Can Dynamic Whole-Body FDG PET Imaging Differentiate between Malignant and Inflammatory Lesions?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
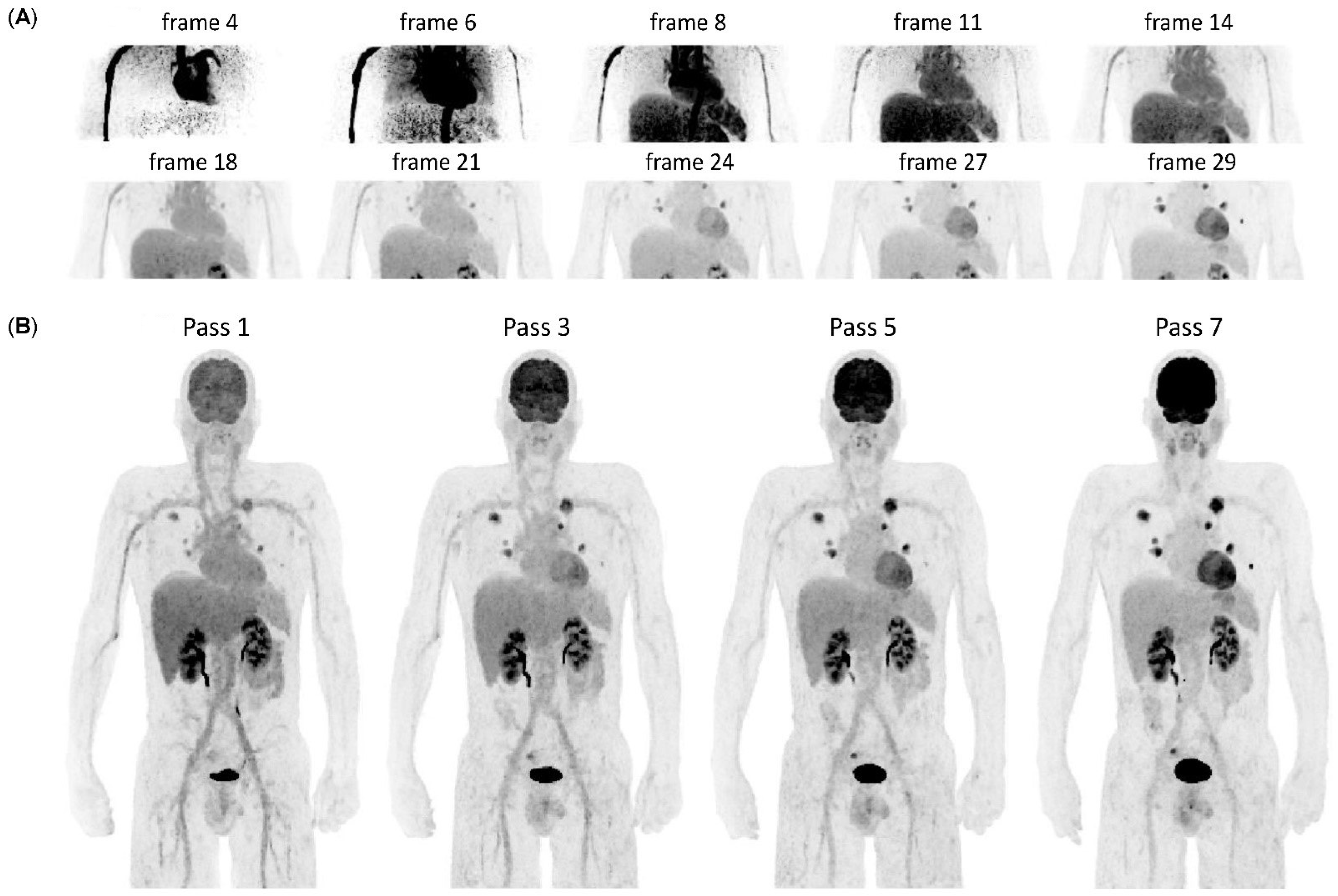
2.2. WBD PET Acquisition and Image Reconstruction
2.3. Generation of Patlak Parametric Images
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Description of Lesions Assessed in the Study Cohort
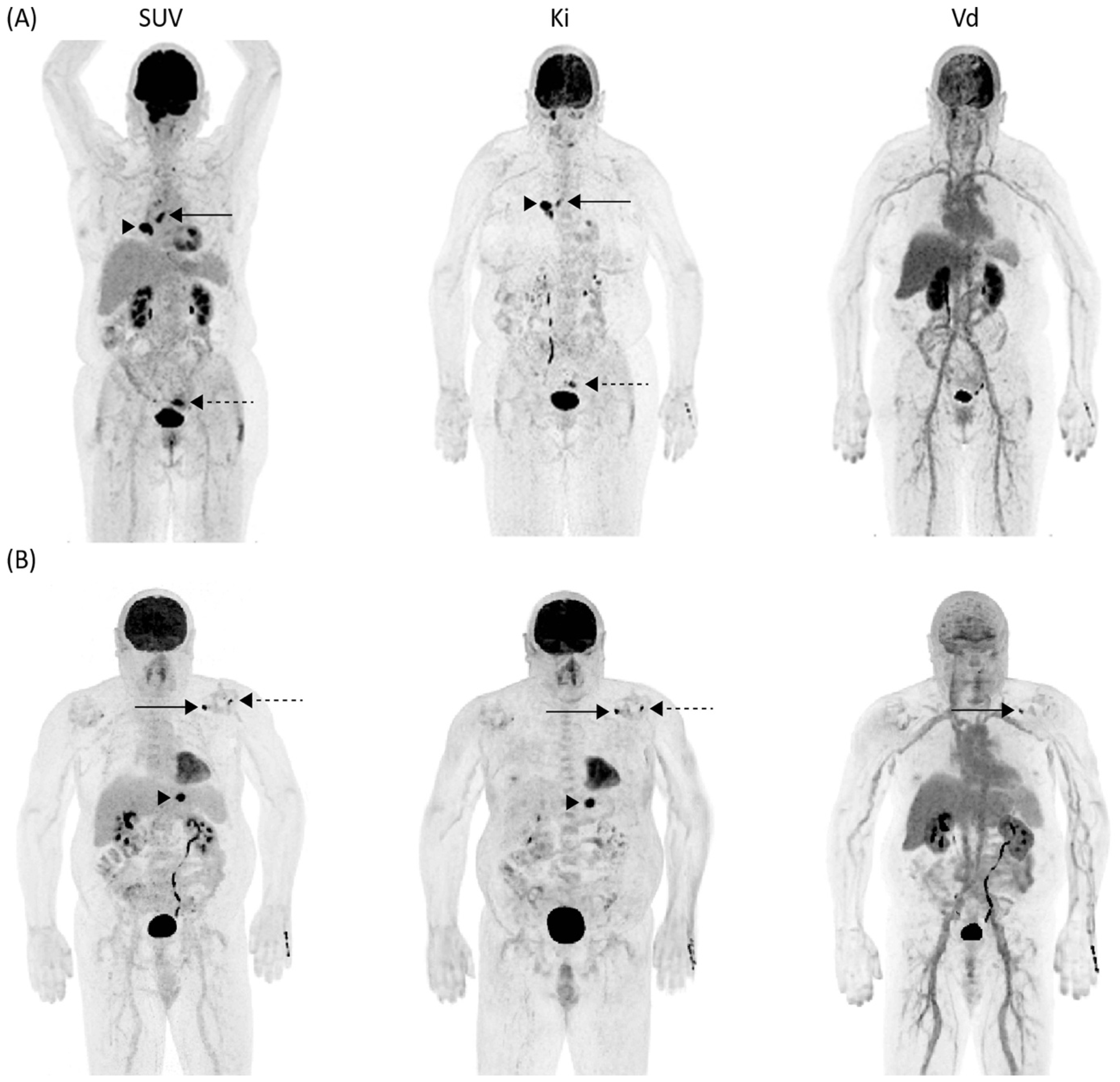
3.3. Quantitative Results of SUV, Ki, and Vd
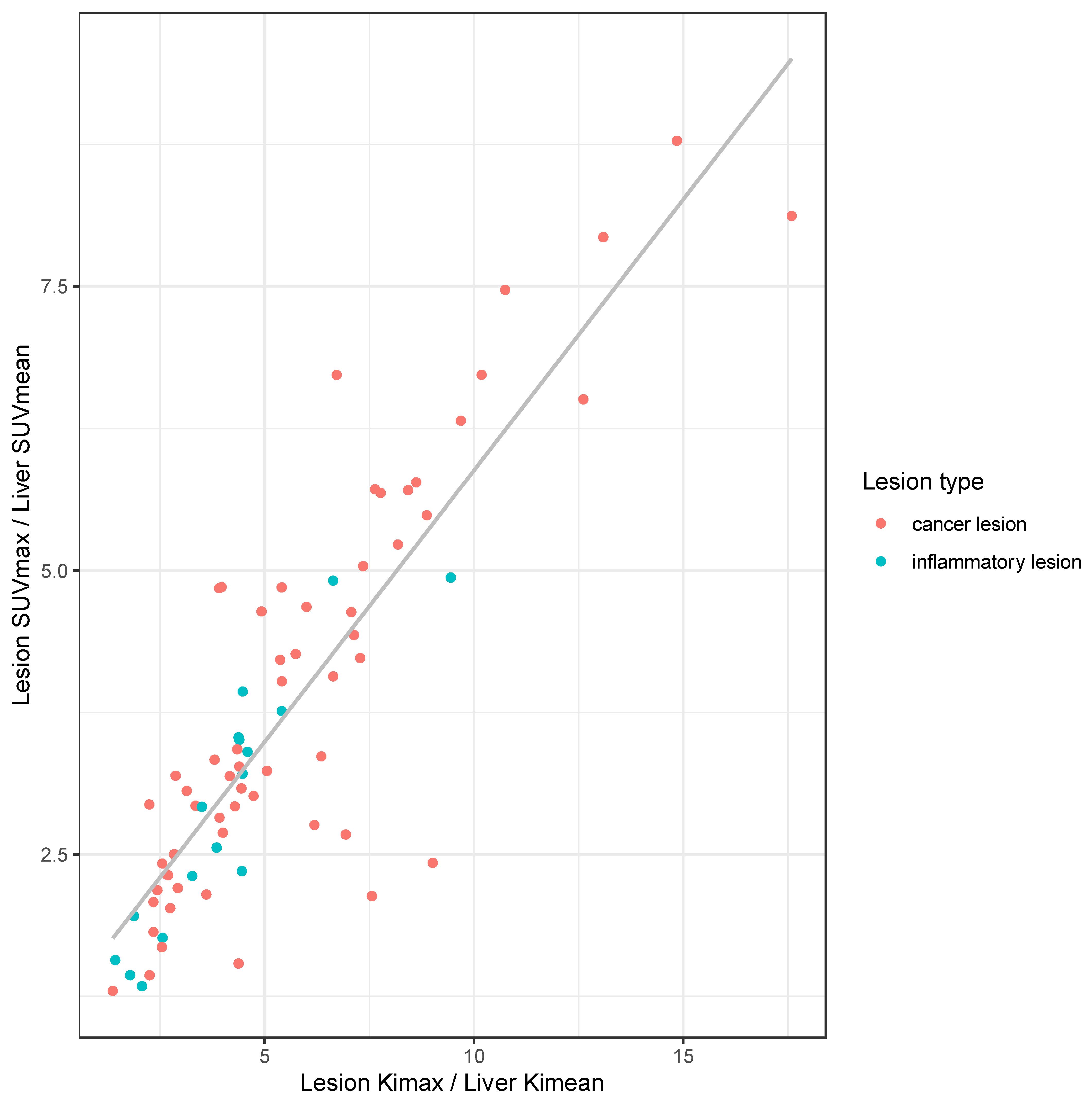
3.4. Differentiation of Malignant vs. Inflammatory Lesion in Clinical and Dynamic Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juweid, M.E.; Cheson, B.D. Positron-emission tomography and assessment of cancer therapy. N. Engl. J. Med. 2006, 354, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.A.; Wieder, H. Monitoring chemotherapy and radiotherapy of solid tumors. Eur. J. Nucl. Med. Mol. Imaging 2006, 33 (Suppl. S1), 27–37. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.A. Positron emission tomography as an imaging biomarker. J. Clin. Oncol. 2006, 24, 3282–3292. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Kim, C.K.; Gupta, N.C. Dependency of standardized uptake values of fluorine-18 fluorodeoxyglucose on body size: Comparison of body surface area correction and lean body mass correction. Nucl. Med. Commun. 1996, 17, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.C.; Turkington, T.G.; Wilson, J.M.; Wong, T.Z. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am. J. Roentgenol. 2010, 195, 310–320. [Google Scholar] [CrossRef]
- Keramida, G.; Anagnostopoulos, C.D.; Peters, A.M. The extent to which standardized uptake values reflect FDG phosphorylation in the liver and spleen as functions of time after injection of (18)F-fluorodeoxyglucose. EJNMMI Res. 2017, 7, 13. [Google Scholar] [CrossRef]
- Doot, R.K.; Dunnwald, L.K.; Schubert, E.K.; Muzi, M.; Peterson, L.M.; Kinahan, P.E.; Kurland, B.F.; Mankoff, D.A. Dynamic and static approaches to quantifying 18F-FDG uptake for measuring cancer response to therapy, including the effect of granulocyte CSF. J. Nucl. Med. 2007, 48, 920–925. [Google Scholar] [CrossRef]
- Keramida, G.; Dizdarevic, S.; Bush, J.; Peters, A.M. Quantification of tumour (18) F-FDG uptake: Normalise to blood glucose or scale to liver uptake? Eur. Radiol. 2015, 25, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoff, J.; Oehme, L.; Schramm, G.; Maus, J.; Lougovski, A.; Petr, J.; Beuthien-Baumann, B.; Hofheinz, F. The PET-derived tumor-to-blood standard uptake ratio (SUR) is superior to tumor SUV as a surrogate parameter of the metabolic rate of FDG. EJNMMI Res. 2013, 3, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikulin, P.; Hofheinz, F.; Maus, J.; Li, Y.; Butof, R.; Lange, C.; Furth, C.; Zschaeck, S.; Kreissl, M.C.; Kotzerke, J.; et al. A convolutional neural network for fully automated blood SUV determination to facilitate SUR computation in oncological FDG-PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Butof, R.; Hofheinz, F.; Zophel, K.; Schmollack, J.; Jentsch, C.; Zschaeck, S.; Kotzerke, J.; van den Hoff, J.; Baumann, M. Prognostic value of SUR in patients with trimodality treatment of locally advanced esophageal carcinoma. J. Nucl. Med. 2019, 60, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Hofheinz, F.; Apostolova, I.; Oehme, L.; Kotzerke, J.; van den Hoff, J. Test-Retest Variability in Lesion SUV and Lesion SUR in (18)F-FDG PET: An Analysis of Data from Two Prospective Multicenter Trials. J. Nucl. Med. 2017, 58, 1770–1775. [Google Scholar] [CrossRef]
- Rahmim, A.; Lodge, M.A.; Karakatsanis, N.A.; Panin, V.Y.; Zhou, Y.; McMillan, A.; Cho, S.; Zaidi, H.; Casey, M.E.; Wahl, R.L. Dynamic whole-body PET imaging: Principles, potentials and applications. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Lammertsma, A.A.; Hoekstra, C.J.; Giaccone, G.; Hoekstra, O.S. How should we analyse FDG PET studies for monitoring tumour response? Eur. J. Nucl. Med. Mol. Imaging 2006, 33 (Suppl. S1), 16–21. [Google Scholar] [CrossRef]
- Freedman, N.M.; Sundaram, S.K.; Kurdziel, K.; Carrasquillo, J.A.; Whatley, M.; Carson, J.M.; Sellers, D.; Libutti, S.K.; Yang, J.C.; Bacharach, S.L. Comparison of SUV and Patlak slope for monitoring of cancer therapy using serial PET scans. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 46–53. [Google Scholar] [CrossRef]
- Nakajo, M.; Ojima, S.; Kawakami, H.; Tani, A.; Hirayama, A.; Jinguji, M.; Kubozono, T.; Ohishi, M.; Yoshiura, T. Value of Patlak Ki images from (18)F-FDG-PET/CT for evaluation of the relationships between disease activity and clinical events in cardiac sarcoidosis. Sci. Rep. 2021, 11, 2729. [Google Scholar] [CrossRef]
- Mankoff, D.A.; Muzi, M.; Krohn, K.A. Quantitative positron emission tomography imaging to measure tumor response to therapy: What is the best method? Mol. Imaging Biol. 2003, 5, 281–285. [Google Scholar] [CrossRef]
- Karakatsanis, N.A.; Lodge, M.A.; Tahari, A.K.; Zhou, Y.; Wahl, R.L.; Rahmim, A. Dynamic whole-body PET parametric imaging: I. Concept, acquisition protocol optimization and clinical application. Phys. Med. Biol. 2013, 58, 7391–7418. [Google Scholar] [CrossRef]
- Patlak, C.S.; Blasberg, R.G.; Fenstermacher, J.D. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J. Cereb. Blood Flow Metab. 1983, 3, 1–7. [Google Scholar] [CrossRef]
- Burger, I.A.; Burger, C.; Berthold, T.; Buck, A. Simplified quantification of FDG metabolism in tumors using the autoradiographic method is less dependent on the acquisition time than SUV. Nucl. Med. Biol. 2011, 38, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, M.; Gallezot, J.D.; Shah, V.; Mulnix, T.; Young, C.; Dias, M.; Chen, M.K.; Smith, A.M.; Carson, R.E. Assessment of population-based input functions for Patlak imaging of whole body dynamic (18)F-FDG PET. EJNMMI Phys. 2020, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Van Sluis, J.; Yaqub, M.; Brouwers, A.H.; Dierckx, R.; Noordzij, W.; Boellaard, R. Use of population input functions for reduced scan duration whole-body Patlak (18)F-FDG PET imaging. EJNMMI Phys. 2021, 8, 11. [Google Scholar] [CrossRef]
- Dias, A.H.; Pedersen, M.F.; Danielsen, H.; Munk, O.L.; Gormsen, L.C. Clinical feasibility and impact of fully automated multiparametric PET imaging using direct Patlak reconstruction: Evaluation of 103 dynamic whole-body (18)F-FDG PET/CT scans. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 837–850. [Google Scholar] [CrossRef]
- Karakatsanis, N.A.; Casey, M.E.; Lodge, M.A.; Rahmim, A.; Zaidi, H. Whole-body direct 4D parametric PET imaging employing nested generalized Patlak expectation-maximization reconstruction. Phys. Med. Biol. 2016, 61, 5456–5485. [Google Scholar] [CrossRef]
- Karakatsanis, N.A.; Zhou, Y.; Lodge, M.A.; Casey, M.E.; Wahl, R.L.; Zaidi, H.; Rahmim, A. Generalized whole-body Patlak parametric imaging for enhanced quantification in clinical PET. Phys. Med. Biol. 2015, 60, 8643–8673. [Google Scholar] [CrossRef]
- Zaker, N.; Kotasidis, F.; Garibotto, V.; Zaidi, H. Assessment of Lesion Detectability in Dynamic Whole-Body PET Imaging Using Compartmental and Patlak Parametric Mapping. Clin. Nucl. Med. 2020, 45, e221–e231. [Google Scholar] [CrossRef]
- Fahrni, G.; Karakatsanis, N.A.; Di Domenicantonio, G.; Garibotto, V.; Zaidi, H. Does whole-body Patlak (18)F-FDG PET imaging improve lesion detectability in clinical oncology? Eur. Radiol. 2019, 29, 4812–4821. [Google Scholar] [CrossRef]
- Marin, A.; Murchison, J.T.; Skwarski, K.M.; Tavares, A.A.S.; Fletcher, A.; Wallace, W.A.; Salapura, V.; van Beek, E.J.R.; Mirsadraee, S. Can dynamic imaging, using (18)F-FDG PET/CT and CT perfusion differentiate between benign and malignant pulmonary nodules? Radiol. Oncol. 2021, 55, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Messerli, M.; Stolzmann, P.; Egger-Sigg, M.; Trinckauf, J.; D’Aguanno, S.; Burger, I.A.; von Schulthess, G.K.; Kaufmann, P.A.; Huellner, M.W. Impact of a Bayesian penalized likelihood reconstruction algorithm on image quality in novel digital PET/CT: Clinical implications for the assessment of lung tumors. EJNMMI Phys. 2018, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Aiello, M.; Alfano, V.; Salvatore, E.; Cavaliere, C.; Picardi, M.; Della Pepa, R.; Nicolai, E.; Soricelli, A.; Vella, A.; Salvatore, M.; et al. [(18)F]FDG uptake of the normal spinal cord in PET/MR imaging: Comparison with PET/CT imaging. EJNMMI Res. 2020, 10, 91. [Google Scholar] [CrossRef]
- Viglianti, B.L.; Wale, D.J.; Wong, K.K.; Johnson, T.D.; Ky, C.; Frey, K.A.; Gross, M.D. Effects of Tumor Burden on Reference Tissue Standardized Uptake for PET Imaging: Modification of PERCIST Criteria. Radiology 2018, 287, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Messerli, M.; Kotasidis, F.; Ferraro, D.A.; Kudura, K.; Treyer, V.; Trinckauf, J.; Weyermann, C.; Hullner, M.; Kaufmann, P.; Burger, I.A. Whole-body parametric [(18)F]-FDG PET/CT improves interpretation of a distant lesion as venous embolus in a lung cancer patient. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2047–2048. [Google Scholar] [CrossRef]
- Yang, Z.; Zan, Y.; Zheng, X.; Hai, W.; Chen, K.; Huang, Q.; Xu, Y.; Peng, J. Dynamic FDG-PET Imaging to Differentiate Malignancies from Inflammation in Subcutaneous and In Situ Mouse Model for Non-Small Cell Lung Carcinoma (NSCLC). PLoS ONE 2015, 10, e0139089. [Google Scholar] [CrossRef]
- Ilan, E.; Velikyan, I.; Sandstrom, M.; Sundin, A.; Lubberink, M. Tumor-to-Blood Ratio for Assessment of Somatostatin Receptor Density in Neuroendocrine Tumors Using (68)Ga-DOTATOC and (68)Ga-DOTATATE. J. Nucl. Med. 2020, 61, 217–221. [Google Scholar] [CrossRef]
- Velikyan, I.; Sundin, A.; Sorensen, J.; Lubberink, M.; Sandstrom, M.; Garske-Roman, U.; Lundqvist, H.; Granberg, D.; Eriksson, B. Quantitative and qualitative intrapatient comparison of 68Ga-DOTATOC and 68Ga-DOTATATE: Net uptake rate for accurate quantification. J. Nucl. Med. 2014, 55, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Azad, G.K.; Siddique, M.; Taylor, B.; Green, A.; O’Doherty, J.; Gariani, J.; Blake, G.M.; Mansi, J.; Goh, V.; Cook, G.J.R. Is Response Assessment of Breast Cancer Bone Metastases Better with Measurement of (18)F-Fluoride Metabolic Flux Than with Measurement of (18)F-Fluoride PET/CT SUV? J. Nucl. Med. 2019, 60, 322–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Female/male, n (%) | 8 (33%)/16 (66%) |
| Age, years | 66 ± 16 (47–80) |
| Body weight, kg | 82 ± 25 (55–146) |
| Body height, m | 1.71 ± 0.1 (1.57–1.86) |
| BMI, kg/m2 | 27.6 ± 6.0 (18.8–42.2) |
| Blood glucose level at time of injection, mg/dL | 107 ± 17 (81–155) |
| Injected tracer activity, MBq | 210 ± 75 (107–303) |
| Indication for PET | |
| Lung cancer | 11 (46%) |
| Breast cancer | 3 (13%) |
| Esophageal cancer | 2 (8%) |
| Urogenital cancer | 2 (8%) |
| Head and neck cancer | 1 (4%) |
| Neuroendocrine carcinoma | 1 (4%) |
| Cholangiocarcinoma | 1 (4%) |
| Mesothelioma | 1 (4%) |
| Malignant melanoma | 1 (4%) |
| Lymphoma | 1 (4%) |
| Lesion Etiology | Lesion Type | Organ | n |
|---|---|---|---|
| Inflammation | 17 (22%) | ||
| Infectious | Pleura | 2 (3%) | |
| Lung | 1 (1%) | ||
| Inflammatory | Gastrointestinal tract | 5 (6%) | |
| Lymph node | 3 (4%) | ||
| Thyroid | 2 (3%) | ||
| Joint | 4 (5%) | ||
| Cancer | 60 (78%) | ||
| Primary | Lung | 8 (10%) | |
| Breast | 2 (3%) | ||
| Gastrointestinal tract | 1 (1%) | ||
| Tonsil | 1 (1%) | ||
| Metastasis | Lymph node | 16 (21%) | |
| Bone | 10 (13%) | ||
| Lung | 8 (10%) | ||
| Pleura | 7 (9%) | ||
| Liver | 3 (4%) | ||
| Soft tissue | 3 (4%) | ||
| Gastrointestinal tract | 1 (1%) |
| SUVmax | Kimax [×10−2] | Vdmax | |
|---|---|---|---|
| Blood | 2.4 (2.0–4) | 0.7 (0.4–1.5)) | 1.0 (0.5–1.5) |
| Bone | 2.5 (1.8–4.7) | 1.1 (0.2–2.5) | 0.4 (0.2–1.4) |
| Brain | 8.5 (5.5–13.1) | 3.1 (0.5–4.4) | 1.0 (0.6–1.4) |
| Cancer | 7.8 (3.2–19.1) | 3.0 (0.7–11.4) | 0.9 (0.3–2.2) |
| Subcutaneous fat | 0.5 (0.1–0.8) | 0.2 (0.1–0.9) | 0.2 (0.1–0.6) |
| Inflammation | 6.9 (2.6–13.4) | 2.0 (0.8–4.2) | 0.7 (0.3–1.3) |
| Liver | 3.1 (2.4–4.6) | 1.0 (0.6–1.8) | 1.1 (0.7–1.5) |
| Lung | 0.8 (0.4–1.6) | 0.3 (0.1–0.7) | 0.3 (0.1–0.3) |
| Muscle | 1.2 (0.6–1.8) | 0.5 (0.1–1.3) | 0.2 (0.1–0.4) |
| Spleen | 2.9 (0.1–19.1) | 0.8 (0.5–2.2) | 0.3 (0.2–0.5) |
| Cancer Lesions (n = 60) | Inflammatory/Infectious Lesions (n = 17) | p-Value | |
|---|---|---|---|
| SUVmax [g/mL] | 7.8 (IQR 5.9) | 6.9 (IQR 2.9) | 0.11 |
| LBR (SUVmax) | 3.3 (IQR 2.4) | 2.9 (IQR 1.6) | 0.06 |
| Kimax [10−2 mL/min/mL] | 3.0 (IQR 2.2) | 2.0 (IQR 1.1) | 0.002 * |
| LBR (Kimax) | 5.0 (IQR 4.1) | 4.4 (IQR 1.9) | 0.05 |
| Vdmax [mL/mL] | 0.9 (IQR 0.5) | 0.7 (IQR 0.4) | 0.13 |
| LBR (Vdmax) | 1.1 (IQR 0.5) | 1.0 (IQR 0.4) | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skawran, S.; Messerli, M.; Kotasidis, F.; Trinckauf, J.; Weyermann, C.; Kudura, K.; Ferraro, D.A.; Pitteloud, J.; Treyer, V.; Maurer, A.; et al. Can Dynamic Whole-Body FDG PET Imaging Differentiate between Malignant and Inflammatory Lesions? Life 2022, 12, 1350. https://doi.org/10.3390/life12091350
Skawran S, Messerli M, Kotasidis F, Trinckauf J, Weyermann C, Kudura K, Ferraro DA, Pitteloud J, Treyer V, Maurer A, et al. Can Dynamic Whole-Body FDG PET Imaging Differentiate between Malignant and Inflammatory Lesions? Life. 2022; 12(9):1350. https://doi.org/10.3390/life12091350
Chicago/Turabian StyleSkawran, Stephan, Michael Messerli, Fotis Kotasidis, Josephine Trinckauf, Corina Weyermann, Ken Kudura, Daniela A. Ferraro, Janique Pitteloud, Valerie Treyer, Alexander Maurer, and et al. 2022. "Can Dynamic Whole-Body FDG PET Imaging Differentiate between Malignant and Inflammatory Lesions?" Life 12, no. 9: 1350. https://doi.org/10.3390/life12091350
APA StyleSkawran, S., Messerli, M., Kotasidis, F., Trinckauf, J., Weyermann, C., Kudura, K., Ferraro, D. A., Pitteloud, J., Treyer, V., Maurer, A., Huellner, M. W., & Burger, I. A. (2022). Can Dynamic Whole-Body FDG PET Imaging Differentiate between Malignant and Inflammatory Lesions? Life, 12(9), 1350. https://doi.org/10.3390/life12091350





