Factorial Optimization of Ultrasound-Assisted Extraction of Phycocyanin from Synechocystis salina: Towards a Biorefinery Approach
Abstract
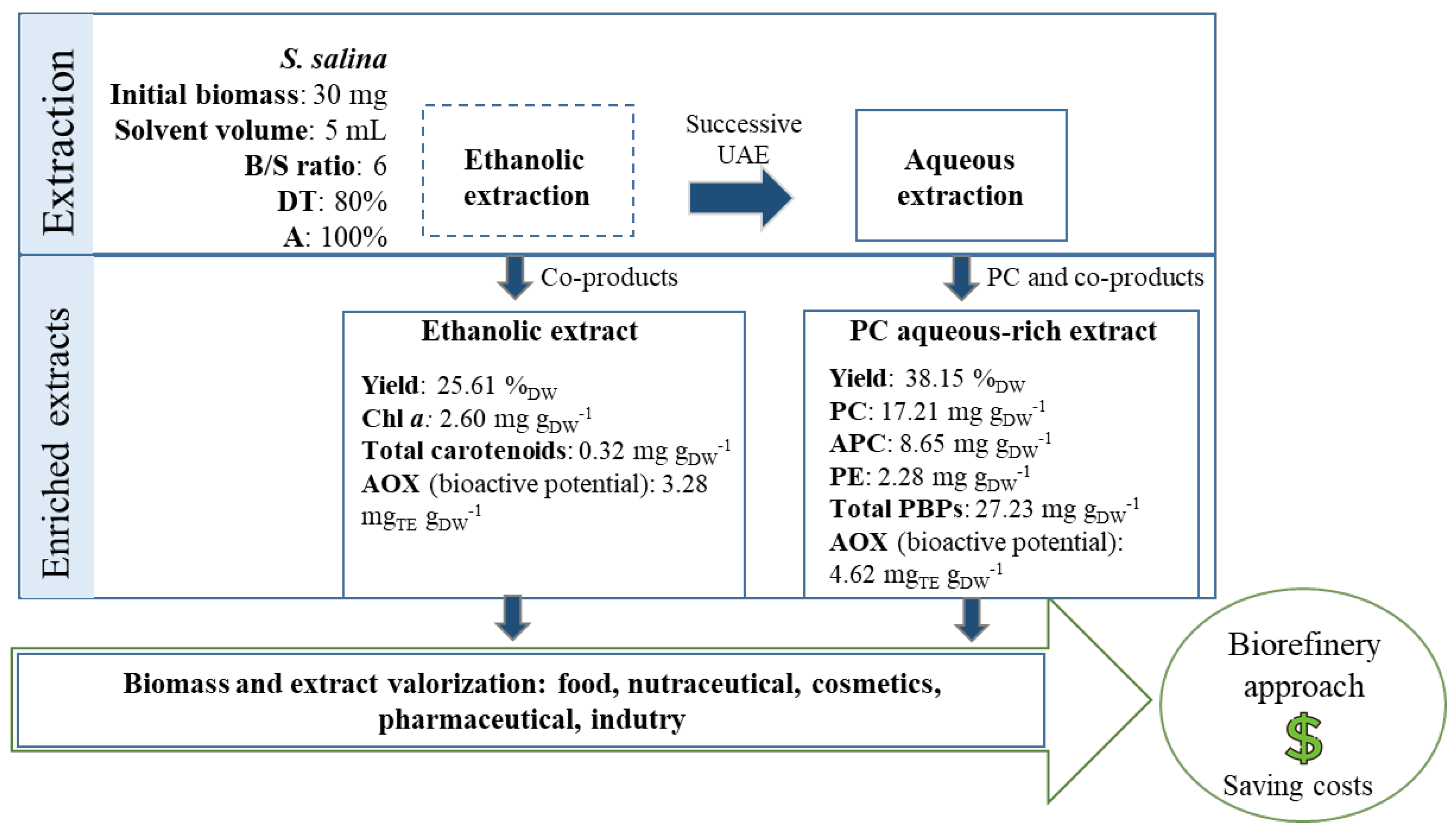
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Biomass Production
2.2. Experimental Design
2.3. Extraction Process
2.3.1. Ultrasound Processor and UAE
2.3.2. Classical Extraction of Bioactive Pigments
2.4. Analytical Methods for Extract Composition Determination
2.4.1. Determination of Chl a and Total Carotenoids
2.4.2. Determination of Phycobiliproteins
2.5. Extract Yield Determination
2.6. Determination of Antioxidant (AOX) Capacity
2.7. Statistical Analysis
3. Results
3.1. Design Experiment
3.2. Bioactive Pigment Extraction Yields
3.2.1. Chlorophyll a Extraction Yields
3.2.2. Total Carotenoids Extraction Yields
3.2.3. Phycocyanin Extraction Yields
3.2.4. Allophycocyanin Extraction Yields
3.2.5. Phycoerythrin Extraction Yields
3.2.6. Total Phycobiliprotein Extraction Yields
3.3. Extract Yields
3.3.1. Ethanolic Extracts Yield
3.3.2. Water Extracts Yield
3.4. Antioxidant Capacity (AOX)
3.4.1. Antioxidant Capacity of Ethanolic Extract Yield
3.4.2. Antioxidant Capacity of Water Extract Yield
3.5. Model Optimum Conditions
3.6. Comparison of Ultrasound-Assisted Extraction of Phycocyanin and Conventional Extraction
4. Discussion
4.1. Effect of Different Factors in Bioactive Pigments, Yields and AOX
4.2. Comparison of Ultrasound-Assisted Extraction of Phycocyanin to Conventional Extractions
4.3. Box–Behnken Design as Optimization Tool
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Assunção, J.; Amaro, H.M.; Malcata, F.X.; Guedes, A.C. Extraction of Microalgal Bioactive Compounds towards Functional Ingredients: A Biorefinery Approach, with Prospects and Challenges. In Algal Functional Foods and Nutraceuticals: Benefits, Opportunities and Challenges; Mishra, A., Ed.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022; Volume 1, pp. 120–175. [Google Scholar]
- Mandal, M.K.; Chanu, N.K.; Chaurasia, N. Cyanobacterial pigments and their fluorescence characteristics: Applications in research and industry. In Advances in Cyanobacterial Biology; Singh, P.K., Kumar, A., Singh, V.K., Shrivastava, A.K., Eds.; Academic Press Inc.: New York, NY, USA, 2020; pp. 55–72. [Google Scholar]
- Costa, J.A.V.; Freitas, B.C.B.; Rosa, G.M.; Moraes, L.; Morais, M.G.; Mitchell, B.G. Operational and economic aspects of Spirulina-based biorefinery. Bioresour. Technol. 2019, 292, 121946. [Google Scholar] [CrossRef] [PubMed]
- Assunção, J.; Amaro, H.M.; Malcata, F.X.; Guedes, A.C. Cyanobacterial pigments: Photosynthetic function and biotechnological purposes. In The Pharmacological Potential of Cyanobacteria; Lopes, G., Silva, M., Vasconcelos, V., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2022; ISBN 9780128214916. [Google Scholar]
- Assunção, J.; Amaro, H.M.; Lopes, G.; Tavares, T.; Malcata, F.X.; Guedes, A.C. Synechocystis salina: Potential bioactivity and combined extraction of added-value metabolites. J. Appl. Phycol. 2021, 33, 3731–3746. [Google Scholar] [CrossRef]
- Pagels, F.; Salvaterra, D.; Amaro, H.M.; Lopes, G.; Sousa-Pinto, I.; Vasconcelos, V.; Guedes, A.C. Bioactive potential of Cyanobium sp. pigment-rich extracts. J. Appl. Phycol. 2020, 32, 3031–3040. [Google Scholar] [CrossRef]
- Assunção, J.; Amaro, H.M.; Lopes, G.; Tavares, T.; Malcata, F.X.; Guedes, A.C. Exploration of marine genus Chroococcidiopsis sp.: A valuable source for antioxidant industry? J. Appl. Phycol. 2021, 33, 2169–2187. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef]
- Saini, D.K.; Pabbi, S.; Shukla, P. Cyanobacterial pigments: Perspectives and biotechnological approaches. Food Chem. Toxicol. 2018, 120, 616–624. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Insights, F.M. Phycocyanin Market: Food & Beverage Application to Hold Close to 85% Value Share throughout the Forecast Period: Global Industry Analysis (2013–2017) & Opportunity Assessment (2018–2028). Available online: https://www.marketresearch.com/Future-Market-Insights-v4066/Phycocyanin-Food-Beverage-Application-Hold-11865287/ (accessed on 2 December 2020).
- Kapoore, R.V.; Butler, T.O.; Pandhal, J.; Vaidyanathan, S. Microwave-assisted extraction for microalgae: From biofuels to biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; Pennacchio, A.; Torres-Acosta, M.A.; Parra-Saldívar, R.; de Souza Vandenberghe, L.P.; Faraco, V. Multi-product biorefinery from Arthrospira platensis biomass as feedstock for bioethanol and lactic acid production. Sci. Rep. 2021, 11, 19309. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Miranda, S.M.; Belo, I.; Spigno, G.; Teixeira, J.A.; Rocha, C.M.R. Sequential multi-stage extraction of biocompounds from Spirulina platensis: Combined effect of ohmic heating and enzymatic treatment. Innov. Food Sci. Emerg. Technol. 2021, 71, 102707. [Google Scholar] [CrossRef]
- Barba, F.J.; Grimi, N. New approaches for the use of non-conventional cell disruption technologies to extract potential food additives and nutraceuticals from microalgae. Food Eng. Rev. 2015, 7, 45–62. [Google Scholar] [CrossRef]
- Pagels, F.; Salvaterra, D.; Amaro, H.M.; Guedes, A.C. Pigments from microalgae. In Handbook of Microalgae-Based Processes and Products; Jacob-Lopes, E., Maroneze, M.M., Queiroz, M.I., Zepka, L.Q., Eds.; Academic Press Inc.: Cambridge, MA, USA, 2020; pp. 465–492. ISBN 9780128185360. [Google Scholar]
- Chemat, F.; Rombaut, N.; Sicaire, A.; Meullemiestre, A.; Abert-vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Pez, D.; Rech, R.; Damasceno, L.; Marczak, F.; Domeneghini, G. Ultrasound as an alternative technology to extract carotenoids and lipids from Heterochlorella luteoviridis. Biosource Technol. 2017, 224, 753–757. [Google Scholar]
- Dey, S.; Rathod, V.K. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason. Sonochem. 2013, 20, 271–276. [Google Scholar] [CrossRef]
- Kong, W.; Liu, N.; Zhang, J.; Yang, Q.; Hua, S.; Song, H.; Xia, C. Optimization of ultrasound-assisted extraction parameters of chlorophyll from Chlorella vulgaris residue after lipid separation using response surface methodology. J. Food Sci. Technol. 2014, 51, 2006–2013. [Google Scholar] [CrossRef]
- Stramarkou, M.; Papadaki, S.; Kyriakopoulou, K.; Krokida, M. Recovery of Functional Pigments from four Different Species of Microalgae. IOSR J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 26–30. [Google Scholar] [CrossRef]
- Hadiyanto; Suttrisnorhadi; Sutanto, H.; Suzery, M. Phyocyanin extraction from microalgae Spirulina platensis assisted by ultrasound irradiation: Effect of time and temperature. Songklanakarin J. Sci. Technol. 2016, 38, 391–398. [Google Scholar]
- Sharma, N.; Mehariya, S.; Martino, M.; Larocca, V.; Di Sanzo, G.; Balducchi, R.; Iovine, A.; Karatza, D.; Marino, T.; Musmarra, D.; et al. Extraction of Carotenes and Lutein from Dunaliella Salina Microalgae Using Pressurized Liquid Extraction. In Proceedings of the 27th European Biomass Conference and Exhibition, Lisbon, Portugal, 27–31 May 2021; pp. 112–118. [Google Scholar]
- Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Gayen, K.; Bhowmick, T.K. Downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: A review. Food Bioprod. Process. 2018, 110, 60–84. [Google Scholar] [CrossRef]
- Munir, N.; Sharif, N.; Shagufta, N.; Saleem, F.; Manzoor, F. Harvesting and processing of microalgae biomass fractions for biodiesel production (a review). Sci. Technol. Dev. 2013, 32, 235–243. [Google Scholar]
- Gam, D.H.; Kim, S.Y.; Kim, J.W. Optimization of ultrasound-assisted extraction condition for phenolic compounds, antioxidant activity, and epigallocatechin gallate in lipid-extracted microalgae. Molecules 2020, 25, e25030454. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Patiño, J.C.; Gullón, B.; Romero, I.; Ruiz, E.; Brnčić, M.; Žlabur, J.Š.; Castro, E. Optimization of ultrasound-assisted extraction of biomass from olive trees using response surface methodology. Ultrason. Sonochem. 2019, 51, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, G.A.; Ambati, R.R.; Alzahrani, M.A.J.; Perera, C.O. Ultrasound-Assisted Extraction of Bioactive Compounds from Microalgae. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; pp. 81–88. [Google Scholar]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Fratelli, C.; Burck, M.; Amarante, M.C.A.; Braga, A.R.C. Antioxidant potential of nature’s “something blue”: Something new in the marriage of biological activity and extraction methods applied to C-phycocyanin. Trends Food Sci. Technol. 2021, 107, 309–323. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. Biotechnological potential of Synechocystis salina co-cultures with selected microalgae and cyanobacteria: Nutrients removal, biomass and lipid production. Bioresour. Technol. 2016, 200, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Kotai, J. Instructions for preparation of modified nutrient solution Z8 for algae. Oikos 1972, 15, 155–164. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 10th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; ISBN 9781119492443. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids Measurement and UV-VIS characterization Lichtenthaler 2001. Curr. Protoc. Food Anal. Chem. 2001, F4.3.1–F4.3.8, 1–8. [Google Scholar]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1994, 58, 419–435. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Gião, M.S.; Malcata, F.X. Optimization of ABTS radical cation assay specifically for determination of antioxidant capacity of intracellular extracts of microalgae and cyanobacteria. Food Chem. 2013, 138, 638–643. [Google Scholar] [CrossRef]
- Granados-Guzman, G.; Salazar-Aranda, R.; Garza-Tapia, M.; Castro-Rios, R.; Waksman de Torres, N. Optimization and validation of two high-throughput methods indicating antiradical activity. Curr. Anal. Chem. 2017, 13, 499–507. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggete, A.; Panala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying and improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Deenu, A.; Naruenartwongsakul, S.; Kim, S.M. Optimization and economic evaluation of ultrasound extraction of lutein from Chlorella vulgaris. Biotechnol. Bioprocess Eng. 2013, 18, 1151–1162. [Google Scholar] [CrossRef]
- Sharma, R.; Bhunia, B.; Mondal, A.; Kanti Bandyopadhyay, T.; Devi, I.; Oinam, G.; Prasanna, R.; Abraham, G.; Nath Tiwari, O. Statistical optimization of process parameters for improvement of phycobiliproteins (PBPs) yield using ultrasound-assisted extraction and its kinetic study. Ultrason. Sonochem. 2020, 60, 104762. [Google Scholar] [CrossRef]
- Ardiles, P.; Cerezal-Mezquita, P.; Salinas-Fuentes, F.; Órdenes, D.; Renato, G.; Ruiz-Domínguez, M.C. Biochemical composition and phycoerythrin extraction from red microalgae: A comparative study using green extraction technologies. Processes 2020, 8, 1628. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Paciulli, M.; Abbaspourrad, A. Extraction of phycocyanin—A natural blue colorant from dried spirulina biomass: Influence of processing parameters and extraction techniques. J. Food Sci. 2020, 85, 727–735. [Google Scholar] [CrossRef]
- Kobus, Z.; Krzywicka, M.; Starek-Wójcicka, A.; Sagan, A. Effect of the duty cycle of the ultrasonic processor on the efficiency of extraction of phenolic compounds from Sorbus intermedia. Sci. Rep. 2022, 12, 8311. [Google Scholar] [CrossRef]
- Sommer, M.C.; Balazinski, M.; Rataj, R.; Wenske, S.; Kolb, J.F.; Zocher, K. Assessment of phycocyanin extraction from cyanidium caldarium by spark discharges, compared to freeze-thaw cycles, sonication and pulsed electric fields. Microorganisms 2021, 9, 1452. [Google Scholar] [CrossRef]
- Sarada, R.; Pillai, M.G.; Ravishankar, G.A. Phycocyanin from Spirulina sp: Influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Process Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Mittal, R.; Chandrasekhar, J.; Raghavarao, K.S.M.S. Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthospira platensis. Algal Res. 2018, 31, 239–251. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Sinha, R.P. Thermokinetic stability of phycocyanin and phycoerythrin in food-grade preservatives. J. Appl. Phycol. 2016, 28, 1063–1070. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. Physico-chemical factors affecting the in vitro stability of phycobiliproteins from Phormidium rubidum A09DM. Bioresour. Technol. 2015, 190, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Pagels, F.; Pereira, R.N.; Vicente, A.A.; Guedes, A.C. Extraction of pigments from microalgae and cyanobacteria-a review on current methodologies. Appl. Sci. 2021, 11, 5187. [Google Scholar] [CrossRef]
- da Costa Ores, J.; de Amarante, M.C.A.; Kalil, S.J. Co-production of carbonic anhydrase and phycobiliproteins by Spirulina sp. and Synechococcus nidulans. Bioresour. Technol. 2016, 219, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Hardiningtyas, S.D.; Setyaningsih, I.; Susila, A.A.R. Effect of ultrasonication-assisted extraction time on the profile of phycocyanin from wet biomass Spirulina plantesis. IOP Conf. Ser. Earth Environ. Sci. 2022, 1033, 012055. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hosano, N.; Hosano, H. Recovering Microalgal Bioresources: A Review of Cell Disruption Methods and Extraction Technologies. Molecules 2022, 27, 2786. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Pan, Z.; Qu, W.; Ma, H.; Atungulu, G.G.; McHugh, T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2011, 18, 1249–1257. [Google Scholar] [CrossRef]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from cyanobacteria: Biotechnological potential and optimization strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef]
- Prabha, S.; Vijay, A.K.; Paul, R.R.; George, B. Cyanobacterial biorefinery: Towards economic feasibility through the maximum valorization of biomass. Sci. Total Environ. 2022, 814, 152795. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Amaro, H.M.; Sousa-Pinto, I.; Malcata, F.X. Applications of Spent Biomass. In Biofuels from Algae; Pandey, A., Lee, D.J., Socool, C.R., Chisti, Y., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2013; ISBN 9780444595584. [Google Scholar]
- Hilali, S.; Wils, L.; Chevalley, A.; Clément-Larosière, B.; Boudesocque-Delaye, L. Glycerol-based NaDES as green solvents for ultrasound-assisted extraction of phycocyanin from Arthrospira platensis—RSM optimization and ANN modelling. Biomass Convers. Biorefin. 2022, 12, 157–170. [Google Scholar] [CrossRef]
- Hadiyanto, S.H. Response surface optimization of ultrasound assisted extraction (UAE) of phycocyanin from microalgae Spirulina platensis. Emir. J. Food Agric. 2016, 28, 227–234. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Cerezal, P.; Salinas, F.; Medina, E.; Renato-Castro, G. Application of box-behnken design and desirability function for green prospection of bioactive compounds from isochrysis galbana. Appl. Sci. 2020, 10, 2789. [Google Scholar] [CrossRef] [Green Version]
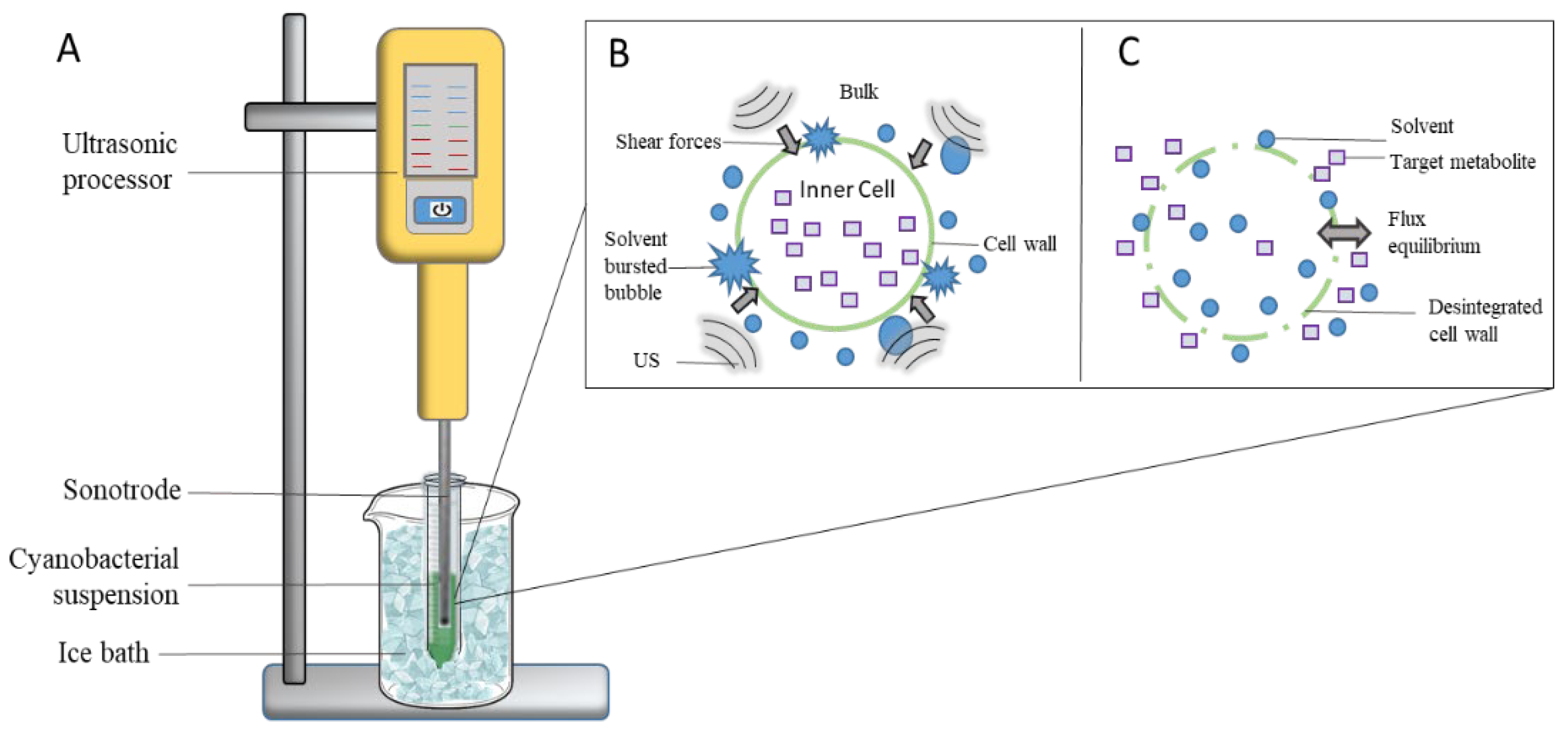
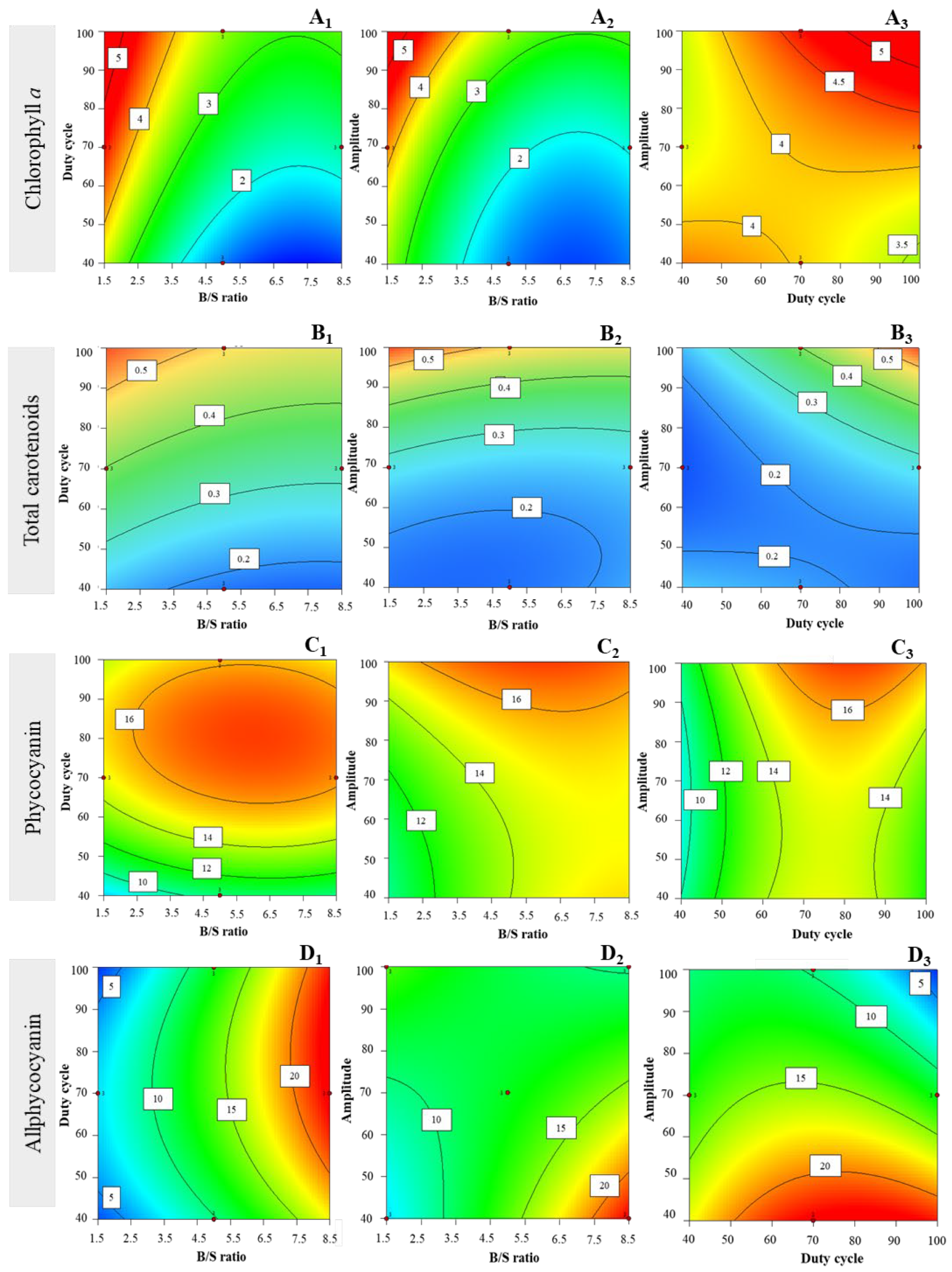
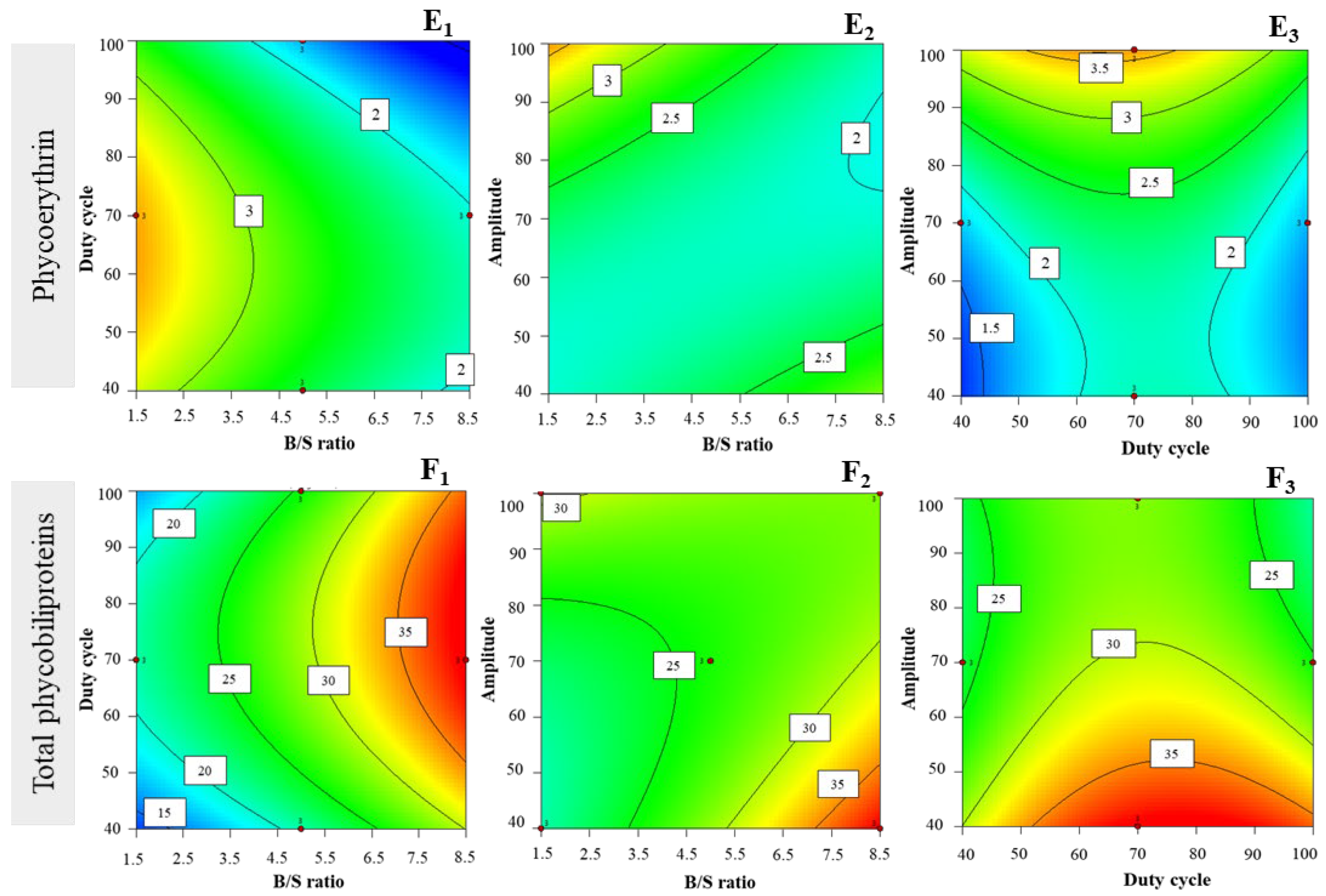

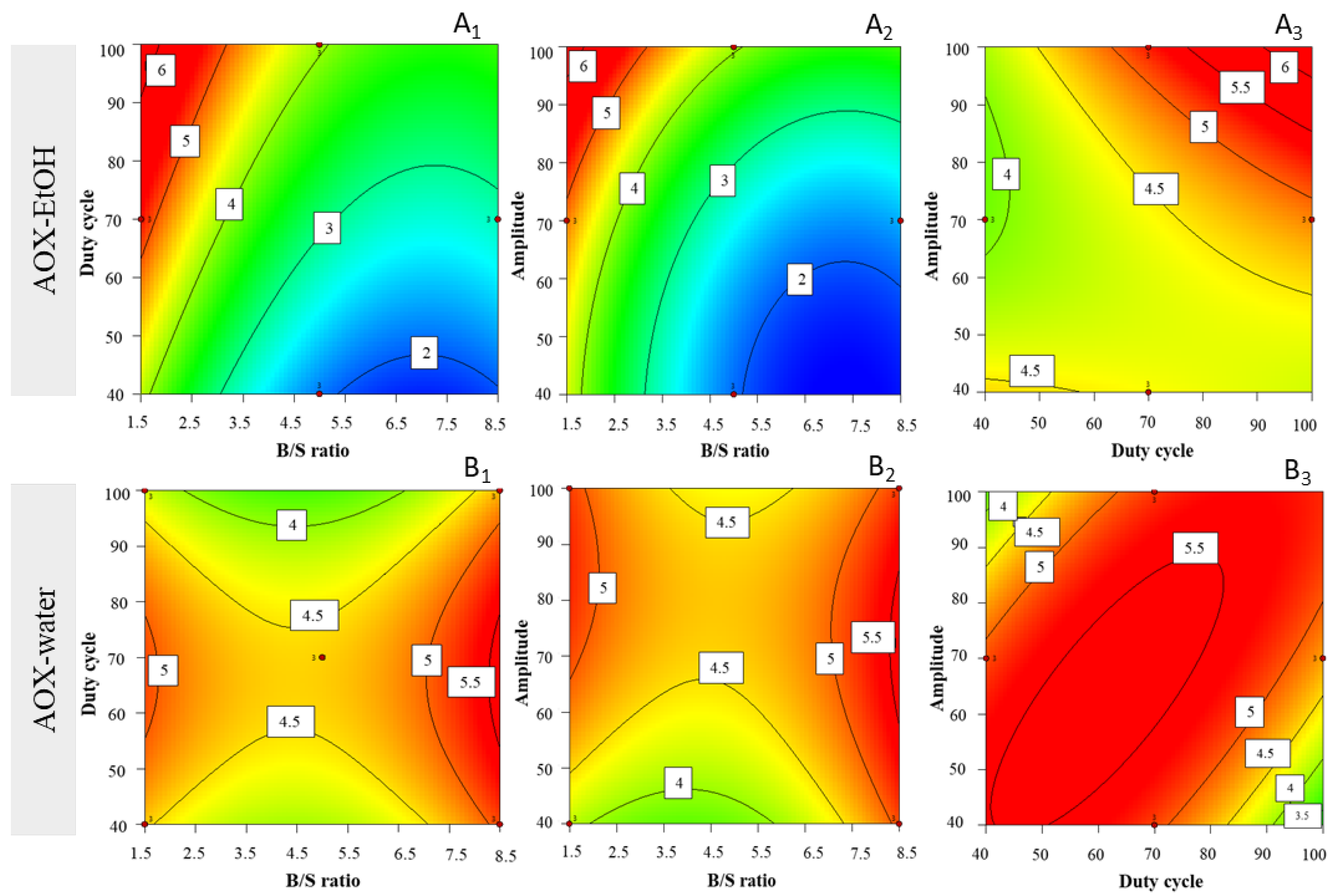
| Experimental Factors | |||
|---|---|---|---|
| Runs | A: Biomass to Solvent Ratio (mg·mL−1) | B: Duty Cycle (%) | C: Amplitude (%) |
| 1 | 1.5 | 40 | 70 |
| 2 | 1.5 | 70 | 40 |
| 3 | 1.5 | 70 | 100 |
| 4 | 1.5 | 100 | 70 |
| 5 | 5 | 40 | 100 |
| 6 | 5 | 100 | 40 |
| 7 | 5 | 40 | 40 |
| 8 | 5 | 70 | 70 |
| 9 | 5 | 100 | 100 |
| 10 | 8.5 | 40 | 70 |
| 11 | 8.5 | 70 | 40 |
| 12 | 8.5 | 70 | 100 |
| 13 | 8.5 | 100 | 70 |
| Parameters (Objective Function) | p-Value | R2 | Equation |
|---|---|---|---|
| Chl a (mg·gDW−1) | <0.0001 | 0.90 | 2.01 − 1.10[B/S] + 0.2847DT + 0.2617A + 0.0389[B/S]*DT − 0.0914[B/S]*A + 0.6809DT*A + 0.9328[B/S]2 − 0.1211DT2 + 0.2689A2 |
| Total carotenoids (mg·gDW−1) | <0.0001 | 0.70 | 0.1888 − 0.0067[B/S] + 0.0581DT + 0.0587A + 0.0042[B/S]*DT − 0.0326 [B/S]*A + 0.0975 DT*A + 0.0221[B/S]2 − 0.0026DT2 + 0.0885A2 |
| PC (mg·gDW−1) | <0.0001 | 0.94 | 14.26 − 1.68[B/S] + 1.92DT + 1.32A − 0.2897[B/S]*DT − 0.9223[B/S]*A + 0.7611 DT*A − 1.15[B/S]2 − 3.49DT2 + 1.03A2 |
| APC (mg·gDW−1) | <0.0001 | 0.84 | 11.87 + 3.04[B/S] − 1.79DT − 1.56A + 1.31[B/S]*DT − 5.00[B/S]*A − 2.92 DT*A + 0.7294[B/S]2 − 3.19DT2 + 0.6361A2 |
| PE (mg·gDW−1) | <0.0001 | 0.84 | 2.09 − 0.1765[B/S] − 0.1766DT + 0.1335A − 0.1498[B/S]*DT − 0.6114[B/S]*A − 0.1958DT*A + 0.1098[B/S]2 − 0.6245DT2 + 0.4885A2 |
| TPBP (mg·gDW−1) | <0.0001 | 0.86 | 25.81 − 3.92[B/S] + 0.0590DT − 0.910A + 0.7327[B/S]*DT − 5.04[B/S]*A − 2.18DT*A + 0.8581[B/S]2 − 5.93DT2 + 3.15A2 |
| Yield-EtOH (%DW) | <0.0001 | 0.91 | 23.41 − 19.28[B/S] + 1.56DT + 0.0758A − 2.74[B/S]*DT + 0.9497[B/S]*A − 1.88DT*A + 13.64[B/S]2 − 3.01DT2 + 5.71A2 |
| Yield-Water (%DW) | <0.0001 | 0.96 | 35 + 11.72[B/S] − 3.21DT − 2.73A + 4.19[B/S]*DT − 1.97[B/S]*A − 2.59DT*A − 8.15[B/S]2 + 5.17DT2 + 4.72A2 |
| AOX-EtOH (mgTE·gDW−1) | <0.0001 | 0.93 | 2.29 − 1.17[B/S] + 0.2865DT + 0.4444A + 0.0184[B/S]*DT − 0.1597[B/S]*A + 0.6220DT*A + 0.9532[B/S]2 − 0.1468DT2 + 0.5602A2 |
| AOX-Water (mgTE·gDW−1) | <0.0001 | 0.78 | 4.57 + 0.2569[B/S] − 0.1722DT + 0.2800A − 0.0152[B/S]*DT –0.2309[B/S]*A + 0.8906DT*A + 0.7665[B/S]2 − 0.67937DT2 − 0.4600A2 |
| Experimental Factors | Variables Evaluated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B/S (mg·mL−1) | Duty Cycle (%) | Amplitude (%) | Chl a (mg·gDW−1) | Total Carotenoids (mg·gDW−1) | PC (mg·gDW−1) | APC (mg·gDW−1) | PE (mg·gDW−1) | TPBP (mg·gDW−1) | Yield-EtOH (%DW) | Yield-Water (%DW) | AOX-EtOH (mgTE·gDW−1) | AOX-Water (mgTE·gDW−1) |
| 1.5 | 40 | 70 | 4.27 ± 0.13 | 0.21 ± 0.04 | 6.26 ± 0.27 | 5.99 ± 0.25 | 1.47 ± 0.08 | 13.74 ± 1.53 | 47.65 ± 9.16 | 24.59 ± 1.30 | 4.47 ± 0.26 | 3.84 ± 0.22 |
| 1.5 | 70 | 40 | 3.61 ± 0.25 | 0.26 ± 0.07 | 9.90 ± 0.20 | 9.60 ± 0.67 | 2.30 ± 0.00 | 23.62 ± 0.97 | 69.71 ± 0.00 | 22.24 ± 0.28 | 4.35 ± 0.10 | 4.43 ± 0.02 |
| 1.5 | 70 | 100 | 4.46 ± 0.21 | 0.32 ± 0.01 | 14.47 ± 0.32 | 15.24 ± 0.16 | 3.98 ± 0.07 | 32.82 ± 1.33 | 62.97 ± 1.55 | 18.77 ± 0.90 | 5.27 ± 0.10 | 5.00 ± 0.11 |
| 1.5 | 100 | 70 | 4.13 ± 0.04 | 0.31 ± 0.00 | 10.93 ± 0.06 | 2.31 ± 0.01 | 1.46 ± 0.19 | 15.24 ± 1.18 | 50.34 ± 3.10 | 14.68 ± 0.31 | 4.39 ± 0.06 | 4.50 ± 0.79 |
| 5 | 40 | 100 | 1.07 ± 0.05 | 0.17 ± 0.01 | 10.11 ± 0.11 | 14.36 ± 0.03 | 2.38 ± 0.01 | 26.65 ± 0.02 | 22.28 ± 1.84 | 51.37 ± 0.72 | 2.08 ± 0.07 | 1.54 ± 0.04 |
| 5 | 100 | 40 | 1.89 ± 0.10 | 0.19 ± 0.01 | 11.95 ± 0.49 | 10.12 ± 0.29 | 1.91 ± 0.05 | 23.80 ± 0.50 | 26.17 ± 1.01 | 43.57 ± 1.45 | 2.09 ± 0.11 | 3.94 ± 0.02 |
| 5 | 40 | 40 | 2.05 ± 0.01 | 0.17 ± 0.01 | 9.79 ± 0.54 | 10.39 ± 0.20 | 1.92 ± 0.08 | 22.16 ± 0.27 | 20.90 ± 1.67 | 49.69 ± 0.45 | 2.13 ± 0.06 | 4.47 ± 0.12 |
| 5 | 70 | 70 | 2.05 ± 0.07 | 0.19 ± 0.00 | 14.26 ± 0.33 | 11.87± 0.51 | 2.09 ± 0.23 | 25.81 ± 0.28 | 23.41 ± 0.42 | 35.00 ± 0.25 | 2.29 ± 0.13 | 4.57 ± 0.08 |
| 5 | 100 | 100 | 3.63 ± 0.17 | 0.57 ± 0.06 | 15.31 ± 0.47 | 2.42 ± 0.10 | 1.59 ± 0.07 | 19.55 ± 0.67 | 35.06 ± 0.85 | 34.90 ± 0.82 | 4.50 ± 0.06 | 4.18 ± 0.05 |
| 8.5 | 40 | 70 | 1.44 ± 0.03 | 0.16 ± 0.00 | 8.95 ± 0.01 | 13.91 ± 0.49 | 1.98 ± 0.06 | 24.78 ± 0.82 | 23.20 ± 0.39 | 4.98 ± 0.63 | 1.77 ± 0.03 | 4.85 ± 0.15 |
| 8.5 | 70 | 40 | 2.15 ± 0.03 | 0.36 ± 0.04 | 15.64 ± 0.78 | 21.23 ± 0.88 | 2.61 ± 0.29 | 36.90 ± 1.12 | 20.63 ± 1.05 | 48.30 ± 0.40 | 2.66 ± 0.18 | 5.09 ± 0.15 |
| 8.5 | 70 | 100 | 2.63 ± 0.04 | 0.32 ± 0.01 | 17.22 ± 0.54 | 6.87 ± 0.31 | 1.84 ± 0.15 | 25.94 ± 0.39 | 17.70 ± 0.72 | 36.95 ± 0.84 | 2.93 ± 0.08 | 4.89 ± 0.12 |
| 8.5 | 100 | 70 | 1.45 ± 0.05 | 0.16 ± 0.00 | 12.38 ± 0.02 | 15.45 ± 0.15 | 1.37 ± 0.15 | 29.21 ± 0.31 | 14.92 ± 1.35 | 47.83 ± 0.71 | 1.75 ± 0.02 | 5.28 ± 0.07 |
| Model Prediction | |||||
|---|---|---|---|---|---|
| Analyzed Objective Functions | B/S Ratio (mg·mL−1) | Duty Cycle (%) | Amplitude (%) | Max. Predicted Value | Desirability |
| Water extracts | |||||
| PC (mg·gDW−1) | 6 | 80 | 100 | 17.21 ± 0.86 | 0.942 |
| APC (mg·gDW−1) | 8.5 | 70 | 40 | 22.82 ± 2.52 | 1 |
| PE (mg·gDW−1) | 1.5 | 60 | 100 | 3.61 ± 0.32 | 1 |
| Total PBPs (mg·gDW−1) | 8.5 | 70 | 40 | 38.97 ± 2.74 | 1 |
| Yield-water (%DW) | 8.3 | 100 | 40 | 54.96 ± 2.72 | 1 |
| AOX-water (mgTE·gDW−1) | 8.2 | 70 | 70 | 5.44 ± 0.05 | 1 |
| Ethanolic extracts | |||||
| Chl a (mg·gDW−1) | 1.5 | 100 | 100 | 5.47 ± 0.42 | 1 |
| Total carotenoids (mg·gDW−1) | 1.5 | 100 | 100 | 0.57 ± 0.06 | 1 |
| Yield-EtOH (%DW) | 1.5 | 100 | 100 | 64.32 ± 6.31 | 0.905 |
| AOX-EtOH (mgTE·gDW−1) | 1.5 | 100 | 100 | 6.32 ± 0.38 | 1 |
| Parameters | UAE (max. Predicted) | Classical | Precellys |
|---|---|---|---|
| Aqueous extracts | |||
| PC (mg·gDW−1) | 17.21 ± 0.86 a | 15.30 ± 1.51 a | 11.64 ± 0.08 b |
| APC (mg·gDW−1) | 8.65 ± 2.52 a | 18.15 ± 0.72 b | 4.36 ± 0.45 c |
| PE (mg·gDW−1) | 2.28 ± 0.32 a | 7.40 ± 0.46 b | 2.57 ± 0.13 a |
| Total PBPs (mg·gDW−1) | 27.23 ± 2.74 a | 38.56 ± 2.96 b | 20.02 ± 1.81 c |
| Yield-water (%DW) | 38.15 ± 2.72 a | 27.15 ± 0.74 b | 36.31 ± 0.43 a |
| AOX-water (mgTE·gDW−1) | 4.62 ± 0.55 a | 4.32 ± 0.32 a | 1.81 ± 0.07 b |
| Ethanolic extracts | |||
| Chl a (mg·gDW−1) | 2.60 ± 0.42 a | 4.75 ± 0.21 b | 3.27 ± 0.18 a |
| Total carotenoids (mg·gDW−1) | 0.32 ± 0.06 a | 0.23 ± 0.01 a | 0.18 ± 0.01 a, b |
| Yield-EtOH (%DW) | 25.61 ± 6.31 a | 43.27 ± 5.14 b | 42.48 ± 0.85 b |
| AOX-EtOH (mgTE·gDW−1) | 3.28 ± 0.38 a | 2.92 ± 0.22 a | 0.33 ± 0.01 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assunção, J.; Amaro, H.M.; Malcata, F.X.; Guedes, A.C. Factorial Optimization of Ultrasound-Assisted Extraction of Phycocyanin from Synechocystis salina: Towards a Biorefinery Approach. Life 2022, 12, 1389. https://doi.org/10.3390/life12091389
Assunção J, Amaro HM, Malcata FX, Guedes AC. Factorial Optimization of Ultrasound-Assisted Extraction of Phycocyanin from Synechocystis salina: Towards a Biorefinery Approach. Life. 2022; 12(9):1389. https://doi.org/10.3390/life12091389
Chicago/Turabian StyleAssunção, Joana, Helena M. Amaro, Francisco Xavier Malcata, and Ana Catarina Guedes. 2022. "Factorial Optimization of Ultrasound-Assisted Extraction of Phycocyanin from Synechocystis salina: Towards a Biorefinery Approach" Life 12, no. 9: 1389. https://doi.org/10.3390/life12091389
APA StyleAssunção, J., Amaro, H. M., Malcata, F. X., & Guedes, A. C. (2022). Factorial Optimization of Ultrasound-Assisted Extraction of Phycocyanin from Synechocystis salina: Towards a Biorefinery Approach. Life, 12(9), 1389. https://doi.org/10.3390/life12091389










