HER2 in Metastatic Colorectal Cancer: Pathology, Somatic Alterations, and Perspectives for Novel Therapeutic Schemes
Abstract
:Simple Summary
Abstract
1. Introduction
2. HER2 Targeting in Metastatic Colorectal Cancer
3. Spectrum and Heterogeneity of HER2 Expression in Colorectal Cancer
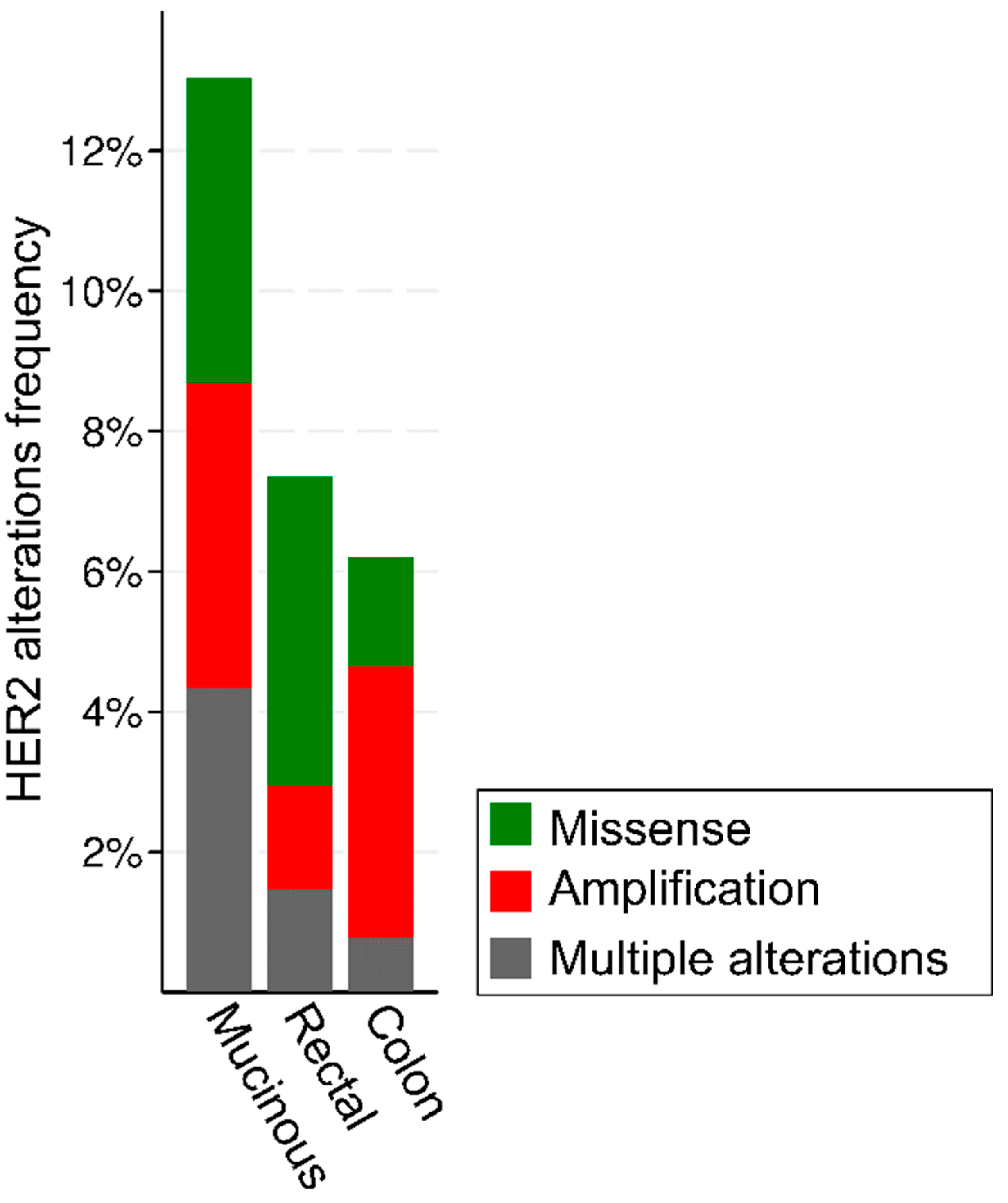
4. Genetics and Actionable Mutations in HER2-Positive Colorectal Cancer
5. Perspectives for Individualized Therapeutic Schemes
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaye, E.; Penel, N.; Lebellec, L. Novel treatment approaches for HER2 positive solid tumors (excluding breast cancer). Curr. Opin. Oncol. 2022, 34, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chowdhuri, S.; Davies, K.D.; Ritterhouse, L.L.; Snow, A.N. ERBB2 (HER2) Alterations in Colorectal Cancer. J. Mol. Diagn. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Miao, J.; Wen, Y.; Xia, X.; Chen, Y.; Huang, M.; Chen, S.; Zhao, Z.; Zhang, Y.; Chen, C.; et al. Molecular Landscape of ERBB2 Alterations in 14,956 Solid Tumors. Pathol. Oncol. Res. POR 2022, 28, 1610360. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Bang, Y.-J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Vranić, S.; Bešlija, S.; Gatalica, Z. Targeting HER2 expression in cancer: New drugs and new indications. Bosn. J. Basic Med. Sci. 2021, 21, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Seo, A.N.; Kwak, Y.; Kim, D.W.; Kang, S.B.; Choe, G.; Kim, W.H.; Lee, H.S. HER2 status in colorectal cancer: Its clinical significance and the relationship between HER2 gene amplification and expression. PLoS ONE 2014, 9, e98528. [Google Scholar] [CrossRef]
- Fusco, N.; Bosari, S. HER2 aberrations and heterogeneity in cancers of the digestive system: Implications for pathologists and gastroenterologists. World J. Gastroenterol. 2016, 22, 7926–7937. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kavuri, S.M.; Jain, N.; Galimi, F.; Cottino, F.; Leto, S.M.; Migliardi, G.; Searleman, A.C.; Shen, W.; Monsey, J.; Trusolino, L.; et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015, 5, 832–841. [Google Scholar] [CrossRef] [PubMed]
- El-Deiry, W.S.; Vijayvergia, N.; Xiu, J.; Scicchitano, A.; Lim, B.; Yee, N.S.; Harvey, H.A.; Gatalica, Z.; Reddy, S. Molecular profiling of 6892 colorectal cancer samples suggests different possible treatment options specific to metastatic sites. Cancer Biol. Ther. 2015, 16, 1726–1737. [Google Scholar] [CrossRef] [Green Version]
- Afrăsânie, V.A.; Marinca, M.V.; Alexa-Stratulat, T.; Gafton, B.; Păduraru, M.; Adavidoaiei, A.M.; Miron, L.; Rusu, C. KRAS, NRAS, BRAF, HER2 and microsatellite instability in metastatic colorectal cancer—Practical implications for the clinician. Radiol. Oncol. 2019, 53, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef]
- Dienstmann, R.; Ciner, A.; Hochster, H.S. Should next-generation sequencing testing be routinely used in metastatic colorectal cancer? Lancet Oncol. 2018, 19, 1434–1435. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef]
- Henry, N.L.; Somerfield, M.R.; Dayao, Z.; Elias, A.; Kalinsky, K.; McShane, L.M.; Moy, B.; Park, B.H.; Shanahan, K.M.; Sharma, P.; et al. Biomarkers for Systemic Therapy in Metastatic Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, JCO2201063. [Google Scholar] [CrossRef]
- Bartley, A.N.; Washington, M.K.; Colasacco, C.; Ventura, C.B.; Ismaila, N.; Benson, A.B.; Carrato, A.; Gulley, M.L.; Jain, D.; Kakar, S.; et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 2017, 35, 446–464. [Google Scholar] [CrossRef]
- Djaballah, S.A.; Daniel, F.; Milani, A.; Ricagno, G.; Lonardi, S. HER2 in colorectal cancer: The long and winding road from negative predictive factor to positive actionable target. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 219–232. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Chae, Y.S.; Lee, K.S.; et al. Trastuzumab deruxtecan (T-DXd) versus treatment of physician’s choice (TPC) in patients (pts) with HER2-low unresectable and/or metastatic breast cancer (mBC): Results of DESTINY-Breast04, a randomized, phase 3 study. J. Clin. Oncol. 2022, 40, LBA3. [Google Scholar] [CrossRef]
- Clark, J.W.; Niedzwiecki, D.; Hollis, D.M.R. Phase-II trial of 5-fluororuacil (5-FU), leucovorin (LV), oxaliplatin (Ox), and trastuzamab (T) for patients with metastatic colorectal cancer (CRC) refractory to initial therapy. Onkologie 2003, 26, 13–46. [Google Scholar]
- Ramanathan, R.K.; Hwang, J.J.; Zamboni, W.C.; Sinicrope, F.A.; Safran, H.; Wong, M.K.; Earle, M.; Brufsky, A.; Evans, T.; Troetschel, M.; et al. Low overexpression of HER-2/neu in advanced colorectal cancer limits the usefulness of trastuzumab (Herceptin) and irinotecan as therapy. A phase II trial. Cancer Investig. 2004, 22, 858–865. [Google Scholar] [CrossRef]
- Siena, S.; Sartore-Bianchi, A.; Marsoni, S.; Hurwitz, H.I.; McCall, S.J.; Penault-Llorca, F.; Srock, S.; Bardelli, A.; Trusolino, L. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann. Oncol. 2018, 29, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Tosi, F.; Sartore-Bianchi, A.; Lonardi, S.; Amatu, A.; Leone, F.; Ghezzi, S.; Martino, C.; Bencardino, K.; Bonazzina, E.; Bergamo, F.; et al. Long-term Clinical Outcome of Trastuzumab and Lapatinib for HER2-positive Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2020, 19, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Lonardi, S.; Martino, C.; Fenocchio, E.; Tosi, F.; Ghezzi, S.; Leone, F.; Bergamo, F.; Zagonel, V.; Ciardiello, F.; et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: The phase II HERACLES-B trial. ESMO Open 2020, 5, e000911. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef]
- Nakamura, Y.; Okamoto, W.; Kato, T.; Esaki, T.; Kato, K.; Komatsu, Y.; Yuki, S.; Masuishi, T.; Nishina, T.; Ebi, H.; et al. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: A phase 2 trial. Nat. Med. 2021, 27, 1899–1903. [Google Scholar] [CrossRef]
- Gupta, M.; Wang, B.; Carrothers, T.J.; LoRusso, P.M.; Chu, Y.W.; Shih, T.; Loecke, D.; Joshi, A.; Saad, O.; Yi, J.H.; et al. Effects of Trastuzumab Emtansine (T-DM1) on QT Interval and Safety of Pertuzumab Plus T-DM1 in Patients with Previously Treated Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. Clin. Pharmacol. Drug Dev. 2013, 2, 11–24. [Google Scholar] [CrossRef]
- Yoshino, T.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Wainberg, Z.; Elez, E.; et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing metastatic colorectal cancer (mCRC): Final results from a phase 2, multicenter, open-label study (DESTINY-CRC01). In Proceedings of the 2021 ASCO Annual Meeting, Virtual, 4–8 June 2021. [Google Scholar]
- Strickler, J.H.; Zemla, T.; Ou, F.S.; Cercek, A.; Wu, C.; Sanchez, F.A.; Hubbard, J.; Jaszeeski, B.; Bandel, L.; Schweitzer, B.; et al. Trastuzumab and tucatinib for the treatment of HER2 amplified metastatic colorectal cancer (mCRC): Initial results from the MOUNTAINEER trial. In Proceedings of the ESMO Annual Meeting, Barcelona, Spain, 27 September–1 October 2019. [Google Scholar]
- Li, X.; Yang, C.; Wan, H.; Zhang, G.; Feng, J.; Zhang, L.; Chen, X.; Zhong, D.; Lou, L.; Tao, W.; et al. Discovery and development of pyrotinib: A novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur. J. Pharm. Sci. 2017, 110, 51–61. [Google Scholar] [CrossRef]
- Li, W.; Chang, J.; Xu, M.; Zhu, X. Anti-HER2 therapy with pyrotinib and trastuzumab in refractory HER2-positive metastatic colorectal cancer: A preliminary report from HER2-FUSCC-G study. In Proceedings of the 2022 ASCO Gastrointestinal Cancers Symposium, San Francisco, CA, USA, 20–22 January 2022. [Google Scholar]
- Jacobs, S.A.; Lee, J.J.; George, T.J.; Wade, J.L., III; Stella, P.J.; Wang, D.; Sama, A.R.; Piette, F.; Pogue-Geile, K.L.; Kim, R.S.; et al. Neratinib-Plus-Cetuximab in Quadruple-WT (KRAS, NRAS, BRAF, PIK3CA) Metastatic Colorectal Cancer Resistant to Cetuximab or Panitumumab: NSABP FC-7, A Phase Ib Study. Clin. Cancer Res. 2021, 27, 1612–1622. [Google Scholar] [CrossRef]
- Bitar, L.; Zouein, J.; Haddad, F.G.; Eid, R.; Kourie, H.R. HER2 in metastatic colorectal cancer: A new to target to remember. Biomark Med. 2021, 15, 133–136. [Google Scholar] [CrossRef]
- Nazemalhosseini Mojarad, E.; Kuppen, P.J. HER2 and immunotherapy using monoclonal antibodies in colorectal cancer. Immunotherapy 2013, 5, 1267–1269. [Google Scholar] [CrossRef]
- Shi, Y.; Fan, X.; Meng, W.; Deng, H.; Zhang, N.; An, Z. Engagement of immune effector cells by trastuzumab induces HER2/ERBB2 downregulation in cancer cells through STAT1 activation. Breast Cancer Res. 2014, 16, R33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, X.; He, Z.; Qin, C.; Zhang, P.; Sui, C.; Deng, X.; Fang, Y.; Li, G.; Shi, J. Inhibition of PFKFB3 in HER2-positive gastric cancer improves sensitivity to trastuzumab by inducing tumour vessel normalisation. Br. J. Cancer 2022, 127, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.Z.; He, C.Y.; Yang, X.H.; Yang, L.Q.; Lin, J.Z.; Zhou, D.L.; Long, Y.K.; Guan, W.L.; Jin, Y.; Li, Y.H.; et al. Relationship of HER2 Alteration and Microsatellite Instability Status in Colorectal Adenocarcinoma. Oncologist 2021, 26, e1161–e1170. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Passardi, A.; Canale, M.; Valgiusti, M.; Ulivi, P. Immune Checkpoints as a Target for Colorectal Cancer Treatment. Int. J. Mol. Sci. 2017, 18, 1324. [Google Scholar] [CrossRef]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Sahin, I.H.; Akce, M.; Alese, O.; Shaib, W.; Lesinski, G.B.; El-Rayes, B.; Wu, C. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br. J. Cancer 2019, 121, 809–818. [Google Scholar] [CrossRef]
- Sharma, M.; Carvajal, R.D.; Hanna, G.J.; Li, B.T.; Moore, K.N.; Pegram, M.D.; Rasco, D.W.; Spira, A.I.; Alonso, M.; Fang, L.; et al. Preliminary results from a phase 1/2 study of BDC-1001, a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), in patients (pts) with advanced HER2-expressing solid tumors. In Proceedings of the ASCO Annual Meeting, Virtual, 4–8 June 2021. [Google Scholar]
- Lefler, D.S.; Snook, A.E.; Bashir, B. Immune checkpoint inhibitors in luminal gastrointestinal malignancies: Going beyond MSI-H/dMMR, TMB and PD-L1. Immunotherapy 2022, 14, 885–902. [Google Scholar] [CrossRef]
- Guarini, C.; Grassi, T.; Pezzicoli, G.; Porta, C. Beyond RAS and BRAF: HER2, a New Actionable Oncotarget in Advanced Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6813. [Google Scholar] [CrossRef]
- Sajjadi, E.; Venetis, K.; Ivanova, M.; Fusco, N. Improving HER2 testing reproducibility in HER2-low breast cancer. Cancer Drug Resist. 2022, 5, 882–888. [Google Scholar] [CrossRef]
- Fusco, N.; Rocco, E.G.; Del Conte, C.; Pellegrini, C.; Bulfamante, G.; Di Nuovo, F.; Romagnoli, S.; Bosari, S. HER2 in gastric cancer: A digital image analysis in pre-neoplastic, primary and metastatic lesions. Mod. Pathol. 2013, 26, 816–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocaña, A.; Amir, E.; Pandiella, A. HER2 heterogeneity and resistance to anti-HER2 antibody-drug conjugates. Breast Cancer Res. 2020, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.A. HER2 in Colorectal Carcinoma: Are We There yet? Surg. Pathol. Clin. 2020, 13, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Nakamura, Y.; Yamanaka, T.; Kuboki, Y.; Yamaguchi, D.; Yuki, S.; Yoshino, T.; Komatsu, Y.; Sakamoto, N.; Okamoto, W.; et al. Prognostic and Predictive Value of HER2 Amplification in Patients with Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2018, 17, 198–205. [Google Scholar] [CrossRef]
- Valtorta, E.; Martino, C.; Sartore-Bianchi, A.; Penaullt-Llorca, F.; Viale, G.; Risio, M.; Rugge, M.; Grigioni, W.; Bencardino, K.; Lonardi, S.; et al. Assessment of a HER2 scoring system for colorectal cancer: Results from a validation study. Mod. Pathol. 2015, 28, 1481–1491. [Google Scholar] [CrossRef]
- Ross, J.S.; Fakih, M.; Ali, S.M.; Elvin, J.A.; Schrock, A.B.; Suh, J.; Vergilio, J.A.; Ramkissoon, S.; Severson, E.; Daniel, S.; et al. Targeting HER2 in colorectal cancer: The landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer 2018, 124, 1358–1373. [Google Scholar] [CrossRef]
- Owen, D.R.; Wong, H.-L.; Bonakdar, M.; Jones, M.; Hughes, C.S.; Morin, G.B.; Jones, S.J.M.; Renouf, D.J.; Lim, H.; Laskin, J.; et al. Molecular characterization of ERBB2-amplified colorectal cancer identifies potential mechanisms of resistance to targeted therapies: A report of two instructive cases. Cold Spring Harb. Mol. Case Stud. 2018, 4, a002535. [Google Scholar] [CrossRef]
- Schmoll, H.-J. Targeting HER2: Precision oncology for colorectal cancer. Lancet Oncol. 2016, 17, 685–686. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Cenaj, O.; Ligon, A.H.; Hornick, J.L.; Sholl, L.M. Detection of ERBB2 Amplification by Next-Generation Sequencing Predicts HER2 Expression in Colorectal Carcinoma. Am. J. Clin. Pathol. 2019, 152, 97–108. [Google Scholar] [CrossRef]
- Fusco, N.; Malapelle, U.; Fassan, M.; Marchiò, C.; Buglioni, S.; Zupo, S.; Criscitiello, C.; Vigneri, P.; Dei Tos, A.P.; Maiorano, E.; et al. PIK3CA Mutations as a Molecular Target for Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Front Oncol. 2021, 11, 644737. [Google Scholar] [CrossRef] [PubMed]
- Fusco, N.; Sajjadi, E.; Venetis, K.; Gaudioso, G.; Lopez, G.; Corti, C.; Rocco, E.G.; Criscitiello, C.; Malapelle, U.; Invernizzi, M. PTEN Alterations and Their Role in Cancer Management: Are We Making Headway on Precision Medicine? Genes 2020, 11, 719. [Google Scholar] [CrossRef]
- Loree, J.M.; Bailey, A.M.; Johnson, A.M.; Yu, Y.; Wu, W.; Bristow, C.A.; Davis, J.S.; Shaw, K.R.; Broaddus, R.; Banks, K.C.; et al. Molecular Landscape of ERBB2/ERBB3 Mutated Colorectal Cancer. J. Natl. Cancer Inst. 2018, 110, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, E.; Venetis, K.; Piciotti, R.; Invernizzi, M.; Guerini-Rocco, E.; Haricharan, S.; Fusco, N. Mismatch repair-deficient hormone receptor-positive breast cancers: Biology and pathological characterization. Cancer Cell Int. 2021, 21, 266. [Google Scholar] [CrossRef]
- Piciotti, R.; Venetis, K.; Sajjadi, E.; Fusco, N. Mismatch Repair Status Characterization in Oncologic Pathology: Taking Stock of the Real-World Possibilities. J. Mol. Pathol. 2021, 2, 93–100. [Google Scholar] [CrossRef]
- Lopez, G.; Venetis, K.; Sajjadi, E.; Fusco, N. Mismatch Repair System Genomic Scars in Gastroesophageal Cancers: Biology and Clinical Testing. Gastrointest. Disord. 2020, 2, 341–352. [Google Scholar] [CrossRef]
- Corti, C.; Sajjadi, E.; Fusco, N. Determination of Mismatch Repair Status in Human Cancer and Its Clinical Significance: Does One Size Fit All? Adv. Anat. Pathol. 2019, 26, 270–279. [Google Scholar] [CrossRef]
- Zaidi, S.H.; Harrison, T.A.; Phipps, A.I.; Steinfelder, R.; Trinh, Q.M.; Qu, C.; Banbury, B.L.; Georgeson, P.; Grasso, C.S.; Giannakis, M.; et al. Landscape of somatic single nucleotide variants and indels in colorectal cancer and impact on survival. Nat. Commun. 2020, 11, 3644. [Google Scholar] [CrossRef]
- Siena, S.; Bardelli, A.; Sartore-Bianchi, A.; Martino, C.; Siravegna, G.; Magrì, A.; Leone, F.; Zagonel, V.; Lonardi, S.; Amatu, A.; et al. HER2 amplification as a ‘molecular bait’ for trastuzumab-emtansine (T-DM1) precision chemotherapy to overcome anti-HER2 resistance in HER2 positive metastatic colorectal cancer: The HERACLES-RESCUE trial. In Proceedings of the 2016 Gastrointestinal Cancers Symposium, San Francisco, CA, USA, 21–23 June 2016. [Google Scholar]
- Saude-Conde, R.; Rasschaert, G.; Bregni, G.; Hendlisz, A.; Sclafani, F. Mind the target: Human epidermal growth factor receptor 2 in colorectal cancer. Curr. Opin. Oncol. 2022, 34, 382–388. [Google Scholar] [CrossRef]
- Antonarelli, G.; Giugliano, F.; Corti, C.; Repetto, M.; Tarantino, P.; Curigliano, G. Research and Clinical Landscape of Bispecific Antibodies for the Treatment of Solid Malignancies. Pharmaceuticals 2021, 14, 884. [Google Scholar] [CrossRef]
- Corti, C.; Venetis, K.; Sajjadi, E.; Zattoni, L.; Curigliano, G.; Fusco, N. CAR-T cell therapy for triple-negative breast cancer and other solid tumors: Preclinical and clinical progress. Expert Opin. Investig. Drugs 2022, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Venetis, K.; Invernizzi, M.; Sajjadi, E.; Curigliano, G.; Fusco, N. Cellular immunotherapy in breast cancer: The quest for consistent biomarkers. Cancer Treat. Rev. 2020, 90, 102089. [Google Scholar] [CrossRef] [PubMed]
- Venetis, K.; Crimini, E.; Sajjadi, E.; Corti, C.; Guerini-Rocco, E.; Viale, G.; Curigliano, G.; Criscitiello, C.; Fusco, N. HER2 low, ultra-low, and novel complementary biomarkers: Expanding the spectrum of HER2 positivity in breast cancer. Front. Mol. Biosci. 2022, 9, 834651. [Google Scholar] [CrossRef] [PubMed]
- Angerilli, V.; Galuppini, F.; Pagni, F.; Fusco, N.; Malapelle, U.; Fassan, M. The Role of the Pathologist in the Next-Generation Era of Tumor Molecular Characterization. Diagnostics 2021, 11, 339. [Google Scholar] [CrossRef]
- Pisapia, P.; L’Imperio, V.; Galuppini, F.; Sajjadi, E.; Russo, A.; Cerbelli, B.; Fraggetta, F.; d’Amati, G.; Troncone, G.; Fassan, M.; et al. The evolving landscape of anatomic pathology. Crit. Rev. Oncol./Hematol. 2022, 178, 103776. [Google Scholar] [CrossRef]
- Greally, M.; Kelly, C.M.; Cercek, A. HER2: An emerging target in colorectal cancer. Curr. Probl. Cancer 2018, 42, 560–571. [Google Scholar] [CrossRef]


| Reference | Nr. of Patients | HER2 Overexpression (%) | Treatment | ORR (%) | mPFS | mOS |
|---|---|---|---|---|---|---|
| HERACLES-A | 32 | 22 (2+); 78 (3+) | trastuzumab + lapatinib | 28 | 4.7 | 10 |
| HERACLES-B | 31 | 20 (2+); 80 (3+) | pertuzumab + TDM-1 | 9.7 | 4.1 | - |
| MyPathway | 57 | 100 | trastuzumab + pertuzumab | 32 | 2.9 | 11.5 |
| TRIUMPH | 18 | 100 | trastuzumab + pertuzumab | 35 | 4 | - |
| TAPUR | 28 | 100 | trastuzumab + pertuzumab | 14 | 3.8 | - |
| DESTINY-CRC01 | 78 | A: 68 (3+/2+ IHC+); B: 9 (2+ IHC−); C: 23 (1+) | trastuzumab deruxtecan | A: 45.3; B/C: − | 6.3 - | 15.5 - |
| MOUNTAINEER (recruiting) | 26 | 100 | trastuzumab + tucatinib | 52.2 | 8.1 | 18.7 |
| HER2-FUSCC-G (recruiting) | 11 | 100 | Trastuzumab + pirotinib | 45.5 | 7.8 | 14.9 |
| Cancer Type | Drug | Level |
|---|---|---|
| NSCLC | Ado-Trastuzumab Emtansine | 2 |
| NSCLC | Trastuzumab Deruxtecan | 2 |
| NSCLC | Neratinib | 3A |
| NSCLC | Trastuzumab + Pertuzumab + Docetaxel | 3A |
| Breast cancer | Neratinib | 3A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, M.; Venetis, K.; Guerini-Rocco, E.; Bottiglieri, L.; Mastropasqua, M.G.; Garrone, O.; Fusco, N.; Ghidini, M. HER2 in Metastatic Colorectal Cancer: Pathology, Somatic Alterations, and Perspectives for Novel Therapeutic Schemes. Life 2022, 12, 1403. https://doi.org/10.3390/life12091403
Ivanova M, Venetis K, Guerini-Rocco E, Bottiglieri L, Mastropasqua MG, Garrone O, Fusco N, Ghidini M. HER2 in Metastatic Colorectal Cancer: Pathology, Somatic Alterations, and Perspectives for Novel Therapeutic Schemes. Life. 2022; 12(9):1403. https://doi.org/10.3390/life12091403
Chicago/Turabian StyleIvanova, Mariia, Konstantinos Venetis, Elena Guerini-Rocco, Luca Bottiglieri, Mauro Giuseppe Mastropasqua, Ornella Garrone, Nicola Fusco, and Michele Ghidini. 2022. "HER2 in Metastatic Colorectal Cancer: Pathology, Somatic Alterations, and Perspectives for Novel Therapeutic Schemes" Life 12, no. 9: 1403. https://doi.org/10.3390/life12091403
APA StyleIvanova, M., Venetis, K., Guerini-Rocco, E., Bottiglieri, L., Mastropasqua, M. G., Garrone, O., Fusco, N., & Ghidini, M. (2022). HER2 in Metastatic Colorectal Cancer: Pathology, Somatic Alterations, and Perspectives for Novel Therapeutic Schemes. Life, 12(9), 1403. https://doi.org/10.3390/life12091403










