Assessment of Pharmacological Potential of Novel Exopolysaccharide Isolated from Marine Kocuria sp. Strain AG5: Broad-Spectrum Biological Investigations
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation and Sampling
2.2. Recognition of Bacterial Isolates
| Forward primer: | 5′-TCCGTAGGTGAACTTTGCGG-3′ |
| Reverse primer: | 5′-TCCTCCGCTTATTGATATGC-3′ |
2.3. Production and Fractionation of EPS
2.4. Evaluation of EPSR5
2.5. Investigation of Antioxidant Effect Using DPPH Test
2.6. Investigation of Cytotoxicity on Various Human Cell Lines
2.7. Investigation of Anti-Inflammatory Property
2.7.1. Lipoxygenase (LOX) Activity In Vitro
2.7.2. Cyclooxygenase (COX-2) Activity In Vitro
2.8. Membrane Stabilization
2.9. Acetylcholine Esterase Activity
2.10. Statistical Analysis
3. Results
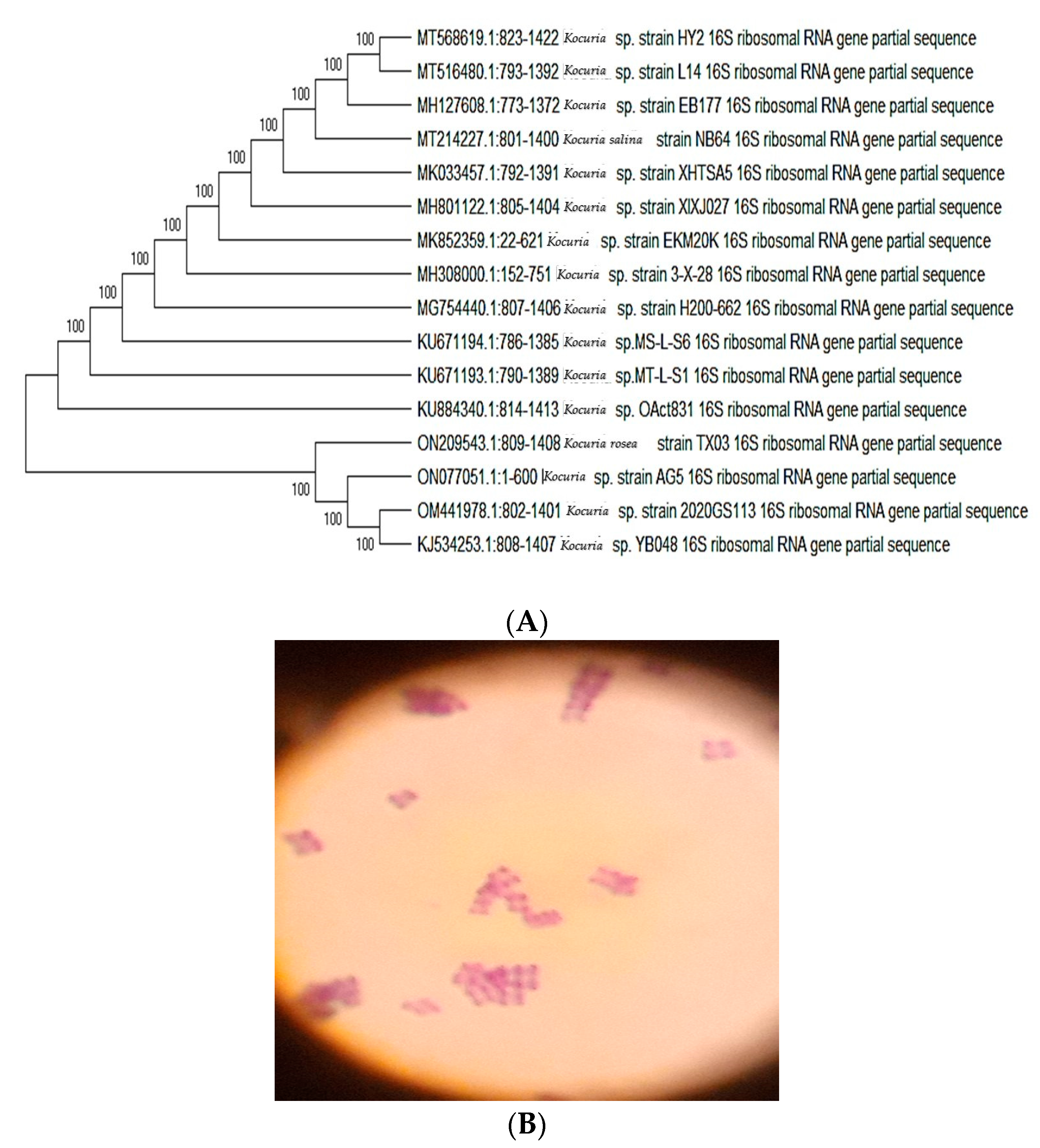
3.1. Isolation and Phylogenetic Analysis of the Bacteria
3.2. Manufacture and Chemical Composition of EPSR5
3.3. Antioxidant Activity of EPSR5
3.4. Anti-Inflammatory Activity of EPSR5
3.5. Membrane Stabilizing Effect of EPSR5
3.6. Antitumor Activity
3.7. Assessment of Acetylcholine Esterase Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.; Dalvi, Y.B. Natural Products as Anticancer Agents. Curr. Drug Targets 2021, 22, 1272–1287. [Google Scholar] [CrossRef] [PubMed]
- Gaber, A.; Alsanie, W.F.; Kumar, D.N.; Refat, M.S.; Saied, E.M. Novel Papaverine Metal Complexes with Potential Anticancer Activities. Molecules 2020, 25, 5447. [Google Scholar] [CrossRef] [PubMed]
- Saied, E.M.; El-Maradny, Y.A.; Osman, A.A.; Darwish, A.M.G.; Abo Nahas, H.H.; Niedbała, G.; Piekutowska, M.; Abdel-Rahman, M.A.; Balbool, B.A.; Abdel-Azeem, A.M. A Comprehensive Review about the Molecular Structure of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Insights into Natural Products against COVID-19. Pharmaceutics 2021, 13, 1759. [Google Scholar] [CrossRef]
- Chakraborty, J.; Kungwani, N.; Kumari, S.; Kumar, H.; Das, S.; Dash, H. Marine Bacterial Exopolysaccharides: Functional Diversity and Prospects in Environmental Restoration. In Marine Glycobiology: Principles and Applications; CRS Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial Exopolysaccharides: Functionality and Prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.M.; Kai, G.-Y.; Al-Hammady, M.A.M.; et al. Cyanobacteria—From the Oceans to the Potential Biotechnological and Biomedical Applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef]
- Bhagat, N.; Raghav, M.; Dubey, S.; Bedi, N. Bacterial Exopolysaccharides: Insight into Their Role in Plant Abiotic Stress Tolerance. J. Microbiol. Biotechnol. 2021, 31, 221–244. [Google Scholar] [CrossRef]
- More, T.T.; Yadav, J.S.S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular Polymeric Substances of Bacteria and Their Potential Environmental Applications. J. Environ. Manag. 2014, 144, 1–25. [Google Scholar] [CrossRef]
- Suresh Kumar, A.; Mody, K.; Jha, B. Bacterial Exopolysaccharides—A Perception. J. Basic Microbiol. 2007, 47, 103–117. [Google Scholar] [CrossRef]
- Elkazzaz, S.K.; Khodeer, D.M.; El Fayoumi, H.M.; Moustafa, Y.M. Role of Sodium Glucose Cotransporter Type 2 Inhibitors Dapagliflozin on Diabetic Nephropathy in Rats; Inflammation, Angiogenesis and Apoptosis. Life Sci. 2021, 280, 119018. [Google Scholar] [CrossRef]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial Exopolysaccharides from Extreme Marine Habitats: Production, Characterization and Biological Activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef]
- Mohd Nadzir, M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical Applications of Bacterial Exopolysaccharides: A Review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef]
- Sharar, M.; Saied, E.M.; Rodriguez, M.C.; Arenz, C.; Montes-Bayón, M.; Linscheid, M.W. Elemental Labelling and Mass Spectrometry for the Specific Detection of Sulfenic Acid Groups in Model Peptides: A Proof of Concept. Anal. Bioanal. Chem. 2017, 409, 2015–2027. [Google Scholar] [CrossRef]
- Poad, B.L.J.; Maccarone, A.T.; Yu, H.; Mitchell, T.W.; Saied, E.M.; Arenz, C.; Hornemann, T.; Bull, J.N.; Bieske, E.J.; Blanksby, S.J. Differential-Mobility Spectrometry of 1-Deoxysphingosine Isomers: New Insights into the Gas Phase Structures of Ionized Lipids. Anal. Chem. 2018, 90, 5343–5351. [Google Scholar] [CrossRef]
- Gugliandolo, C.; Spanò, A.; Lentini, V.; Arena, A.; Maugeri, T.L. Antiviral and Immunomodulatory Effects of a Novel Bacterial Exopolysaccharide of Shallow Marine Vent Origin. J. Appl. Microbiol. 2014, 116, 1028–1034. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 453. [Google Scholar] [CrossRef]
- Laurienzo, P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef]
- Onesti, M.G.; Fioramonti, P.; Carella, S.; Fino, P.; Sorvillo, V.; Scuderi, N. A New Association between Hyaluronic Acid and Collagenase in Wound Repair: An Open Study. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 210–216. [Google Scholar]
- Selim, S.; Almuhayawi, M.S.; Alharbi, M.T.; Nagshabandi, M.K.; Alanazi, A.; Warrad, M.; Hagagy, N.; Ghareeb, A.; Ali, A.S. In Vitro Assessment of Antistaphylococci, Antitumor, Immunological and Structural Characterization of Acidic Bioactive Exopolysaccharides from Marine Bacillus Cereus Isolated from Saudi Arabia. Metabolites 2022, 12, 132. [Google Scholar] [CrossRef]
- Karadayi, Y.I.; Aykutoglu, G.; Arslan, N.P.; Baltaci, M.O.; Adiguzel, A.; Taskin, M. Production of Water-Soluble Sulfated Exopolysaccharide with Anticancer Activity from Anoxybacillus Gonensis YK25. J. Chem. Technol. Biotechnol. 2021, 96, 1258–1266. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Yuan, Q.; Shan, L.-T.; Lin, M.-A.; Cheng, D.-Q.; Li, C.-Y. Antitumor Activity of Bacterial Exopolysaccharides from the Endophyte Bacillus Amyloliquefaciens sp. Isolated from Ophiopogon Japonicus. Oncol. Lett. 2013, 5, 1787–1792. [Google Scholar] [CrossRef]
- Pei, F.; Ma, Y.; Chen, X.; Liu, H. Purification and Structural Characterization and Antioxidant Activity of Levan from Bacillus Megaterium PFY-147. Int. J. Biol. Macromol. 2020, 161, 1181–1188. [Google Scholar] [CrossRef]
- Liu, G.; Liu, R.; Shan, Y.; Sun, C. Marine Bacterial Exopolysaccharide EPS11 Inhibits Migration and Invasion of Liver Cancer Cells by Directly Targeting Collagen I. J. Biol. Chem. 2021, 297, 101133. [Google Scholar] [CrossRef]
- Asker, M.; Mahmoud, M.; Ibrahim, A.; Mohamed, S.S. Inhibitory Effect of Exopolysaccharide from Achromobacter Piechaudii NRC2 against Cyclooxygenases and Acetylcholinesterase with Evaluation of Its Antioxidant Properties and Structure Elucidation. Pharm. Lett. 2015, 7, 129–141. [Google Scholar]
- Hamad, A.A.; Sharaf, M.; Hamza, M.A.; Selim, S.; Hetta, H.F.; El-Kazzaz, W. Investigation of the Bacterial Contamination and Antibiotic Susceptibility Profile of Bacteria Isolated from Bottled Drinking Water. Microbiol. Spectr. 2022, 10, e0151621. [Google Scholar] [CrossRef]
- Castellani, R.; Hirai, K.; Aliev, G.; Drew, K.L.; Nunomura, A.; Takeda, A.; Cash, A.D.; Obrenovich, M.E.; Perry, G.; Smith, M.A. Role of Mitochondrial Dysfunction in Alzheimer’s Disease. J. Neurosci. Res. 2002, 70, 357–360. [Google Scholar] [CrossRef]
- Jeandel, C.; Nicolas, M.B.; Dubois, F.; Nabet-Belleville, F.; Penin, F.; Cuny, G. Lipid Peroxidation and Free Radical Scavengers in Alzheimer’s Disease. Gerontology 1989, 35, 275–282. [Google Scholar] [CrossRef]
- Pasinetti, G.M. Cyclooxygenase and Inflammation in Alzheimer’s Disease: Experimental Approaches and Clinical Interventions. J. Neurosci. Res. 1998, 54, 1–6. [Google Scholar] [CrossRef]
- Blandón Garcia, L.; Noseda, M.; Islan, G.; Castro, G.; Pereira, G.; Thomaz-Soccol, V.; Soccol, C. Optimization of Culture Conditions for Kefiran Production in Whey: The Structural and Biocidal Properties of the Resulting Polysaccharide. Bioact. Carbohydr. Diet. Fibre 2018, 16, 14–21. [Google Scholar] [CrossRef]
- Noel, S.; Fortier, C.; Murschel, F.; Belzil, A.; Gaudet, G.; Jolicoeur, M.; De Crescenzo, G. Co-Immobilization of Adhesive Peptides and VEGF within a Dextran-Based Coating for Vascular Applications. Acta Biomater. 2016, 37, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Saveleva, M.; Vladescu, A.; Cotrut, C.; Van der Meeren, L.; Surmeneva, M.; Surmenev, R.; Parakhonskiy, B.; Skirtach, A.G. The Effect of Hybrid Coatings Based on Hydrogel, Biopolymer and Inorganic Components on the Corrosion Behavior of Titanium Bone Implants. J. Mater. Chem. B 2019, 7, 6778–6788. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Nonomura, H. Humic Acid-Vitamin Agar, a New Medium for the Selective Isolation of Soil Actinomycetes. J. Ferment. Technol. 1987, 65, 501–509. [Google Scholar] [CrossRef]
- Kim, S.-W.; Ahn, S.-G.; Seo, W.-T.; Kwon, G.-S.; Park, Y.-H. Rheological Properties of a Novel High Viscosity Polysaccharide, A49-Pol, Produced by Bacillus Polymyxa. J. Microbiol. Biotechnol. 1998, 8, 178–181. [Google Scholar]
- Bergey, D.H.; Holt, J.G. (Eds.) Bergey’s Manual of Determinative Bacteriology, 9th ed.; Williams & Wilkins: Baltimore, MD, USA, 1994; ISBN 978-0-683-00603-2. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Banhart, S.; Saied, E.M.; Martini, A.; Koch, S.; Aeberhard, L.; Madela, K.; Arenz, C.; Heuer, D. Improved Plaque Assay Identifies a Novel Anti-Chlamydia Ceramide Derivative with Altered Intracellular Localization. Antimicrob. Agents Chemother. 2014, 58, 5537–5546. [Google Scholar] [CrossRef]
- Saied, E.M.; Banhart, S.; Bürkle, S.E.; Heuer, D.; Arenz, C. A Series of Ceramide Analogs Modified at the 1-Position with Potent Activity against the Intracellular Growth of Chlamydia Trachomatis. Future Med. Chem. 2015, 7, 1971–1980. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes—Application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Lu, L.; Liu, Y.; Wang, F.; Xiao, M. Isolation, Structural Characterization and Immunological Activity of an Exopolysaccharide Produced by Bacillus Licheniformis 8-37-0-1. Bioresour. Technol. 2010, 101, 5528–5533. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, X.; Mu, H.; Liang, X.; Guan, H. Structure and Protective Effect of Exopolysaccharide from P. Agglomerans Strain KFS-9 against UV Radiation. Microbiol. Res. 2007, 162, 124–129. [Google Scholar] [CrossRef]
- Nicely, W.B. Infrared Spectra of Carbohydrates. In Advances in Carbohydrate Chemistry; Wolfrom, M.L., Tipson, R.S., Eds.; Academic Press: Cambridge, MA, USA, 1957; Volume 12, pp. 13–33. [Google Scholar]
- Refat, M.S.; Ibrahim, H.K.; Sowellim, S.Z.A.; Soliman, M.H.; Saeed, E.M. Spectroscopic and Thermal Studies of Mn(II), Fe(III), Cr(III) and Zn(II) Complexes Derived from the Ligand Resulted by the Reaction Between 4-Acetyl Pyridine and Thiosemicarbazide. J. Inorg. Organomet. Polym. Mater. 2009, 19, 521. [Google Scholar] [CrossRef]
- Salem, M.; El-Maaty, D.; El-Deen, Y.; Elesawy, B.; El Askary, A.; Saleh, A.; Saied, E.M.; El Behery, M. Novel 1,3-Thiazole Analogues with Potent Activity against Breast Cancer: A Design, Synthesis, In Vitro, and In Silico Study. Molecules 2022, 27, 4898. [Google Scholar] [CrossRef]
- El Azab, I.H.; Saied, E.M.; Osman, A.A.; Mehana, A.E.; Saad, H.A.; Elkanzi, N.A. Novel N-Bridged Pyrazole-1-Carbothioamides with Potential Antiproliferative Activity: Design, Synthesis, In Vitro and In Silico Studies. Future Med. Chem. 2021, 13, 1743–1766. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.; Carpita, N.C. Measurement of Uronic Acids without Interference from Neutral Sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A Note on the Determination of the Ester Sulphate Content of Sulphated Polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Randall, R.C.; Phillips, G.O.; Williams, P.A. The Role of the Proteinaceous Component on the Emulsifying Properties of Gum Arabic. Food Hydrocoll. 1988, 2, 131–140. [Google Scholar] [CrossRef]
- Masamune, H.; Yosizawa, Z.; Tokita, K. Biochemical Studies on Carbohydrates. Tohoku J. Exp. Med. 1957, 65, 187–194. [Google Scholar] [CrossRef][Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.A.; Abd El-Kareem, H.F.; Alzamami, A.; Fahmy, C.A.; Elesawy, B.H.; Mostafa Mahmoud, M.; Ghareeb, A.; El Askary, A.; Abo Nahas, H.H.; Attallah, N.G.M.; et al. Novel Exopolysaccharide from Marine Bacillus Subtilis with Broad Potential Biological Activities: Insights into Antioxidant, Anti-Inflammatory, Cytotoxicity, and Anti-Alzheimer Activity. Metabolites 2022, 12, 715. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Kassem, I.I.; Kumar, A.; Rajashekara, G. In Vitro Evaluation of the Impact of the Probiotic E. Coli Nissle 1917 on Campylobacter Jejuni’s Invasion and Intracellular Survival in Human Colonic Cells. Front. Microbiol. 2017, 8, 1588. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Kassem, I.I.; Rajashekara, G. Immuno-Modulatory Effect of Probiotic E. Coli Nissle 1917 in Polarized Human Colonic Cells against Campylobacter Jejuni Infection. Gut Microbes 2021, 13, 1857514. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Gaber, A.; Refat, M.S.; Belal, A.A.M.; El-Deen, I.M.; Hassan, N.; Zakaria, R.; Alhomrani, M.; Alamri, A.S.; Alsanie, W.F.; Saied, M.E. New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study. Molecules 2021, 26, 2288. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Kathayat, D.; Ghanem, M.; Jung, K.; Closs, G.; Deblais, L.; Srivastava, V.; El-Gazzar, M.; Rajashekara, G. Identification and Characterization of Novel Small Molecule Inhibitors to Control Mycoplasma Gallisepticum Infection in Chickens. Vet. Microbiol. 2020, 247, 108799. [Google Scholar] [CrossRef] [PubMed]
- Granica, S.; Czerwińska, M.E.; Piwowarski, J.P.; Ziaja, M.; Kiss, A.K. Chemical Composition, Antioxidative and Anti-Inflammatory Activity of Extracts Prepared from Aerial Parts of Oenothera Biennis L. and Oenothera Paradoxa Hudziok Obtained after Seeds Cultivation. J. Agric. Food Chem. 2013, 61, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Koki, A.T.; Masferrer, J.L. Celecoxib: A Specific COX-2 Inhibitor with Anticancer Properties. Cancer Control J. Moffitt Cancer Cent. 2002, 9, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Amessis-Ouchemoukh, N.; Madani, K.; Falé, P.L.V.; Serralheiro, M.L.; Araújo, M.E.M. Antioxidant Capacity and Phenolic Contents of Some Mediterranean Medicinal Plants and Their Potential Role in the Inhibition of Cyclooxygenase-1 and Acetylcholinesterase Activities. Ind. Crops Prod. 2014, 53, 6–15. [Google Scholar] [CrossRef]
- Petrovic, N.; Murray, M. Using N,N,N’,N’-Tetramethyl-p-Phenylenediamine (TMPD) to Assay Cyclooxygenase Activity In Vitro. Methods Mol. Biol. 2010, 594, 129–140. [Google Scholar] [CrossRef]
- Shinde, U.A.; Phadke, A.S.; Nair, A.M.; Mungantiwar, A.A.; Dikshit, V.J.; Saraf, M.N. Membrane Stabilizing Activity—A Possible Mechanism of Action for the Anti-Inflammatory Activity of Cedrus Deodara Wood Oil. Fitoterapia 1999, 70, 251–257. [Google Scholar] [CrossRef]
- Zhou, Y.; Plowman, S.J.; Lichtenberger, L.M.; Hancock, J.F. The Anti-Inflammatory Drug Indomethacin Alters Nanoclustering in Synthetic and Cell Plasma Membranes. J. Biol. Chem. 2010, 285, 35188–35195. [Google Scholar] [CrossRef]
- Monserrat, J.M.; Bianchini, A.; Bainy, A.C.D. Kinetic and Toxicological Characteristics of Acetylcholinesterase from the Gills of Oysters (Crassostrea Rhizophorae) and Other Aquatic Species. Mar. Environ. Res. 2002, 54, 781–785. [Google Scholar] [CrossRef]
- Mohamed, D.I.; Alaa El-Din Aly El-Waseef, D.; Nabih, E.S.; El-Kharashi, O.A.; Abd El-Kareem, H.F.; Abo Nahas, H.H.; Abdel-Wahab, B.A.; Helmy, Y.A.; Alshawwa, S.Z.; Saied, E.M. Acetylsalicylic Acid Suppresses Alcoholism-Induced Cognitive Impairment Associated with Atorvastatin Intake by Targeting Cerebral MiRNA155 and NLRP3: In Vivo, and In Silico Study. Pharmaceutics 2022, 14, 529. [Google Scholar] [CrossRef]
- Mohamed, D.I.; Ezzat, S.F.; Elayat, W.M.; El-Kharashi, O.A.; El-Kareem, H.F.A.; Nahas, H.H.A.; Abdel-Wahab, B.A.; Alshawwa, S.Z.; Saleh, A.; Helmy, Y.A.; et al. Hepatoprotective Role of Carvedilol against Ischemic Hepatitis Associated with Acute Heart Failure via Targeting MiRNA-17 and Mitochondrial Dynamics-Related Proteins: An In Vivo and In Silico Study. Pharmaceuticals 2022, 15, 832. [Google Scholar] [CrossRef]
- Mohamed, D.I.; Abou-Bakr, D.A.; Ezzat, S.F.; El-Kareem, H.F.A.; Nahas, H.H.A.; Saad, H.A.; Mehana, A.E.; Saied, E.M. Vitamin D3 Prevents the Deleterious Effects of Testicular Torsion on Testis by Targeting MiRNA-145 and ADAM17: In Silico and In Vivo Study. Pharmaceuticals 2021, 14, 1222. [Google Scholar] [CrossRef]
- Al-Abkal, F.; Abdel-Wahab, B.A.; El-Kareem, H.F.A.; Moustafa, Y.M.; Khodeer, D.M. Protective Effect of Pycnogenol against Methotrexate-Induced Hepatic, Renal, and Cardiac Toxicity: An In Vivo Study. Pharmaceuticals 2022, 15, 674. [Google Scholar] [CrossRef]
- Manju, S.L.; Ethiraj, K.R.; Elias, G. Safer Anti-Inflammatory Therapy through Dual COX-2/5-LOX Inhibitors: A Structure-Based Approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar] [CrossRef]
- Sharaf, M.; Hamouda, H.; Shabana, S.; Khan, S.; Arif, M.; Rozan, H.; Abdalla, M.; Chi, Z.; Liu, C. Design of Lipid-Based Nanocarrier for Drug Delivery Has a Double Therapy for Six Common Pathogens Eradication. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126662. [Google Scholar] [CrossRef]
- Chen, X. Structure and Function Relationships of Exopolysaccharides Produced by Lactic Acid Bacteria. Available online: https://era.library.ualberta.ca/items/ee2c709d-daa8-4185-ae35-6f0e1f2fe23f (accessed on 25 May 2022).
- Rajoka, M.S.R.; Mehwish, H.M.; Hayat, H.F.; Hussain, N.; Sarwar, S.; Aslam, H.; Nadeem, A.; Shi, J. Characterization, the Antioxidant and Antimicrobial Activity of Exopolysaccharide Isolated from Poultry Origin Lactobacilli. Probiotics Antimicrob. Proteins 2019, 11, 1132–1142. [Google Scholar] [CrossRef]
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxid. Med. Cell. Longev. 2015, 2016, e5692852. [Google Scholar] [CrossRef]
- Dilna, S.V.; Surya, H.; Aswathy, R.G.; Varsha, K.K.; Sakthikumar, D.N.; Pandey, A.; Nampoothiri, K.M. Characterization of an Exopolysaccharide with Potential Health-Benefit Properties from a Probiotic Lactobacillus Plantarum RJF4. LWT Food Sci. Technol. 2015, 64, 1179–1186. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Yang, Y.; Zhao, A.; Yang, Z. Characterization and Bioactivities of an Exopolysaccharide Produced by Lactobacillus Plantarum YW32. Int. J. Biol. Macromol. 2015, 74, 119–126. [Google Scholar] [CrossRef]
- Vinothkanna, A.; Sathiyanarayanan, G.; Rai, A.K.; Mathivanan, K.; Saravanan, K.; Sudharsan, K.; Kalimuthu, P.; Ma, Y.; Sekar, S. Exopolysaccharide Produced by Probiotic Bacillus Albus DM-15 Isolated from Ayurvedic Fermented Dasamoolarishta: Characterization, Antioxidant, and Anticancer Activities. Front. Microbiol. 2022, 13, 832109. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M.; Arif, M.; Hamouda, H.I.; Khan, S.; Abdalla, M.; Shabana, S.; Rozan, H.E.; Khan, T.U.; Chi, Z.; Liu, C. Preparation, Urease Inhibition Mechanisms, and Anti-Helicobacter Pylori Activities of Hesperetin-7-Rhamnoglucoside. Curr. Res. Microb. Sci. 2022, 3, 100103. [Google Scholar] [CrossRef] [PubMed]
- Jenab, A.; Roghanian, R.; Emtiazi, G. Bacterial Natural Compounds with Anti-Inflammatory and Immunomodulatory Properties (Mini Review). Drug Des. Devel. Ther. 2020, 14, 3787–3801. [Google Scholar] [CrossRef] [PubMed]
- Soria-Mercado, I.E.; Villarreal-Gómez, L.J.; Rivas, G.G.; Sánchez, N.E.A. Bioactive Compounds from Bacteria Associated to Marine Algae; IntechOpen: London, UK, 2012; ISBN 978-953-51-0151-2. [Google Scholar]
- Li, M.; van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and Anti-Inflammatory Effects of Short Chain Fatty Acids on Immune and Endothelial Cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res. IJPR 2011, 10, 655–683. [Google Scholar]
- Sharaf, M.; Sewid, A.H.; Hamouda, H.I.; Elharrif, M.G.; El-Demerdash, A.S.; Alharthi, A.; Hashim, N.; Hamad, A.A.; Selim, S.; Alkhalifah, D.H.M.; et al. Rhamnolipid-Coated Iron Oxide Nanoparticles as a Novel Multitarget Candidate against Major Foodborne E. Coli Serotypes and Methicillin-Resistant S. Aureus. Microbiol. Spectr. 2022, 10, e0025022. [Google Scholar] [CrossRef]
- Gangalla, R.; Macha, B.; Kasarla, S.; Eerla, R.; Thampu, R.K. Anti-inflammatory activity of the exopolysaccharides (EPS) produced from polluted soil. Int. J. Pharm. Biol. Sci. 2018, 8, 623–631. [Google Scholar]
- Di, W.; Zhang, L.; Yi, H.; Han, X.; Zhang, Y.; Xin, L. Exopolysaccharides Produced by Lactobacillus Strains Suppress HT-29 Cell Growth via Induction of G0/G1 Cell Cycle Arrest and Apoptosis. Oncol. Lett. 2018, 16, 3577–3586. [Google Scholar] [CrossRef]
- Sungur, T.; Aslim, B.; Karaaslan, C.; Aktas, B. Impact of Exopolysaccharides (EPSs) of Lactobacillus Gasseri Strains Isolated from Human Vagina on Cervical Tumor Cells (HeLa). Anaerobe 2017, 47, 137–144. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Ye, L.; Wang, C. The Anti-Cancer Effects and Mechanisms of Lactic Acid Bacteria Exopolysaccharides in Vitro: A Review. Carbohydr. Polym. 2021, 253, 117308. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Characterization, Anti-Inflammatory and Antiproliferative Activities of Natural and Sulfonated Exo-Polysaccharides from Streptococcus Thermophilus ASCC 1275. J. Food Sci. 2016, 81, M1167–M1176. [Google Scholar] [CrossRef]
- Zaki, A.G.; El-Sayed, E.-S.R.; Abd Elkodous, M.; El-Sayyad, G.S. Microbial Acetylcholinesterase Inhibitors for Alzheimer’s Therapy: Recent Trends on Extraction, Detection, Irradiation-Assisted Production Improvement and Nano-Structured Drug Delivery. Appl. Microbiol. Biotechnol. 2020, 104, 4717–4735. [Google Scholar] [CrossRef]
- Zhu, B.; Li, Z.; Qian, P.-Y.; Herrup, K. Marine Bacterial Extracts as a New Rich Source of Drugs against Alzheimer’s Disease. J. Neurochem. 2020, 152, 493–508. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; Abdel-Fattah, A.-F.M.; Saied, E.M.; El-Seedi, H.R.; Abdel-Daim, M.M. Acrylamide-Induced Peripheral Neuropathy: Manifestations, Mechanisms, and Potential Treatment Modalities. Environ. Sci. Pollut. Res. 2021, 28, 13031–13046. [Google Scholar] [CrossRef]
- Almasi, F.; Mohammadipanah, F.; Adhami, H.-R.; Hamedi, J. Introduction of Marine-Derived Streptomyces sp. UTMC 1334 as a Source of Pyrrole Derivatives with Anti-Acetylcholinesterase Activity. J. Appl. Microbiol. 2018, 125, 1370–1382. [Google Scholar] [CrossRef]
- Drukarch, B.; Schepens, E.; Stoof, J.C.; Langeveld, C.H.; Van Muiswinkel, F.L. Astrocyte-Enhanced Neuronal Survival Is Mediated by Scavenging of Extracellular Reactive Oxygen Species. Free Radic. Biol. Med. 1998, 25, 217–220. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Tan, V.; Heng, B.; Mohammadipanah, F.; Guillemin, G.J. Protective Effects of Myxobacterial Extracts on Hydrogen Peroxide-Induced Toxicity on Human Primary Astrocytes. Neuroscience 2019, 399, 106–117. [Google Scholar] [CrossRef]
- Yildiz, H.; Karatas, N. Microbial Exopolysaccharides: Resources and Bioactive Properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshawwa, S.Z.; Alshallash, K.S.; Ghareeb, A.; Elazzazy, A.M.; Sharaf, M.; Alharthi, A.; Abdelgawad, F.E.; El-Hossary, D.; Jaremko, M.; Emwas, A.-H.; et al. Assessment of Pharmacological Potential of Novel Exopolysaccharide Isolated from Marine Kocuria sp. Strain AG5: Broad-Spectrum Biological Investigations. Life 2022, 12, 1387. https://doi.org/10.3390/life12091387
Alshawwa SZ, Alshallash KS, Ghareeb A, Elazzazy AM, Sharaf M, Alharthi A, Abdelgawad FE, El-Hossary D, Jaremko M, Emwas A-H, et al. Assessment of Pharmacological Potential of Novel Exopolysaccharide Isolated from Marine Kocuria sp. Strain AG5: Broad-Spectrum Biological Investigations. Life. 2022; 12(9):1387. https://doi.org/10.3390/life12091387
Chicago/Turabian StyleAlshawwa, Samar Zuhair, Khalid S. Alshallash, Ahmed Ghareeb, Ahmed M. Elazzazy, Mohamed Sharaf, Afaf Alharthi, Fathy Elsayed Abdelgawad, Dalia El-Hossary, Mariusz Jaremko, Abdul-Hamid Emwas, and et al. 2022. "Assessment of Pharmacological Potential of Novel Exopolysaccharide Isolated from Marine Kocuria sp. Strain AG5: Broad-Spectrum Biological Investigations" Life 12, no. 9: 1387. https://doi.org/10.3390/life12091387
APA StyleAlshawwa, S. Z., Alshallash, K. S., Ghareeb, A., Elazzazy, A. M., Sharaf, M., Alharthi, A., Abdelgawad, F. E., El-Hossary, D., Jaremko, M., Emwas, A.-H., & Helmy, Y. A. (2022). Assessment of Pharmacological Potential of Novel Exopolysaccharide Isolated from Marine Kocuria sp. Strain AG5: Broad-Spectrum Biological Investigations. Life, 12(9), 1387. https://doi.org/10.3390/life12091387











