Effects of Cadmium Stress on Root and Root Border Cells of Some Vegetable Species with Different Types of Root Meristem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sterilization and Germination of the Seeds
2.3. Measure the Number and Viability of RBCs
2.4. Treatments
2.5. Measurement of Root Length, Malondialdehyde (MDA), and Cd Content
2.6. Data Processing
3. Results
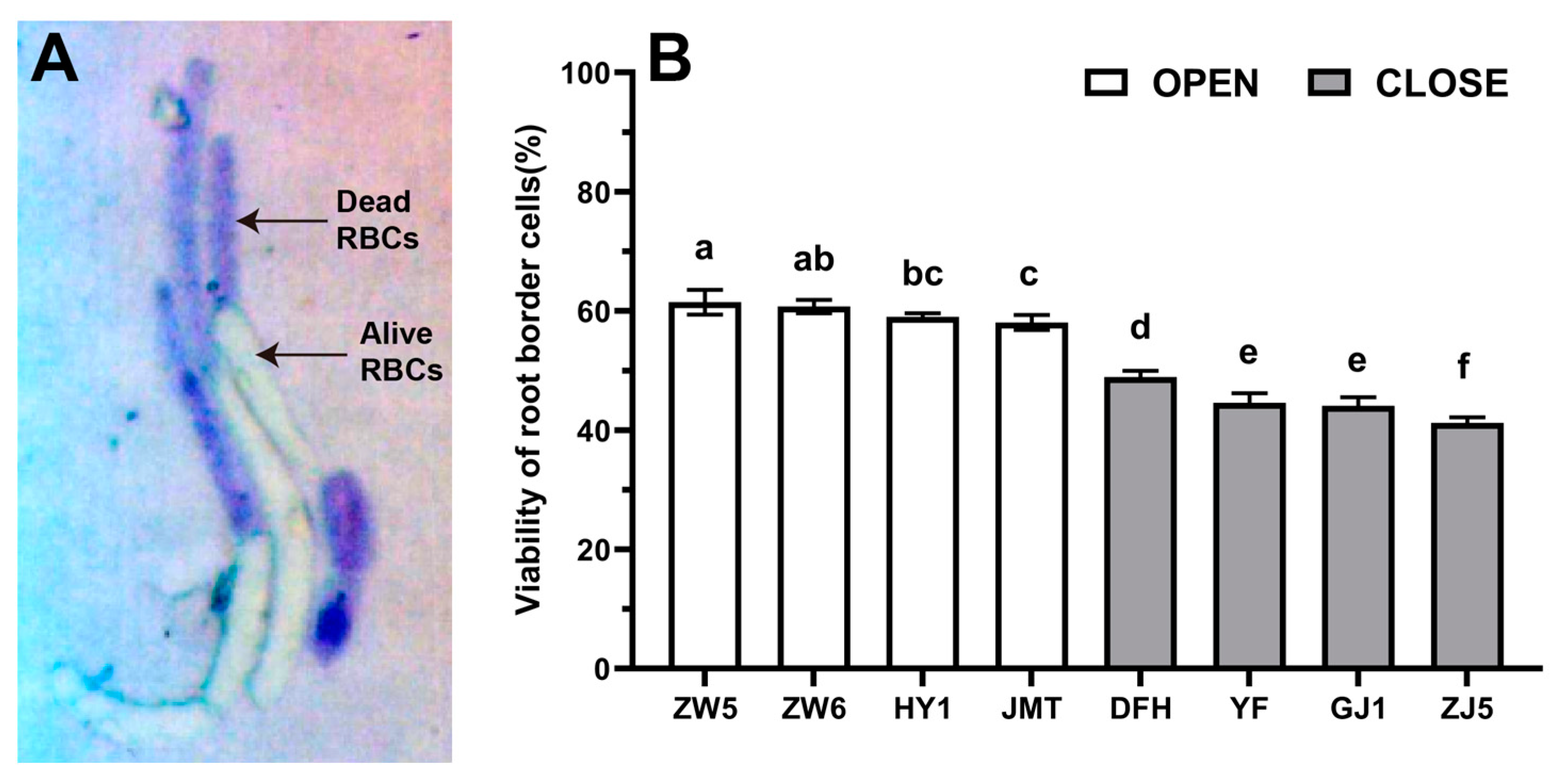
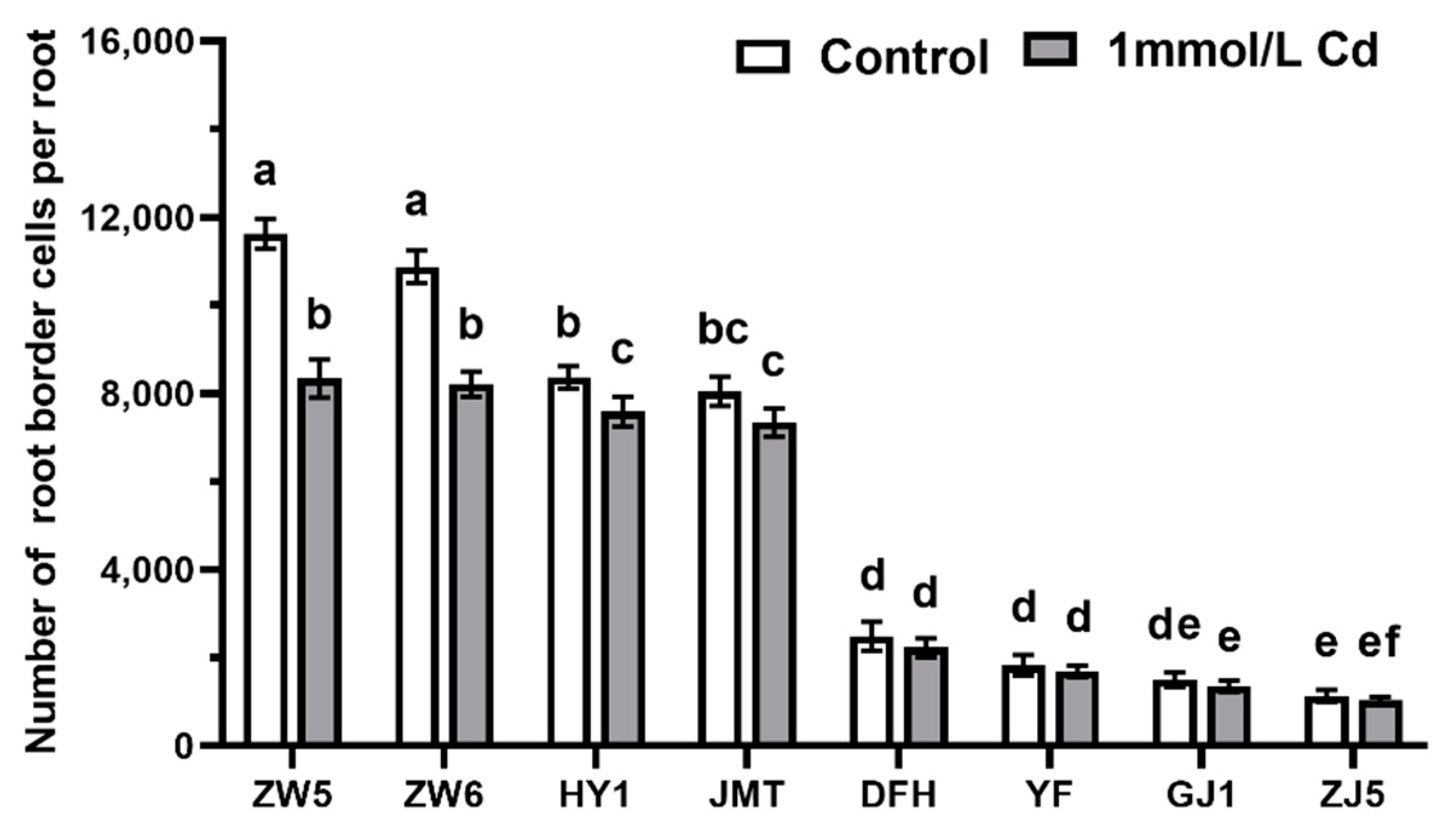
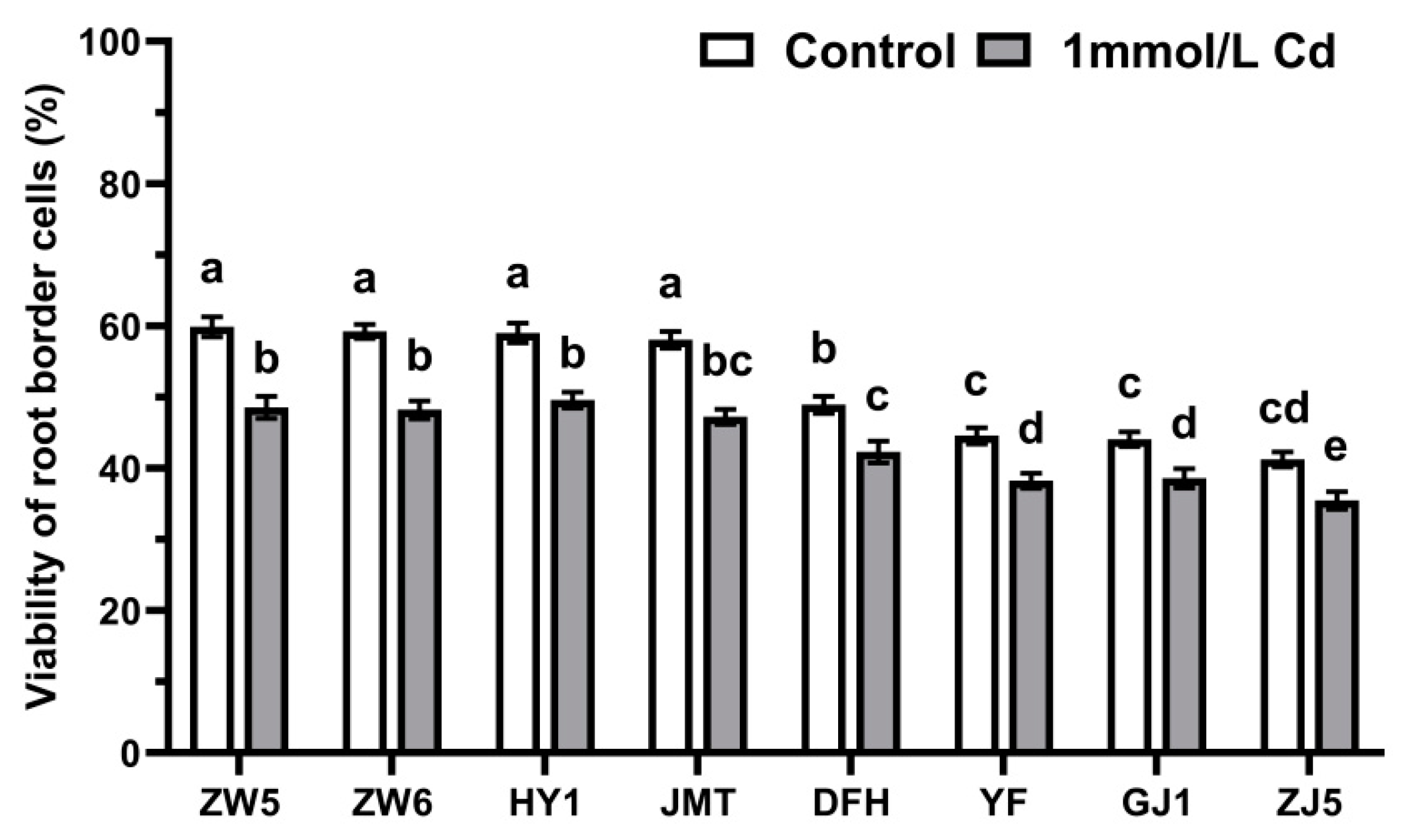
3.1. Differences in the Quantity and Viability of RBCs among Different Vegetable Species
3.2. Effects of Cadmium Treatment on the Growth of Root-Tip of Different Vegetables
3.3. The Effects of Cd Treatments on MDA Contents in Roots of Vegetables
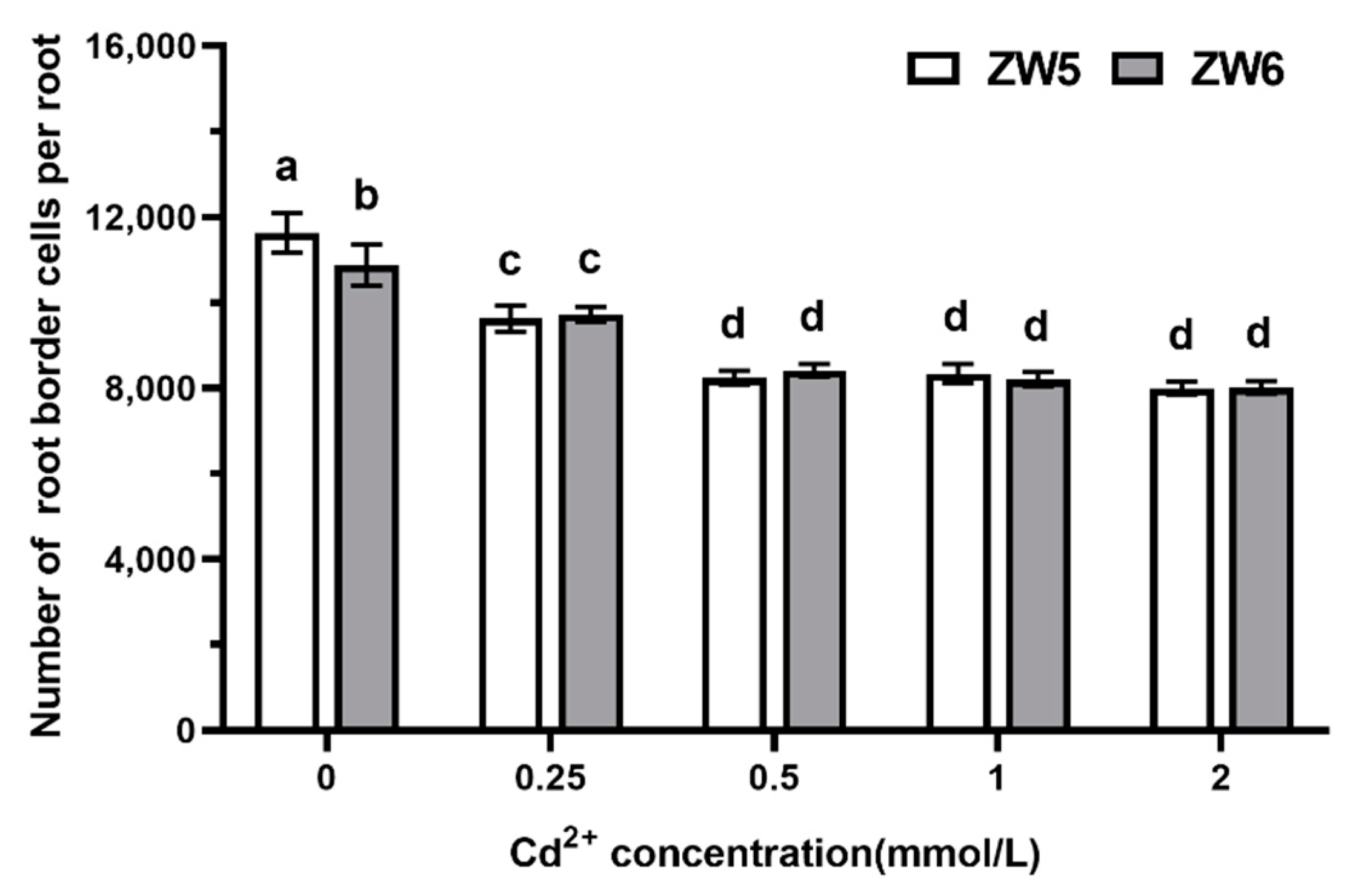
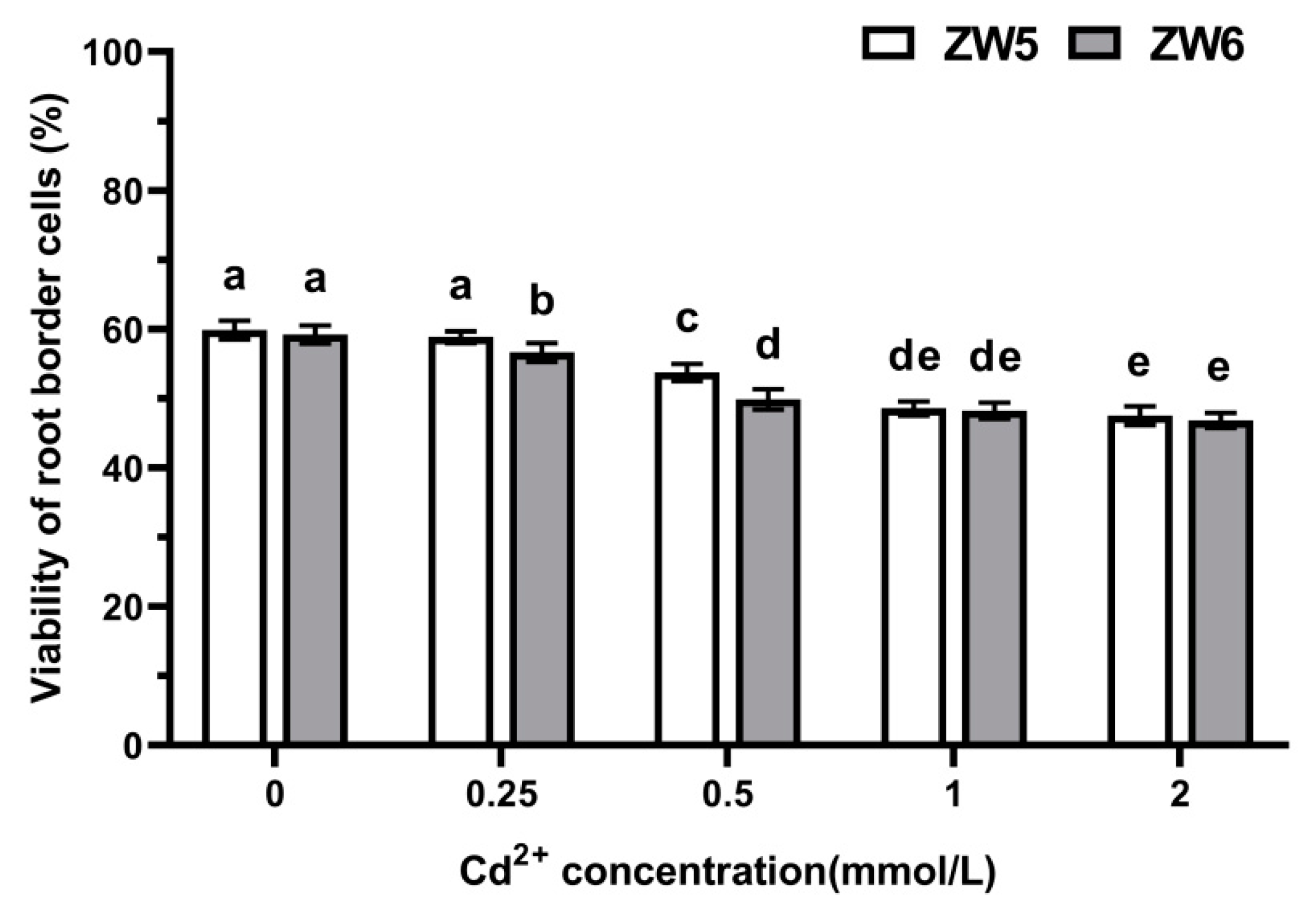
3.4. The Effects of Cd Concentrations on the Number of RBCs of Pea
3.5. The Response of RBCs to Cadmium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kah, M.; Levy, L.; Brown, C. Potential for effects of land contamination on human health. 1. The case of cadmium. J. Toxicol. Environ. Health Part B Crit. Rev. 2012, 15, 348–363. [Google Scholar] [CrossRef]
- Shimpei, U.; Shinsuke, M.; Masato, K.; Akira, K.; Tomohito, A.; Satoru, I. Root-to-shoot-Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 2009, 60, 2677–2688. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, V.; Bose, B.; Singhal, R.K. Cadmium toxicity in plants and alleviation through seed priming approach. Plant Physiol. Rep. 2021, 26, 647–660. [Google Scholar] [CrossRef]
- Liu, Z.; He, X.; Chen, W.; Zhao, M. Ecotoxicological responses of three ornamental herb species to cadmium. Environ. Toxicol. Chem. 2013, 32, 1746–1751. [Google Scholar] [CrossRef]
- Dalcorso, G.; Farinati, S.; Furini, A. Regulatory networks of cadmium stress in plants. Plant Signal. Behav. 2010, 5, 663–667. [Google Scholar] [CrossRef]
- Groot, E.P.; Doyle, J.A.; Nichol, S.A.; Rost, T.L. Phylogenetic distribution and evolution of root apical meristem organization in dicotyledonous angiosperms. Int. J. Plant Sci. 2004, 165, 97–105. [Google Scholar] [CrossRef]
- Feng, Y.M.; Chen, X.Y.; Li, X.W.; Li, Y.L.; Nong, W.; Tang, J.; Han, H.X.; Shi, L.; Shabala, S.; Yu, M. Root border cells as a convenient single cell system to study plant-environmental interactions: A case study for aluminum tolerance. Front. Soil Sci. 2022, 2, 909530. [Google Scholar] [CrossRef]
- Ninmanont, P.; Wongchai, C.; Pfeiffer, W.; Chaidee, A. Salt stress of two rice varieties: Root border cell response and multi-logistic quantification. Protoplasma 2021, 258, 1119–1131. [Google Scholar] [CrossRef]
- Driouich, A.; Follet-Gueye, M.L.; Vicré-Gibouin, M.; Hawes, M. Root border cells and secretions as critical elements in plant host defense. Curr. Opin. Plant Biol. 2013, 16, 489–495. [Google Scholar] [CrossRef]
- Feng, Y.M.; Li, X.W.; Guo, S.X.; Chen, X.Y.; Chen, T.X.; He, Y.M.; Shabala, S.; Yu, M. Extracellular silica nanocoat formed by layer-by-layer (LBL) self-assembly confers aluminum resistance in root border cells of pea (Pisum sativum). J. Nanobiotechnol. 2019, 17, 53. [Google Scholar] [CrossRef]
- Hawes, M.C.; Gunawardena, U.; Miyasaka, S. The role of root border cells in plant defense. Trends Plant Sci. 2000, 5, 128–133. [Google Scholar] [CrossRef]
- Li, X.W.; Li, Y.L.; Mai, J.W.; Tao, L.; Qu, M.; Liu, J.Y.; Shen, R.F.; Xu, G.L.; Feng, Y.M.; Xiao, H.D.; et al. Boron alleviates aluminum toxicity by promoting root alkalization in transition zone via polar auxin transport. Plant Physiol. 2018, 177, 1254–1266. [Google Scholar] [CrossRef]
- Yu, M.; Feng, Y.M.; Goldbach, H.E. Mist culture for mass harvesting of root border cells: Aluminum effects. J. Plant Nutr. Soil Sci. 2006, 169, 670–674. [Google Scholar] [CrossRef]
- Ma, Q.J.; Sun, M.H.; Lu, J.; Zhu, X.P.; Gao, W.S.; Hao, Y.J. Ectopic expression of apple MDSUT2 gene influences development and abiotic stress resistance in tomato. Sci. Hortic. 2017, 220, 259–266. [Google Scholar] [CrossRef]
- Hamamoto, L.; Hawes, M.C.; Rost, T.L. The production and release of living root cap border cells is a function of root apical meristem type in dicotyledonous angiosperm plants. Ann. Bot. 2006, 97, 917–923. [Google Scholar] [CrossRef]
- Qin, S.Y.; Liu, H.G.; Nie, Z.J.; Rengel, Z.; Gao, W.; Li, C.; Zhao, P. Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: A review. Pedosphere 2020, 30, 13–25. [Google Scholar] [CrossRef]
- Ishikawa, S.; Suzuki, N.; Tanabata, S.; Ishii, S.; Shu, F. Real-time imaging and analysis of differences in cadmium dynamics in rice cultivars (Oryza sativa) using positron-emitting 107Cd tracer. BMC Plant Biol. 2012, 11, 172–183. [Google Scholar] [CrossRef]
- Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Méndez, A.A.; Gallego, S.M. Oxidative post translational modifications of proteins related to cell cycle are involved in cadmium toxicity in wheat seedlings. Plant Sci. 2012, 196, 1–7. [Google Scholar] [CrossRef]
- Fotiadis, E.; Lolas, P.C. Phytoremediation of Cd contaminated soil through certain weed and crop species. J. Agric. Sci. Technol. 2011, 1, 811–817. [Google Scholar] [CrossRef]
- Feng, Y.; Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Kosobryukhov, A.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Effects of iron oxide nanoparticles (Fe3O4) on growth, photosynthesis, antioxidant activity and distribution of mineral elements in wheat (Triticum aestivum) Plants. Plants 2022, 11, 1894. [Google Scholar] [CrossRef]
- Hussain, S.; Irfan, M.; Sattar, A.; Hussain, S.; Ullah, S.; Abbas, T.; Ur-Rehman, H.; Nawaz, F.; Al-Hashimi, A.; Elshikh, M.S.; et al. Alleviation of cadmium stress in wheat through the combined application of boron and biochar via regulating morpho-physiological and antioxidant defense mechanisms. Agronomy 2022, 12, 434. [Google Scholar] [CrossRef]
- Yu, M.; Shen, R.F.; Liu, J.Y.; Chen, R.F.; Xu, M.M.; Yang, Y.; Xiao, H.D.; Wang, H.Z.; Wang, H.Y.; Wang, C.Q. The role of root border cells of pea (Pisum sativum) in protection of roots from aluminum toxicity under mist culture. J. Plant Nutr. Soil Sci. 2009, 172, 528–534. [Google Scholar] [CrossRef]
- Nagayama, T.; Nakamura, A.; Yamaji, N.; Satoh, S.; Furukawa, J.; Iwai, H. Changes in the distribution of pectin in root border cells under aluminum stress. Front. Plant Sci. 2019, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Tamás, L.; Budíková, S.; Huttová, J.; Mistrík, I.; Šimonovičová, M.; Široká, B. Aluminum-induced cell death of barley-root border cells is corre lated with peroxidase- and oxalate oxidase-mediated hydrogen peroxide production. Plant Cell Rep. 2005, 24, 189–194. [Google Scholar] [CrossRef]
- Darshan, K.; Singh, J.; Yadav, S.; Venugopala, K.M.; Aggarwal, R. Root border cells: A pioneer’s of plant defense in rhizosphere. Indian J. Agric. Sci. 2020, 90, 14–19. [Google Scholar]
- Pan, J.W.; Zhu, M.Y.; Peng, H.Z.; Wang, L.L. Developmental regulation and biological functions of root border cells in higher plants. Acta Bot. Scinica 2002, 44, 1–8. [Google Scholar]

| Variety | Control | 0.25 mmol/L CdCl2 | 0.5 mmol/L CdCl2 | 1 mmol/L CdCl2 | 2 mmol/L CdCl2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Root Length (cm) | Root Length (cm) | RRL (%) | Root Length (cm) | RRL (%) | Root Length (cm) | RRL (%) | Root Length (cm) | RRL (%) | |
| ZW5 | 4.35 ± 0.08a | 4.29 ± 0.07a | 98.62 | 3.57 ± 0.05b | 82.07 | 2.73 ± 0.04c | 62.76 | 2.59 ± 0.04d | 59.54 |
| ZW6 | 4.26 ± 0.06a | 3.94 ± 0.05b | 92.49 | 3.54 ± 0.06c | 83.10 | 2.57 ± 0.03d | 60.33 | 2.40 ± 0.04e | 56.34 |
| HY1 | 4.35 ± 0.05a | 3.88 ± 0.05b | 89.20 | 3.00 ± 0.03c | 68.97 | 2.71 ± 0.04d | 62.30 | 2.62 ± 0.03e | 60.23 |
| JMT | 3.94 ± 0.06a | 3.66 ± 0.04b | 92.89 | 2.87 ± 0.04c | 72.84 | 2.46 ± 0.03d | 62.44 | 2.32 ± 0.04e | 58.88 |
| DFH | 2.22 ± 0.03a | 1.85 ± 0.02b | 83.33 | 1.45 ± 0.03c | 65.32 | 1.28 ± 0.03d | 57.66 | 1.18 ± 0.02e | 53.15 |
| YF | 2.15 ± 0.07a | 1.80 ± 0.04b | 83.72 | 1.41 ± 0.03c | 65.58 | 1.29 ± 0.02d | 60.00 | 1.15 ± 0.03e | 53.49 |
| GJ1 | 3.03 ± 0.06a | 2.62 ± 0.07b | 86.47 | 2.25 ± 0.05c | 74.26 | 1.81 ± 0.07d | 59.74 | 1.64 ± 0.07e | 54.13 |
| ZJ5 | 1.52 ± 0.08a | 1.31 ± 0.06b | 86.18 | 1.14 ± 0.02c | 75.00 | 0.88 ± 0.06d | 57.89 | 0.82 ± 0.08e | 53.95 |
| Variety | Control | 0.25 mmol/L CdCl2 | 0.5 mmol/L CdCl2 | 1 mmol/L CdCl2 | 2 mmol/L CdCl2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| MDA Content (μmol/g·FW) | MDA Content (μmol/g·FW) | Relative Increasing Rate (%) | MDA Content (μmol/g·FW) | Relative Increasing Rate (%) | MDA Content (μmol/g·FW) | Relative Increasing Rate (%) | MDA Content (μmol/g·FW) | Relative Increasing Rate (%) | |
| ZW5 | 7.81 ± 0.94e | 9.68 ± 0.81d | 23.94 | 12.95 ± 0.73c | 65.81 | 18.78 ± 1.71a | 140.46 | 16.33 ± 1.68b | 109.09 |
| ZW6 | 7.63 ± 0.61e | 10.38 ± 0.78d | 36.04 | 12.96 ± 0.28c | 69.86 | 19.01 ± 0.48a | 149.15 | 17.52 ± 1.12b | 129.62 |
| HY1 | 5.89 ± 0.56e | 6.75 ± 0.86d | 14.60 | 9.86 ± 0.91c | 67.40 | 12.52 ± 0.21b | 112.56 | 14.32 ± 0.33a | 143.12 |
| JMT | 7.30 ± 0.24e | 10.54 ± 0.42d | 44.38 | 13.85 ± 0.53c | 89.73 | 16.69 ± 0.97b | 128.63 | 21.58 ± 0.81a | 195.62 |
| DFH | 5.69 ± 0.48e | 8.45 ± 0.17d | 48.51 | 10.47 ± 0.86c | 84.01 | 12.53 ± 0.75b | 120.21 | 15.56 ± 0.91a | 173.46 |
| YF | 4.95 ± 0.74e | 6.52 ± 0.15d | 31.72 | 10.55 ± 0.64c | 113.13 | 15.59 ± 0.66a | 214.95 | 14.19 ± 0.74b | 186.67 |
| GJ1 | 5.56 ± 0.45d | 7.56 ± 0.14c | 35.97 | 9.62 ± 0.81bc | 73.02 | 15.66 ± 0.87a | 181.65 | 10.64 ± 0.15b | 91.37 |
| ZJ5 | 3.40 ± 0.51e | 4.47 ± 0.44d | 31.47 | 6.50 ± 0.72c | 91.18 | 7.51 ± 0.71b | 120.88 | 8.54 ± 0.53a | 151.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Li, H.; Zhang, X.; Li, X.; Zhang, J.; Shi, L.; Chen, X.; Nong, W.; Wang, C.; Shabala, S.; et al. Effects of Cadmium Stress on Root and Root Border Cells of Some Vegetable Species with Different Types of Root Meristem. Life 2022, 12, 1401. https://doi.org/10.3390/life12091401
Feng Y, Li H, Zhang X, Li X, Zhang J, Shi L, Chen X, Nong W, Wang C, Shabala S, et al. Effects of Cadmium Stress on Root and Root Border Cells of Some Vegetable Species with Different Types of Root Meristem. Life. 2022; 12(9):1401. https://doi.org/10.3390/life12091401
Chicago/Turabian StyleFeng, Yingming, Huanxiu Li, Xianshi Zhang, Xuewen Li, Jie Zhang, Lei Shi, Xingyun Chen, Wei Nong, Changquan Wang, Sergey Shabala, and et al. 2022. "Effects of Cadmium Stress on Root and Root Border Cells of Some Vegetable Species with Different Types of Root Meristem" Life 12, no. 9: 1401. https://doi.org/10.3390/life12091401
APA StyleFeng, Y., Li, H., Zhang, X., Li, X., Zhang, J., Shi, L., Chen, X., Nong, W., Wang, C., Shabala, S., & Yu, M. (2022). Effects of Cadmium Stress on Root and Root Border Cells of Some Vegetable Species with Different Types of Root Meristem. Life, 12(9), 1401. https://doi.org/10.3390/life12091401









