1033–1046. [Google Scholar] [CrossRef]
- Hammel, J.U.; Filatov, M.V.; Herzen, J.; Beckmann, F.; Kaandorp, J.A.; Nickel, M. The non-hierarchical, non-uniformly branching topology of a leuconoid sponge aquiferous system revealed by 3D reconstruction and morphometrics using corrosion casting and X-ray microtomography. Acta Zool. 2012, 93, 160–170. [Google Scholar] [CrossRef]
- Delecat, S.; Reitner, J. Sponge communities from the Lower Liassic of Adnet (Northern Calcareous Alps, Austria). Facies 2005, 51, 385–404. [Google Scholar] [CrossRef]
- Natalicchio, M.; Peckmann, J.; Birgel, D.; Kiel, S. Seep deposits from northern Istria, Croatia: A first glimpse into the Eocene seep fauna of the Tethys region. Geol. Mag. 2015, 152, 444–459. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.-H.; Hong, J.; Choh, S.-J.; Lee, D.-C.; Lee, D.-J. An Upper Ordovician sponge-bearing micritic limestone and implication for early Palaeozoic carbonate successions. Sediment. Geol. 2015, 319, 124–133. [Google Scholar] [CrossRef]
- Kiel, S.; Sami, M.; Taviani, M. A serpulid-Anodontia-dominated methane-seep deposit from the upper Miocene of northern Italy. Acta Palaeontol. Pol. 2018, 63, 569–577. [Google Scholar] [CrossRef]
- Luo, C.; Zhao, F.; Zeng, H. The first report of a vauxiid sponge from the Cambrian Chengjiang Biota. J. Paleontol. 2020, 94, 28–33. [Google Scholar] [CrossRef]
- Wei, F.; Zhao, Y.; Chen, A.; Hou, X.; Cong, P. New vauxiid sponges from the Chengjiang Biota and their evolutionary significance. J. Geol. Soc. 2021, 178, jgs2020-162. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Babcock, L.E.; Peng, J. A new vauxiid sponge from the Kaili Biota (Cambrian Stage 5), Guizhou, South China. Geol. Mag. 2017, 156, 1334–1343. [Google Scholar] [CrossRef]
- Bergquist, P.R.; Cook, S.D.C. Order Verongida Bergquist, 1978. In Systema Porifera: A Guide to the Classification of Sponges; Hooper, J.N.A., Van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; p. 1081. [Google Scholar]
- Fromont, J.; Zołtowska-Aksamitowska, S.; Galli, R.; Meissner, H.; Erpenbeck, D.; Vacelet, J.; Diaz, C.; Tsurkan, M.V.; Petrenko, I.; Youssef, D.T.A.; et al. New family and genus of a Dendrilla-like sponge with characters of Verongiida. Part II. Discovery of chitin in the skeleton of Ernstilla lacunosa. Zool. Anz. 2019, 280, 21–29. [Google Scholar] [CrossRef]
- Zhuravlev, A.Y. (Department of Biological Evolution, Faculty of Biology, Lomonosov Moscow State University, Moscow, Russia). Personal communication. 2018. [Google Scholar]
- Kruse, P.D.; Zhuravlev, A.Y.; James, N.P. Primordial metazoan-calcimicrobial reefs: Tommotian (Early Cambrian) of the Siberian Platform. Palaios 1995, 10, 291–321. [Google Scholar] [CrossRef]
- Zhuravleva, I.T. Arkheotsiaty Sibirskoy Platformy (Archaeocyaths of the Siberian Platform); Akademiya Nauk SSSR: Moscow, Russia, 1960; pp. 1–344. [Google Scholar]
- Luo, C.; Yang, A.; Zhuravlev, A.Y.; Reitner, J. Vauxiids as descendants of archaeocyaths: A hypothesis. Lethaia 2021, 54, 700–710. [Google Scholar] [CrossRef]
- Cruz-Barraza, J.A.; Carballo, J.L.; Rocha-Olivares, A.; Ehrlich, H.; Hog, M. Integrative taxonomy and molecular phylogeny of genus Aplysina (Demospongiae: Verongida) from Mexican Pacific. PLoS ONE 2012, 7, e42049. [Google Scholar] [CrossRef]
- Bergquist, P.R. A revision of the supraspecific classification of the orders Dictyoceratida, Dendroceratida, and Verongida (class Demospongiae). N. Z. J. Zool. 1980, 7, 443–503. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.; Pei, Y.; Richoz, S.; Li, Q.; Reitner, J. Identification and Current Palaeobiological Understanding of “Keratosa”-Type Nonspicular Demosponge Fossils in Carbonates: With a New Example from the Lowermost Triassic, Armenia. Life 2022, 12, 1348. https://doi.org/10.3390/life12091348
Luo C, Pei Y, Richoz S, Li Q, Reitner J. Identification and Current Palaeobiological Understanding of “Keratosa”-Type Nonspicular Demosponge Fossils in Carbonates: With a New Example from the Lowermost Triassic, Armenia. Life. 2022; 12(9):1348. https://doi.org/10.3390/life12091348
Chicago/Turabian StyleLuo, Cui, Yu Pei, Sylvain Richoz, Qijian Li, and Joachim Reitner. 2022. "Identification and Current Palaeobiological Understanding of “Keratosa”-Type Nonspicular Demosponge Fossils in Carbonates: With a New Example from the Lowermost Triassic, Armenia" Life 12, no. 9: 1348. https://doi.org/10.3390/life12091348





