Using High-Definition Transcranial Alternating Current Stimulation to Treat Patients with Fibromyalgia: A Randomized Double-Blinded Controlled Study
Abstract
:1. Introduction
2. Methods
2.1. Participants
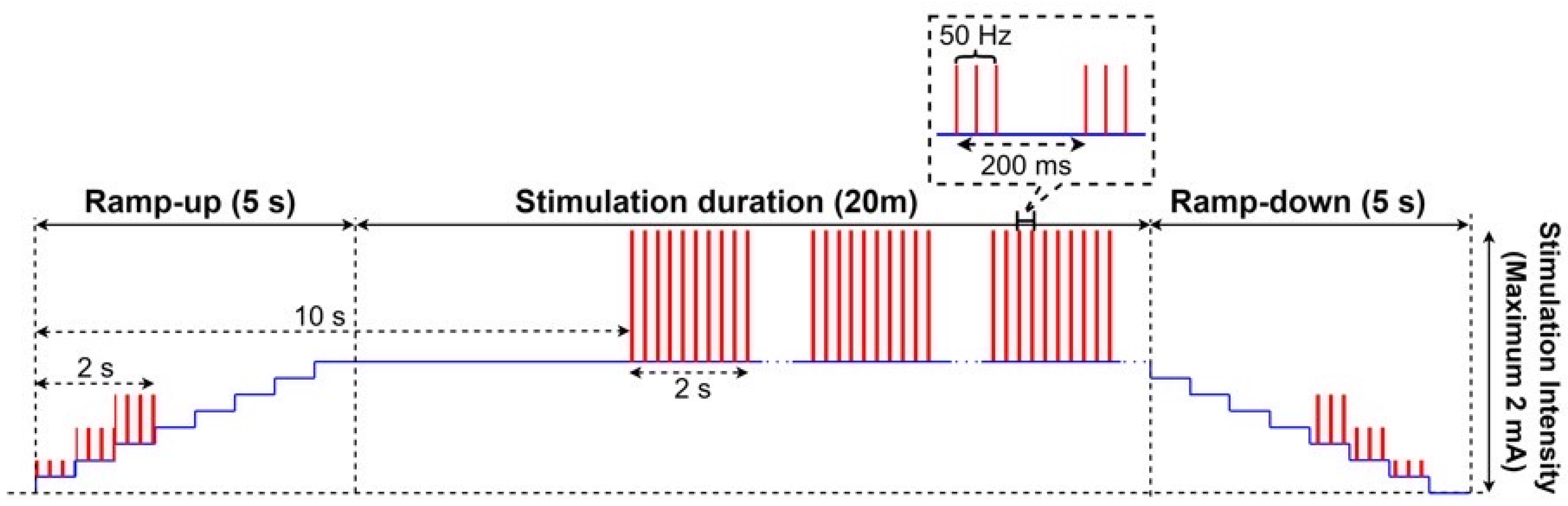
2.2. High-Definition Transcranial Alternating Current (HD-tACS) and Sham Stimulation
2.3. Assessments
2.4. NRS for Pain Assessment
2.5. FIQ for Quality of Life
2.6. BAI
2.7. BDI-II
2.8. PPTs
2.9. Plasma Biomarkers: T-Tau and Beta-Amyloid 1–42
2.10. Adverse Effects
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Sicras-Mainar, A.; Rejas, J.; Navarro, R.; Blanca, M.; Morcillo, A.; Larios, R.; Velasco, S.; Villarroya, C. Treating patients with fibromyalgia in primary care settings under routine medical practice: A claim database cost and burden of illness study. Arthritis Res. Ther. 2009, 11, R54. [Google Scholar] [CrossRef]
- Häuser, W.; Urrútia, G.; Tort, S.; Üçeyler, N.; Walitt, B. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst. Rev. 2018, 2, CD010292. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Derry, S.; Wiffen, P.J.; McQuay, H.J. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2012, 12, Cd008242. [Google Scholar]
- Häuser, W.; Urrútia, G.; Tort, S.; Üçeyler, N.; Walitt, B. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database Syst. Rev. 2013, CD010292. [Google Scholar] [CrossRef] [PubMed]
- Üçeyler, N.; Sommer, C.; Walitt, B.; Häuser, W. Anticonvulsants for fibromyalgia. Cochrane Database Syst. Rev. 2013, Cd010782. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Viganò, A.; Toscano, M.; Puledda, F.; Di Piero, V. Treating Chronic Migraine with Neuromodulation: The Role of Neurophysiological Abnormalities and Maladaptive Plasticity. Front. Pharmacol. 2019, 10, 32. [Google Scholar] [CrossRef]
- Conforto, A.B.; Amaro, E., Jr.; Gonçalves, A.L.; Mercante, J.P.; Guendler, V.Z.; Ferreira, J.R.; Kirschner, C.C.; Peres, M.F. Randomized, proof-of-principle clinical trial of active transcranial magnetic stimulation in chronic migraine. Cephalalgia 2014, 34, 464–472. [Google Scholar] [CrossRef]
- O’Connell, N.E.; Marston, L.; Spencer, S.; DeSouza, L.H.; Wand, B.M. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst. Rev. 2018, 4, Cd008208. [Google Scholar]
- Fagerlund, A.J.; Hansen, O.A.; Aslaksen, P.M. Transcranial direct current stimulation as a treatment for patients with fibromyalgia: A randomized controlled trial. Pain 2015, 156, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Gimenes, R.; Valle, A.C.; Ferreira, M.J.; Rocha, R.R.; Natalle, L.; Bravo, R.; Rigonatti, S.P.; Freedman, S.D.; Nitsche, M.A.; et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006, 54, 3988–3998. [Google Scholar] [CrossRef]
- Villamar, M.F.; Wivatvongvana, P.; Patumanond, J.; Bikson, M.; Truong, D.Q.; Datta, A.; Fregni, F. Focal modulation of the primary motor cortex in fibromyalgia using 4 × 1-ring high-definition transcranial direct current stimulation (HD-tDCS): Immediate and delayed analgesic effects of cathodal and anodal stimulation. J. Pain 2013, 14, 371–383. [Google Scholar] [CrossRef]
- Valle, A.; Roizenblatt, S.; Botte, S.; Zaghi, S.; Riberto, M.; Tufik, S.; Boggio, P.S.; Fregni, F. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: Results of a randomized, sham-controlled longitudinal clinical trial. J. Pain Manag. 2009, 2, 353–361. [Google Scholar] [PubMed]
- Borckardt, J.J.; Bikson, M.; Frohman, H.; Reeves, S.T.; Datta, A.; Bansal, V.; Madan, A.; Barth, K.; George, M.S. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J. Pain 2012, 13, 112–120. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Ayache, S.; Sorel, M.; Farhat, W.; Zouari, H.; de Andrade, D.C.; Ahdab, R.; Ménard-Lefaucheur, I.; Brugières, P.; Goujon, C. Analgesic effects of repetitive transcranial magnetic stimulation of the motor cortex in neuropathic pain: Influence of theta burst stimulation priming. Eur. J. Pain 2012, 16, 1403–1413. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta burst stimulation of the human motor cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef]
- Guerra, A.; Suppa, A.; Bologna, M.; D’Onofrio, V.; Bianchini, E.; Brown, P.; Di Lazzaro, V.; Berardelli, A. Boosting the LTP-like plasticity effect of intermittent theta-burst stimulation using gamma transcranial alternating current stimulation. Brain Stimul. 2018, 11, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Paulus, W. Transcranial alternating current stimulation (tACS). Front. Hum. Neurosci. 2013, 7, 317. [Google Scholar] [CrossRef]
- Villamar, M.F.; Volz, M.S.; Bikson, M.; Datta, A.; DaSilva, A.F.; Fregni, F. Technique and considerations in the use of 4x1 ring high-definition transcranial direct current stimulation (HD-tDCS). J. Vis. Exp. 2013, 77, e50309. [Google Scholar] [CrossRef]
- Castillo-Saavedra, L.; Gebodh, N.; Bikson, M.; Diaz-Cruz, C.; Brandao, R.; Coutinho, L.; Truong, D.; Datta, A.; Shani-Hershkovich, R.; Weiss, M.; et al. Clinically Effective Treatment of Fibromyalgia Pain With High-Definition Transcranial Direct Current Stimulation: Phase II Open-Label Dose Optimization. J. Pain 2016, 17, 14–26. [Google Scholar] [CrossRef]
- Bernardi, L.; Bertuccelli, M.; Formaggio, E.; Rubega, M.; Bosco, G.; Tenconi, E.; Cattelan, M.; Masiero, S.; Del Felice, A. Beyond physiotherapy and pharmacological treatment for fibromyalgia syndrome: Tailored tACS as a new therapeutic tool. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 199–210. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.R.; Mander, B.A.; Helfrich, R.F.; Maass, A.; Harrison, T.M.; Baker, S.L.; Knight, R.T.; Jagust, W.J.; Walker, M.P. Sleep as a Potential Biomarker of Tau and β-Amyloid Burden in the Human Brain. J. Neurosci. 2019, 39, 6315–6324. [Google Scholar] [CrossRef] [PubMed]
- Nguy, B.-H.T.; Liu, W.-T.; Chang, Y.-T.; Lin, C.-P.; Kang, J.-H. Elevated tau and β-amyloid in the serum of fibromyalgia patients. CNS Spectr. 2020, 27, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.F.; Fernandes, A.M.B.; Sá, K.; Okano, A.H.; Brunoni, A.R.; Lara-Solares, A.; Iskandar, A.J.; Guerrero, C.; Amescua-García, C.; Kraychete, D.C.; et al. Latin American and Caribbean consensus on noninvasive central nervous system neuromodulation for chronic pain management (LAC(2)-NIN-CP). Pain Rep. 2019, 4, e692. [Google Scholar] [CrossRef] [PubMed]
- Desmeules, J.A.; Cedraschi, C.; Rapiti, E.; Baumgartner, E.; Finckh, A.; Cohen, P.; Dayer, P.; Vischer, T.L. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003, 48, 1420–1429. [Google Scholar] [CrossRef]
- Polanía, R.; Paulus, W.; Nitsche, M.A. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum. Brain Mapp. 2012, 33, 2499–2508. [Google Scholar] [CrossRef]
- Lloyd, D.M.; Wittkopf, P.G.; Arendsen, L.J.; Jones, A.K. Is Transcranial Direct Current Stimulation (tDCS) Effective for the Treatment of Pain in Fibromyalgia? A Systematic Review and Meta-Analysis. J. Pain 2020, 21, 1085–1100. [Google Scholar] [CrossRef]
- Khedr, E.M.; Omran, E.A.; Ismail, N.M.; El-Hammady, D.H.; Goma, S.H.; Kotb, H.; Galal, H.; Osman, A.M.; Farghaly, H.S.; Karim, A.A.; et al. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: A double blinded, randomized clinical trial. Brain Stimul. 2017, 10, 893–901. [Google Scholar] [CrossRef]
- Roizenblatt, S.; Fregni, F.; Gimenez, R.; Wetzel, T.; Rigonatti, S.P.; Tufik, S.; Boggio, P.; Valle, A.C. Site-specific effects of transcranial direct current stimulation on sleep and pain in fibromyalgia: A randomized, sham-controlled study. Pain Pract. 2007, 7, 297–306. [Google Scholar] [CrossRef]
- Mendonca, M.E.; Simis, M.; Grecco, L.C.; Battistella, L.R.; Baptista, A.F.; Fregni, F. Transcranial Direct Current Stimulation Combined with Aerobic Exercise to Optimize Analgesic Responses in Fibromyalgia: A Randomized Placebo-Controlled Clinical Trial. Front. Hum. Neurosci. 2016, 10, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samartin-Veiga, N.; Pidal-Miranda, M.; González-Villar, A.J.; Bradley, C.; Garcia-Larrea, L.; O’Brien, A.T.; Carrillo-de-la-Peña, M.T. Transcranial direct current stimulation of three cortical targets is no more effective than placebo as treatment for fibromyalgia: A double-blind sham-controlled clinical trial. Pain 2021, 163, e850–e861. [Google Scholar] [CrossRef]
- Kold, S.; Graven-Nielsen, T. Effect of anodal high-definition transcranial direct current stimulation on the pain sensitivity in a healthy population: A double-blind, sham-controlled study. Pain 2021, 162, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Kim, J.S.; Kim, D.J.; Chung, C.K. Increased Low- and High-Frequency Oscillatory Activity in the Prefrontal Cortex of Fibromyalgia Patients. Front. Hum. Neurosci. 2016, 10, 111. [Google Scholar] [CrossRef]
- Ahn, S.; Prim, J.H.; Alexander, M.L.; McCulloch, K.L.; Fröhlich, F. Identifying and Engaging Neuronal Oscillations by Transcranial Alternating Current Stimulation in Patients With Chronic Low Back Pain: A Randomized, Crossover, Double-Blind, Sham-Controlled Pilot Study. J. Pain 2019, 20, 277.e1–277.e11. [Google Scholar] [CrossRef]
- May, E.S.; Hohn, V.D.; Nickel, M.; Tiemann, L.; Gil Ávila, C.; Heitmann, H.; Sauseng, P.; Ploner, M. Modulating Brain Rhythms of Pain Using Transcranial Alternating Current Stimulation (tACS)—A Sham-Controlled Study in Healthy Human Participants. J. Pain 2021, 22, 1256–1272. [Google Scholar] [CrossRef] [PubMed]
- Arendsen, L.J.; Hugh-Jones, S.; Lloyd, D.M. Transcranial Alternating Current Stimulation at Alpha Frequency Reduces Pain When the Intensity of Pain is Uncertain. J. Pain 2018, 19, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Vöröslakos, M.; Kronberg, G.; Henin, S.; Krause, M.R.; Huang, Y.; Opitz, A.; Mehta, A.; Pack, C.C.; Krekelberg, B.; et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat. Commun. 2018, 9, 5092. [Google Scholar] [CrossRef]
- Neuling, T.; Rach, S.; Herrmann, C.S. Orchestrating neuronal networks: Sustained after-effects of transcranial alternating current stimulation depend upon brain states. Front. Hum. Neurosci. 2013, 7, 161. [Google Scholar] [CrossRef]
- Samartin-Veiga, N.; González-Villar, A.J.; Pidal-Miranda, M.; Vázquez-Millán, A.; Carrillo-De-La-Peña, M.T. Active and sham transcranial direct current stimulation (tDCS) improved quality of life in female patients with fibromyalgia. Qual. Life Res. 2022, 31, 2519–2534. [Google Scholar] [CrossRef]
- Kjær, S.W.; Rice, A.S.C.; Wartolowska, K.; Vase, L. Neuromodulation: More than a placebo effect? Pain 2020, 161, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Colloca, L. The Placebo Effect in Pain Therapies. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 191–211. [Google Scholar] [CrossRef] [PubMed]
- DosSantos, M.F.; Eferreira, N.; Toback, R.L.; Carvalho, A.C.; DaSilva, A.F. Potential Mechanisms Supporting the Value of Motor Cortex Stimulation to Treat Chronic Pain Syndromes. Front. Neurosci. 2016, 10, 18. [Google Scholar] [CrossRef]
- Vase, L.; Wartolowska, K. Pain, Placebo, and Test of Treatment Efficacy: A Narrative Review. Br. J. Anaesth. 2019, 123, e254–e262. [Google Scholar] [CrossRef] [PubMed]
- Kortteenniemi, A.; Ortega-Alonso, A.; Javadi, A.-H.; Tolmunen, T.; Ali-Sisto, T.; Kotilainen, T.; Wikgren, J.; Karhunen, L.; Velagapudi, V.; Lehto, S. Anodal tDCS Over the Left Prefrontal Cortex Does Not Cause Clinically Significant Changes in Circulating Metabolites. Front. Psychiatry 2020, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Evans, D.; Dhana, K.; Aggarwal, N.T.; Wilson, R.S.; McAninch, E.; Rajan, K.B. Longitudinal Association of Total Tau Concentrations and Physical Activity With Cognitive Decline in a Population Sample. JAMA Netw. Open 2021, 4, e2120398. [Google Scholar] [CrossRef]
- Reeves, R.R.; Ladner, M.E. Antidepressant-induced suicidality: An update. CNS Neurosci Ther. 2010, 16, 227–234. [Google Scholar] [CrossRef]
- Jick, H.; Kaye, J.A.; Jick, S.S. Antidepressants and the risk of suicidal behaviors. JAMA 2004, 292, 338–343. [Google Scholar] [CrossRef]
- Wischnewski, M.; Compen, B. Effects of theta transcranial alternating current stimulation (tACS) on exploration and exploitation during uncertain decision-making. Behav. Brain Res. 2022, 426, 113840. [Google Scholar] [CrossRef]
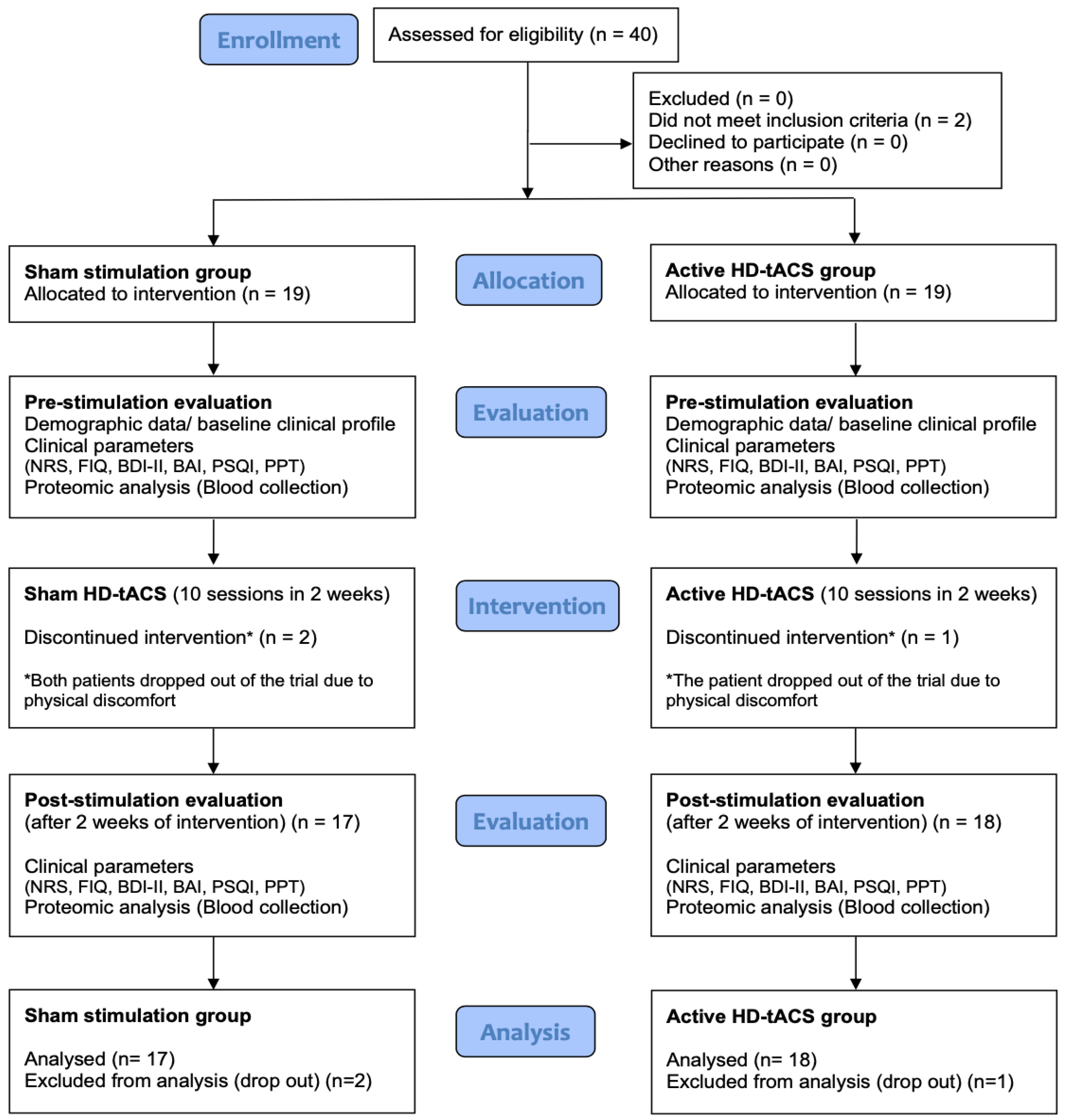
| Characteristic | Sham Stimulation (n = 19) | Active HD-tACS (n = 19) | p-Values * |
|---|---|---|---|
| Age, mean (S.D.), years | 48.9 (12.3) | 48.3 (13.6) | 0.872 |
| Gender, n (%) | 0.118 | ||
| Women | 17 (89) | 13 (68) | |
| Men | 2 (11) | 6 (32) | |
| Marital Status, n (%) | 0.401 | ||
| Single | 7 (37) | 8 (42) | |
| Married | 10 (53) | 10 (53) | |
| Divorced | 0 | 1 (5) | |
| Widowed | 2 (10) | 0 | |
| Clinical Profiles, mean (S.D.) | |||
| WPI | 10.7 (4.3) | 9.4 (3.3) | 0.250 |
| SSS | 6.8 (2.5) | 7.1 (3.2) | 0.821 |
| NRS | 5.3 (1.6) | 5.0 (2.4) | 0.636 |
| FIQ | 49.6 (16.6) | 58.0 (16.5) | 0.125 |
| BDI-II | 18.1 (11.8) | 23.3 (11.2) | 0.171 |
| BAI | 20.6 (11.4) | 20.8 (10.5) | 0.965 |
| PSQI | 11.5 (3.8) | 12.1 (4.2) | 0.662 |
| PPT, kg/cm2 | 3.8 (1.5) | 3.8 (1.6) | 0.959 |
| Outcomes | n | Mean | S.D. | Sig. (2-Tailed) |
|---|---|---|---|---|
| NRS (pre) | 17 | 5.4 | 1.6 | 0.040 * |
| NRS (post) | 17 | 4.2 | 2.4 | |
| FIQ (pre) | 17 | 49.5 | 16.5 | 0.629 |
| FIQ (post) | 17 | 46.7 | 28.5 | |
| BDI (pre) | 17 | 18.1 | 11.0 | 0.000 * |
| BDI (post) | 17 | 12.5 | 9.4 | |
| BAI (pre) | 17 | 20.6 | 11.7 | 0.021 * |
| BAI (post) | 17 | 16.4 | 11.0 | |
| PSQI (pre) | 17 | 11.8 | 3.9 | 0.025 * |
| PSQI (post) | 17 | 9.9 | 4.0 | |
| PPT (kg/cm2) (pre) | 17 | 3.9 | 1.4 | 0.047 * |
| PPT (kg/cm2) (post) | 17 | 3.5 | 1.8 | |
| T-Tau (pg/mL) (pre) | 17 | 23.6 | 3.5 | 0.090 |
| T-Tau (pg/mL) (post) | 17 | 22.3 | 3.4 | |
| ABeta1-42 (pg/mL) (pre) | 17 | 16.6 | 0.4 | 0.123 |
| ABeta1-42 (pg/mL) (post) | 17 | 16.6 | 0.4 | |
| T-Tau*ABeta1-42 (pg/mL)2 (pre) | 17 | 391.5 | 65.3 | 0.854 |
| T-Tau*ABeta1-42(pg/mL)2 (post) | 17 | 371.6 | 63.4 |
| n | Mean | S.D. | Sig. (2-Tailed) | |
|---|---|---|---|---|
| NRS (pre) | 18 | 4.9 | 2.4 | 0.376 |
| NRS (post) | 18 | 4.4 | 2.3 | |
| FIQ (pre) | 18 | 56.9 | 16.3 | 0.010 * |
| FIQ (post) | 18 | 48.7 | 13.6 | |
| BDI (pre) | 18 | 22.8 | 11.3 | 0.002 * |
| BDI (post) | 18 | 17.2 | 9.4 | |
| BAI (pre) | 18 | 20.4 | 10.7 | 0.003 * |
| BAI (post) | 18 | 15.1 | 6.4 | |
| PSQI (pre) | 18 | 11.9 | 4.3 | 0.012 * |
| PSQI (post) | 18 | 10.5 | 3.6 | |
| PPT (kg/cm2) (pre) | 18 | 3.8 | 1.6 | 0.375 |
| PPT (kg/cm2) (post) | 18 | 3.6 | 1.7 | |
| T-Tau (pg/mL) (pre) | 18 | 22.1 | 3.0 | 0.375 |
| T-Tau (pg/mL) (post) | 18 | 22.5 | 2.9 | |
| ABeta1-42 (pg/mL) (pre) | 18 | 16.5 | 0.3 | 0.968 |
| ABeta1-42 (pg/mL) (post) | 18 | 16.5 | 0.3 | |
| T-Tau*ABeta1-42 (pg/mL)2 (pre) | 18 | 365.3 | 52.6 | 0.375 |
| T-Tau*ABeta1-42 (pg/mL)2 (post) | 18 | 372.1 | 52.1 |
| Group | n | Mean | S.D. | Sig. (2-Tailed) | |
|---|---|---|---|---|---|
| NRS | Sham | 17 | −1.1 | 2.1 | 0.413 |
| Active HD-tACS | 18 | −0.5 | 2.3 | ||
| FIQ | Sham | 17 | −2.8 | 23.6 | 0.400 |
| Active HD-tACS | 18 | −8.2 | 11.9 | ||
| BDI | Sham | 17 | −5.6 | 5.0 | 0.963 |
| Active HD-tACS | 18 | −5.6 | 6.3 | ||
| BAI | Sham | 17 | −4.3 | 6.9 | 0.668 |
| Active HD-tACS | 18 | −5.3 | 6.5 | ||
| PSQI | Sham | 17 | −1.9 | 3.2 | 0.552 |
| Active HD-tACS | 18 | −1.4 | 2.1 | ||
| PPT (kg/cm2) | Sham | 17 | −0.3 | 0.7 | 0.527 |
| Active HD-tACS | 18 | −0.2 | 0.8 | ||
| T-Tau (pg/mL) | Sham | 17 | −1.2 | 2.8 | 0.068 |
| Active HD-tACS | 18 | 0.4 | 2.3 | ||
| ABeta1-42 (pg/mL) | Sham | 17 | 0.0 | 0.3 | 0.925 |
| Active HD-tACS | 18 | 0.0 | 0.4 | ||
| T-Tau*ABeta1-42 (pg/mL)2 | Sham | 17 | −30.2 | 45.3 | 0.011 * |
| Active HD-tACS | 18 | 8.5 | 39.3 |
| Adverse Reactions | Active HD-tACS (n = 18) | Sham (n = 17) |
|---|---|---|
| Headache | 1 (5.6%) | 0 (0.0%) |
| Neck pain | 0 (0.0%) | 1 (5.9%) |
| Scalp pain | 2 (11.1%) | 2 (11.8%) |
| Stinging | 3 (16.7%) | 1 (5.9%) |
| Itch | 2 (11.1%) | 2 (11.8%) |
| Burning sensation | 1 (1.0%) | 1 (5.9%) |
| Drowsiness | 2 (11.1%) | 1 (5.9%) |
| Difficulty Concentrating | 1 (1.0%) | 0 (0.0%) |
| Other | 1 (5.6%) # | 2 (11.8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, A.P.; Chiu, C.-C.; Chen, S.-C.; Huang, Y.-J.; Lai, C.-H.; Kang, J.-H. Using High-Definition Transcranial Alternating Current Stimulation to Treat Patients with Fibromyalgia: A Randomized Double-Blinded Controlled Study. Life 2022, 12, 1364. https://doi.org/10.3390/life12091364
Lin AP, Chiu C-C, Chen S-C, Huang Y-J, Lai C-H, Kang J-H. Using High-Definition Transcranial Alternating Current Stimulation to Treat Patients with Fibromyalgia: A Randomized Double-Blinded Controlled Study. Life. 2022; 12(9):1364. https://doi.org/10.3390/life12091364
Chicago/Turabian StyleLin, Ashleigh Peng, Chun-Chieh Chiu, Shih-Ching Chen, Yi-Jing Huang, Chien-Hung Lai, and Jiunn-Horng Kang. 2022. "Using High-Definition Transcranial Alternating Current Stimulation to Treat Patients with Fibromyalgia: A Randomized Double-Blinded Controlled Study" Life 12, no. 9: 1364. https://doi.org/10.3390/life12091364





