The Interplay between Anti-Angiogenics and Immunotherapy in Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
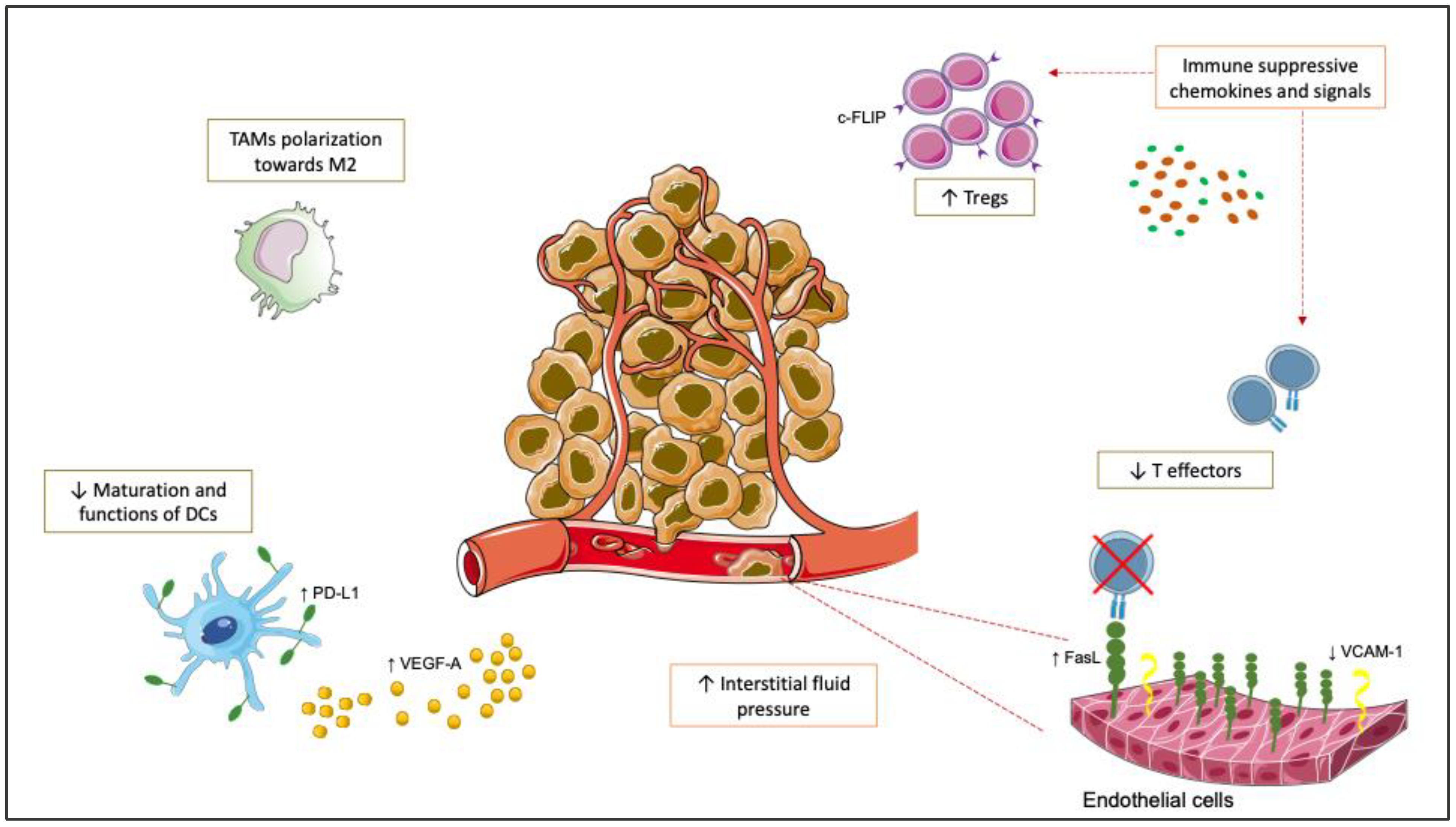
2. Rationale to Combine Anti-Angiogenics and ICIs in mCRC
3. Clinical Trials of Anti-Angiogenics Plus ICIs in mCRC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Ciardiello, F.; Ciardiello, D.; Martini, G.; Napolitano, S.; Tabernero, J.; Cervantes, A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J. Clin. 2022, 72, 372–401. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Saltz, L.B.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.S.; Rivera, F.; et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colo-rectal cancer: A randomized phase III study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef]
- Cremolini, C.; Marmorino, F.; Loupakis, F.; Masi, G.; Antoniotti, C.; Salvatore, L.; Schirripa, M.; Boni, L.; Zagonel, V.; Lonardi, S.; et al. all the investigators of the Gruppo Oncologico del Nord Ovest. TRIBE-2: A phase III, randomized, open-label, strategy trial in unresectable metastatic colorectal cancer patients by the GONO group. BMC Cancer 2017, 17, 408. [Google Scholar] [CrossRef]
- Hegewisch-Becker, S.; Graeven, U.; Lerchenmüller, C.A.; Killing, B.; Depenbusch, R.; Steffens, C.C.; Al-Batran, S.E.; Lange, T.; Dietrich, G.; Stoehlmacher, J.; et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): A randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 1355–1369. [Google Scholar] [CrossRef]
- Simkens, L.H.J.; van Tinteren, H.; May, A.; Tije, A.J.T.; Creemers, G.-J.M.; Loosveld, O.J.L.; E de Jongh, F.; Erdkamp, F.L.G.; Erjavec, Z.; E van der Torren, A.M.; et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): A phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet 2015, 385, 1843–1852. [Google Scholar] [CrossRef]
- Giantonio, B.J.; Catalano, P.J.; Meropol, N.J.; O'Dwyer, P.J.; Mitchell, E.P.; Alberts, S.R.; Schwartz, M.A.; Benson, A.B., III. Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leuco-vorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007, 25, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Bennouna, J.; Sastre, J.; Arnold, D.; Österlund, P.; Greil, R.; Van Cutsem, E.; von Moos, R.; Viéitez, J.M.; Bouché, O.; Borg, C.; et al. ML18147 Study Investigators. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 29–37. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; et al. RAISE Study Investigators. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with meta-static colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015, 16, 499–508, Erratum in: Lancet Oncol. 2015, 16, e262. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Pradeep, J.; Win, T.T.; Aye, S.N.; Sreeramareddy, C.T. Efficacy and Safety of Immune Checkpoint Inhibitors for Advanced Malignant Melanoma: A Meta-Analysis on Monotherapy Vs Combination Therapy. J. Cancer 2022, 13, 3091–3102. [Google Scholar] [CrossRef] [PubMed]
- Lavacchi, D.; Pellegrini, E.; Palmieri, V.E.; Doni, L.; Mela, M.M.; Di Maida, F.; Amedei, A.; Pillozzi, S.; Carini, M.; Antonuzzo, L. Immune Checkpoint Inhibitors in the Treatment of Renal Cancer: Current State and Future Perspective. Int. J. Mol. Sci. 2020, 21, 4691. [Google Scholar] [CrossRef]
- Maiorano, B.A.; De Giorgi, U.; Ciardiello, D.; Schinzari, G.; Cisternino, A.; Tortora, G.; Maiello, E. Immune-Checkpoint Inhibitors in Advanced Bladder Cancer: Seize the Day. Biomedicines 2022, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, B.A.; Maiorano, M.F.P.; Cormio, G.; Maglione, A.; Lorusso, D.; Maiello, E. How Immunotherapy Modified the Therapeutic Scenario of Endometrial Cancer: A Systematic Review. Front. Oncol. 2022, 12, 844801. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, D.; Guerrera, L.P.; Maiorano, B.A.; Parente, P.; Latiano, T.P.; Di Maio, M.; Ciardiello, F.; Troiani, T.; Martinelli, E.; Maiello, E. Immunotherapy in advanced anal cancer: Is the beginning of a new era? Cancer Treat Rev. 2022, 105, 102373. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, D.; Maiorano, B.A.; Parente, P.; Parente, P.; Pia Latiano, T.; Di Maio, M.; Ciardiello, F.; Troiani, T.; Martinelli, E.; Maiello, E. Immunotherapy for Biliary Tract Cancer in the Era of Precision Medicine: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 820. [Google Scholar] [CrossRef]
- Maiorano, B.A.; Maiorano, M.F.P.; Lorusso, D.; Maiello, E. Ovarian Cancer in the Era of Immune Checkpoint Inhibitors: State of the Art and Future Perspectives. Cancers 2021, 13, 4438. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Qin, C.; Hu, H.; Liu, T.; He, Y.; Guo, H.; Yan, H.; Zhang, J.; Tang, S.; Zhou, H. Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Progress, Challenges, and Prospects. Cells 2022, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Hause, R.J.; Pritchard, C.C.; Shendure, J.; Salipante, S.J. Classification and characterization of microsatellite instability across 18 cancer types. Nat. Med. 2016, 22, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Franco, R.; Facchini, G.; Addeo, R.; Ciardiello, F.; Berretta, M.; Zito Marin, F. Microsatellite Instability: From the Implementation of the Detection to a Prognostic and Predictive Role in Cancers. Int. J. Mol. Sci. 2022, 23, 8726. [Google Scholar] [CrossRef]
- Xiao, Y.; Freeman, G.J. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov. 2015, 5, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Drescher, K.M.; Sharma, P.; Watson, P.; Gatalica, Z.; Thibodeau, S.N.; Lynch, H.T. Lymphocyte recruitment into the tumor site is altered in patients with MSI-H colon cancer. Fam. Cancer 2009, 8, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.K.; Kim, T.W.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O'Neil, B.; Kavan, P.; Yoshino, T.; et al. KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Lenz, H.J.; Van Cutsem, E.; Luisa Limon, M.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, D.; Vitiello, P.P.; Cardone, C.; Martini, G.; Troiani, T.; Martinelli, E.; Ciardiello, F. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat. Rev. 2019, 76, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Hurwitz, H. Combinations of Bevacizumab with Cancer Immunotherapy. Cancer J. 2018, 24, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Dvorak, H.F. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002, 20, 4368–4380. [Google Scholar] [CrossRef]
- Fang, J.; Yan, L.; Shing, Y.; Moses, M.A. HIF–1alpha–mediated up–regulation of vascular endothelial growth factor, independent of basic fibroblast growth factor, is important in the switch to the angiogenic phenotype during early tumorigenesis. Cancer Res. 2001, 61, 5731–5735. [Google Scholar]
- Ramjiawan, R.R.; Griffioen, A.W.; Duda, D.G. Anti–angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017, 20, 185–204. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Chen, H.L.; Girgis, K.R.; Cunningham, H.T.; Meny, G.M.; Nadaf, S.; Kavanaugh, D.; Carbone, D.P. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996, 2, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Wei, S.; Dong, H.; Alvarez, X.; Cheng, P.; Mottram, P.; Krzysiek, R.; Knutson, K.L.; Daniel, B.; Zimmermann, M.C.; et al. Blockade of B7–H1 improves myeloid dendritic cell–mediated antitumor immunity. Nat. Med. 2003, 9, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Jiao, D.; Qin, S.; Chu, Q.; Wu, K.; Li, A. Synergistic effect of immune checkpoint blockade and anti–angiogenesis in cancer treatment. Mol. Cancer 2019, 18, 60. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Facciabene, A.; Peng, X.; Hagemann, I.S.; Balint, K.; Barchetti, A.; Wang, L.P.; Gimotty, P.A.; Gilks, C.B.; Lal, P.; Zhang, L.; et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011, 475, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Bendell, J.C.; Powderly, J.D.; Lieu, C.H.; Eckhardt, S.G.; Hurwitz, H.; Hochster, H.S.; Murphy, J.E.; Funke, R.P.; Rossi, C.; Wallin, J.; et al. Safety and efficacy of MPDL3280A (anti–PD–L1) in combination with bevacizumab (bev) and/or FOLFOX in patients (pts) with metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2015, 33, 704. [Google Scholar] [CrossRef]
- Tabernero, J.; Grothey, A.; Arnold, D.; de Gramont, A.; Ducreux, M.; O’Dwyer, P.; Tahiri, A.; Gilberg, F.; Irahara, N.; Schmoll, H.-J.; et al. MODUL cohort 2: An adaptable, randomized, signal-seeking trial of fluoropyrimidine plus bevacizumab with or without atezolizumab maintenance therapy for BRAF metastatic colorectal cancer. ESMO Open 2022, 7, 100559. [Google Scholar] [CrossRef]
- Mettu, N.; Twohy, E.; Ou, F.-S.; Halfdanarson, T.; Lenz, H.; Breakstone, R.; Boland, P.; Crysler, O.; Wu, C.; Grothey, A.; et al. BACCI: A phase II randomized, double–blind, multicenter, placebo–controlled study of capecitabine (C) bevacizumab (B) plus atezolizumab (A) or placebo (P) in refractory metastatic colorectal cancer (mCRC): An ACCRU network study. Ann. Oncol. 2019, 30 (Suppl. 5), v198–v252. [Google Scholar] [CrossRef]
- Antoniotti, C.; Rossini, D.; Pietrantonio, F.; Catteau, A.; Salvatore, L.; Lonardi, S.; Boquet, I.; Tamberi, S.; Marmorino, F.; Moretto, R.; et al. GONO Foundation Investigators. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022, 23, 876–887. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Parikh, A.R.; Spigel, D.R.; Cohn, A.L.; Yoshino, T.; Kochenderfer, M.D.; Elez, E.; Shao, S.H.; Deming, D.A.; Holdridge, R.C.; et al. Nivolumab (NIVO) + 5-fluorouracil/leucovorin/oxaliplatin (mFOLFOX6)/bevacizumab (BEV) versus mFOLFOX6/BEV for first-line (1L) treatment of metastatic colorectal cancer (mCRC): Phase 2 results from CheckMate 9X8. J. Clin. Oncol. 2022, 40 (Suppl. 4), 8. [Google Scholar] [CrossRef]
- Damato, A.; Bergamo, F.; Antonuzzo, L.; Nasti, G.; Pietrantonio, F.; Tonini, G.; Maiello, E.; Bordonaro, R.; Rosati, G.; Romagnani, A.; et al. Phase II study of nivolumab in combination with FOLFOXIRI/bevacizumab as first-line treatment in patients with advanced colorectal cancer RAS/BRAF mutated (mut): NIVACOR trial (GOIRC-03-2018). J. Clin. Oncol. 2022, 40 (Suppl. 16), 3509. [Google Scholar] [CrossRef]
- Bocobo, A.G.; Wang, R.; Behr, S.; Carnevale, J.C.; Cinar, P.; Collisson, E.A.; Fong, L.; Keenan, B.P.; Kidder, W.A.; Ko, A.H.; et al. Phase II study of pembrolizumab plus capecitabine and bevacizumab in microsatellite stable (MSS) metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2022, 40 (Suppl. 16), 3565. [Google Scholar] [CrossRef]
- Redman, J.M.; Tsai, Y.T.; Weinberg, B.A.; Donahue, R.N.; Gandhy, S.; Gatti-Mays, M.E.; Abdul Sater, H.; Bilusic, M.; Cordes, L.M.; Steinberg, S.M.; et al. A Randomized Phase II Trial of mFOLFOX6 + Bevacizumab Alone or with AdCEA Vaccine + Avelumab Immunotherapy for Untreated Metastatic Colorectal Cancer. Oncologist 2022, 27, 198–209. [Google Scholar] [CrossRef]
- Ou, D.; Chen, C.; Hsu, C.; Chung, C.H.; Feng, Z.R.; Lee, B.S.; Cheng, A.L.; Yang, M.H.; Hsu, C. Regorafenib enhances antitumor immunity via inhibition of p38 kinase/Creb1/Klf4 axis in tumor-associated macrophages. J. Immunother. Cancer 2021, 9, e001657. [Google Scholar] [CrossRef]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Kotani, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib Plus Nivolumab in Patients with Advanced Gastric or Colorectal Cancer: An Open–Label, Dose–Escalation, and Dose–Expansion Phase Ib Trial (REGONIVO, EPOC1603). J. Clin. Oncol. 2020, 38, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.D.; Kovari, B.P.; Martinez, M.; Xie, H.; Sahin, I.H.; Mehta, R.; Strosberg, J.; Imanirad, I.; Ghayouri, M.; Kim, Y.C.; et al. A phase I/Ib study of regorafenib and nivolumab in mismatch repair proficient advanced refractory colorectal cancer. Eur. J. Cancer 2022, 169, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.; Raghav, K.P.S.; Chang, D.Z.; Bendell, J.C.; Larson, T.; Cohn, A.L.; Huyck, T.K.; Cosgrove, D.; Fiorillo, J.A.; Garbo, L.E.; et al. Single-arm, phase 2 study of regorafenib plus nivolumab in patients with mismatch repair-proficient (pMMR)/microsatellite stable (MSS) colorectal cancer (CRC). J. Clin. Oncol. 2021, 39 (Suppl. 15). [Google Scholar] [CrossRef]
- Barzi, A.; Azad, N.S.; Yang, Y.; Tsao-Wei, D.; Rehman, R.; Fakih, M.; Iqbal, S.; El-Khoueiry, A.B.; Millstein, J.; Jayachandran, P.; et al. Phase I/II study of regorafenib (rego) and pembrolizumab (pembro) in refractory microsatellite stable colorectal cancer (MSSCRC). J. Clin. Oncol. 2022, 40 (Suppl. 4), 15. [Google Scholar] [CrossRef]
- Cousin, S.; Cantarel, C.; Guegan, J.P.; Gomez-Roca, C.; Metges, J.P.; Adenis, A.; Pernot, S.; Bellera, C.; Kind, M.; Auzanneau, C.; et al. Regorafenib-Avelumab Combination in Patients with Microsatellite Stable Colorectal Cancer (REGOMUNE): A Single-arm, Open-label, Phase II Trial. Clin. Cancer Res. 2021, 27, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; He, M.M.; Yao, Y.C.; Zhao, X.; Wang, Z.Q.; Jin, Y.; Luo, H.Y.; Li, J.B.; Wang, F.H.; Qiu, M.Z.; et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: A phase Ib/II clinical trial and gut microbiome analysis. Cell Rep. Med. 2021, 2, 100383. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roca, C.; Yanez, E.; Im, S.-A.; Alvarez, E.C.; Senellart, H.; Doherty, M.; García-Corbacho, J.; Lopez, J.S.; Basu, B.; Maurice-Dror, C.; et al. LEAP-005: A phase II multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors—Results from the colorectal cancer cohort. J. Clin. Oncol. 2021, 39, 94. [Google Scholar] [CrossRef]
- Yoshino, T.; Fu, R.; Hawk, N.; Adelberg, D.E.; Norwood, K.G.; Heinemann, V. 506TiP Pembrolizumab plus lenvatinib versus standard of care for previously treated metastatic colorectal cancer (mCRC): Phase III LEAP-017 study. Ann. Oncol. 2021, 32, S580. [Google Scholar] [CrossRef]
- Rahma, O.E.; Cleary, J.M.; Schlechter, B.L.; Ng, K.; Eno, J.; Stroiney, A.; Giobbie-Hurder, A.; McDermott, D.F.; Hodi, F.S. Phase Ib study of pembrolizumab and trebananib (angiopoietin-2 inhibitor [Ang-2]): Preliminary analysis of the colorectal cancer (CRC) cohort. J. Clin. Oncol. 2019, 37, e14160. [Google Scholar] [CrossRef]
- Rahma, O.E.; Cleary, J.M.; Ng, K.; Schlechter, B.L.; Eno, J.; Maloney, A.; Giobbie-Hurder, A.; McDermott, D.F.; Hodi, F.S. Phase Ib study to test the safety and activity of pembrolizumab (anti-PD-1) and trebananib (angiopoietin-2 inhibitor [Ang-2]) in patients with advanced solid tumors: Updated analysis of the colorectal cancer (CRC) cohort. J. Clin. Oncol. 2020, 38 (Suppl. 4), 155. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Solimando, A.G.; Summa, S.; Vacca, A.; Ribatti, D. Cancer-Associated Angiogenesis: The Endothelial Cell as a Checkpoint for Immunological Patrolling. Cancers 2020, 12, 3380. [Google Scholar] [CrossRef] [PubMed]
- Duru, G.; van Egmond, M.; Heemskerk, N. A Window of Opportunity: Targeting Cancer Endothelium to Enhance Immunotherapy. Front. Immunol 2020, 11, 584723. [Google Scholar] [CrossRef]
- Amersfoort, J.; Eelen, G.; Carmeliet, P. Immunomodulation by endothelial cells—Partnering up with the immune system? Nat. Rev. Immunol. 2022, 22, 576–588. [Google Scholar] [CrossRef]
- Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 1994, 76, 301–314. [Google Scholar] [CrossRef]
- Carman, C.V.; Martinelli, R. T lymphocyte–endothelial interactions: Emerging understanding of trafficking and antigen-specific immunity. Front. Immunol. 2015, 6, 603. [Google Scholar] [CrossRef]
- Motz, G.T.; Santoro, S.P.; Wang, L.P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014, 20, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, A.W.; Damen, C.A.; Martinotti, S.; Blijham, G.H.; Groenewegen, G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: The role of angiogenic factors. Cancer Res. 1996, 56, 1111–1117. [Google Scholar] [PubMed]
- Derakhshani, A.; Hashemzadeh, S.; Asadzadeh, Z.; Shadbad, M.A.; Rasibonab, F.; Safarpour, H.; Jafarlou, V.; Solimando, A.G.; Racanelli, V.; Singh, P.K.; et al. Cytotoxic T-Lymphocyte Antigen-4 in Colorectal Cancer: Another Therapeutic Side of Capecitabine. Cancers 2021, 13, 2414. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, Z.; Wang, Y.; Wen, X.; Amador, E.H.; Yuan, L.; Ran, X.; Xiong, L.; Ran, Y.; Chen, W.; et al. Colorectal liver metastasis: Molecular mechanism and interventional therapy. Sig. Transduct. Target. Ther. 2022, 7, 70. [Google Scholar] [CrossRef]
- Wang, C.; Sandhu, J.; Ouyang, C.; Ye, J.; Lee, P.P.; Fakih, M. Clinical Response to Immunotherapy Targeting Programmed Cell Death Receptor 1/Programmed Cell Death Ligand 1 in Patients With Treatment-Resistant Microsatellite Stable Colorectal Cancer With and Without Liver Metastases. JAMA Netw. Open 2021, 4, e2118416. [Google Scholar] [CrossRef]
- Panebianco, C.; Ciardiello, D.; Villani, A.; Maiorano, B.A.; Latiano, T.P.; Maiello, E.; Perri, F.; Pazienza, V. Insights into the role of gut and intratumor microbiota in pancreatic ductal adenocarcinoma as new key players in preventive, diagnostic and therapeutic perspective. Semin. Cancer Biol. 2021, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.B.; Zhou, Y.L.; Fang, J.Y. Gut Microbiota in Cancer Immune Response and Immunotherapy. Trends Cancer 2021, 7, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Derosa, L.; Routy, B.; Fidelle, M.; Iebba, V.; Alla, L.; Pasolli, E.; Segata, N.; Desnoyer, A.; Pietrantonio, F.; Ferrere, G.; et al. Gut Bacteria Composition Drives Primary Resistance to Cancer Immunotherapy in Renal Cell Carcinoma Patients. Eur. Urol. 2020, 78, 195–206. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Fasano, M.; Della Corte, C.M.; Di Liello, R.; Barra, G.; Sparano, F.; Viscardi, G.; Iacovino, M.L.; Paragliola, F.; Famiglietti, V.; Ciaramella, V.; et al. Induction of natural killer anti-body-dependent cell cytotoxicity and of clinical activity of cetuximab plus avelumab in non-small cell lung cancer. ESMO Open 2020, 5, e000753. [Google Scholar] [CrossRef]
- Martinelli, E.; Martini, G.; Famiglietti, V.; Troiani, T.; Napolitano, S.; Pietrantonio, F.; Ciardiello, D.; Terminiello, M.; Borrelli, C.; Vitiello, P.P.; et al. Cetuximab Rechallenge Plus Avelumab in Pretreated Patients With RAS Wild-type Metastatic Colorectal Cancer: The Phase 2 Single-Arm Clinical CAVE Trial. JAMA Oncol. 2021, 7, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, D.; Famiglietti, V.; Napolitano, S.; Esposito, L.; Pietrantonio, F.; Avallone, A.; Maiello, E.; Cremolini, C.; Troiani, T.; Martinelli, E.; et al. Final results of the CAVE trial in RAS wild type metastatic colorectal cancer patients treated with cetuximab plus avelumab as rechallenge therapy: Neutrophil to lymphocyte ratio predicts survival. Clin. Colorectal Cancer 2022, 21, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Martini, G.; Ciardiello, D.; Dallio, M.; Famiglietti, V.; Esposito, L.; Corte, C.M.D.; Napolitano, S.; Fasano, M.; Gravina, A.G.; Romano, M.; et al. Gut microbiota correlates with antitumor activity in patients with mCRC and NSCLC treated with cetuximab plus avelumab. Int. J. Cancer 2022, 151, 473–480. [Google Scholar] [CrossRef] [PubMed]
| Trial Name | First Author | Year | Phase | Nr. of Patients | Treatment | ORR, % | mPFS, Months | mOS, Months | Safety |
|---|---|---|---|---|---|---|---|---|---|
| NCT01633970 | Bendell | 2015 | 1b | Arm A (pre-treated): 13 Arm B (naïve): 26 | Arm A: atezolizumab + bevacizumab Arm B: atezolizumab + FOLFOX + bevacizumab | Arm A: 8% Arm B: 36% | NA | NA | ≥G3 AEs: 64% (Arm A), 73% (Arm B) |
| MODUL (NCT02291289) | Grothey | 2018 | 2 | 445 (naïve, BRAF wt) | Maintenance bevacizumab +/− atezolizumab after FOLFOX + bevacizumab | NA | Not met | Immature data | NA |
| CheckMate 9X8 (NCT03414983) | Lenz | 2022 | 2 | Experimental Arm: 127 Control Arm:68 | Experimental Arm: FOLFOX + Bevacizumab + Nivolumab Control Arm: FOLFOX + Bevacizumab | Experimental Arm: 60% vs Control Arm:48% | 11.9 months in both Arm | Immature data | Grade 3−4 AEs 75% experimental Arm Vs. 48% control Arm |
| BACCI (NCT0287319) | Mettu | 2019 | 2 | 133 (pre-treated) | Capecitabine and bevacizumab + atezolizumab or placebo | NA | 4.4 vs. 3.6 | NA | ≥G3 AEs: hypertension (7 vs. 4.3%), diarrhea (7 vs. 4.3%), HFS (7 vs. 4.3%). |
| Atezo TRIBE (NCT03721653) | Antoniotti | 2022 | 2 | 218 (naïve) | FOLFOXIRI + bevacizumab +/− atezolizumab | NA | 13.1 vs. 11.5 (p = 0.012) | NA | ≥G3 AEs: neutropenia, diarrhea; 2 treatment-related deaths |
| CheckMate 9X8 (NCT03414983) | 2022 | 2 | 195 (naïve) | FOLFOX + bevacizumab +/− nivolumab | 60% vs. 46% | 11.9 vs. 11.9 | NA | ≥G3 AEs 75% vs. 48% | |
| NIVACOR (NCT04072198) | Damato | 2022 | 2 | 73 (naïve, RAS/BRAF mut) | FOLFOXIRI + bevacizumab + nivolumab | 76.7% | 10.1 months | NA | ≥G3 AEs: neutropenia, diarrhea, fatigue and hypertension |
| NCT03946917 | Wang | 2021 | 1b/2 | 42 (MSS pre-treated) | Toripalimab + regorafenib | 15.2 | 2.1 | 15.5 | ≥G3 AEs: Hand-foot syndrome; Rash; impaired liver function |
| LEAP-005 (NCT03797326) | Gomez-Roca | 2021 | 2 | 32 (MSS, pre-treated) | Pembrolizumab + lenvatinib | 22 | 2.3 | 7.5 | 50% AEs |
| NCT03396926 | Bocobo | 2022 | 2 | 44 (MSS, pre-treated) | Pembrolizumab + capecitabine + bevacizumab | 5 | 4.3 | 9.6 | 28% ≥G3 AEs, 58% dose reduction/interruption |
| NCT03050814 | Redman | 2022 | 2 | 26 (MSS, naïve) | mFOLFOX6 + bevacizumab +/− avelumab + CEA-targeted vaccine | 50% vs. 50% | No differences | NA | NA |
| REGONIVO (EPOC1603) | Fukuoka | 2020 | 1b | 25 (pre-treated) | Nivolumab + regorafenib | 36 | 7.9 | NA | ≥G3 AEs: rash (12%), proteinuria (12%), PPED (10%) |
| NCT03712943 | Kim | 2022 | 1b | 51 (MSS, pre-treated) | Nivolumab + regorafenib | 10 | 4.3 | 11.1 | ≥G3 AEs: hypertension (16%), rash (19%), anemia (6%) |
| NCT04126733 | Fakih | 2021 | 2 | 70 (MSS, pre-treated) | Nivolumab + regorafenib | 21.7 | 15 weeks | 52 weeks | ≥G3 AEs: rash (14%), fatigue (7%), pneumonia (6%), increased bilirubin (6%) |
| NCT03657641 | Barzi | 2022 | 1/2 | 73 (MSS, pre-treated) | Pembrolizumab + regorafenib | 0 | 2.8 | 9.6 | ≥G3 rash 20%, ≥G3 HFS 7% |
| REGOMUNE (NCT03475953) | Cousin | 2021 | 2 | 48 (MSS, pre-treated) | Avelumab + regorafenib | 0 | 3.6 | 10.8 | ≥G3 AEs: PPED (29.8%), hypertension (23.4%), diarrhea (12.8%) |
| (Wang et al.) | 2021 | 1b/2 | 42 (MSS, pre-treated) | Toripalimab + regorafenib | 15.2 | 2.1 | 15.5 | ≥G3 AEs: 38.5% | |
| NCT03239145 | Rahma | 2020 | 1b | 18 (MSS, pre-treated) | Pembrolizumab + trebananib | NA | NA | 9 | AEs: diarrhea, limber edema, proteinuria, transaminase increase |
| Molecule | Role |
|---|---|
| Chemokines (e.g., CCL2, CXCL4/10) | Attracting and binding immune cells with ECs |
| Circulating pro-inflammatory cytokines (IFNγ, TNFα) | Favoring the activation of ECs with exposure of cell adhesion molecules, immune modulation recruiting MDSCs, Tregs, macrophages shifting towards M2-like subtype |
| VEGF | Recruiting immune suppressive cells such as Tregs, inhibiting expression of cell surface adhesion molecules, reducing T cells recruiting chemokines, inducing FasL expression on ECs |
| Adhesion molecules (E-/P-selectin, VCAM, ICAM) | Recruiting and binding immune cells |
| MHC-I | Overexpression associated with lack of co-stimulatory molecules (CD80/CD86) |
| MHC-II | Decrease on tumor vessels, contributing to immune tolerogenicity |
| PD-L1/2 | Creating an immune suppressive tumor microenvironment through the crosstalk between immune cells, cancer cells, and vessels |
| NO, ROS | Altering immune cells infiltration and suppressing CD8+ T cells |
| IDO, TIM3 | After stimulation of ECs by cytokines such as IFNγ inducing T-cell death, cell cycle arrest, and anergy |
| FasL | Causing T-cell apoptosis |
| Trial Identification | Phase | Drug Combination | Primary Endpoint |
|---|---|---|---|
| NCT03657641 | I/II | Pembrolizumab + Regorafenib | Safety, RD |
| NCT03475004 | II | Pembrolizumab + bevacizumab + binimetinib | Safety |
| NCT03396926 | II | Pembrolizumab + bevacizumab + capecitabine | DLT, ORR |
| NCT04776148 (MK-7902-017/E7080-G000-325/LEAP-017) | III | Pembrolizumab + lenvatinib vs. SOC (Regorafenib or TAS-102) | OS |
| NCT05035381 | II | Pembrolizumab + FOLFIRI + bevacizumab | ORR |
| NCT02298959 | I | Safety, RD | OS |
| NCT04745130 | II | Sintilimab + regorafenib + cetuximab | ORR |
| NCT03712943 | I | Nivolumab + Regorafenib | MTD |
| NCT04963283 | II | Nivolumab + cabozantinib | DCR |
| NCT04362839 | I | Nivolumab + ipilimumab + regorafenib | RD |
| NCT03475953 | I/II | Avelumab + regorafenib | RP2D, ORR |
| NCT02997228 | III | mFOLFOX6 + bevacizumab vs. atezolizumab vs. mFOLFOX6 + bevacizumab + atezolizumab | PFS |
| NCT02873195 | II | Capecitabine + bevacizumab + atezolizumab vs. PBO | PFS |
| NCT04659382 (SIRTCI) | II | Atezolizumab + XELOX + bevacizumab + SIRT | 9 months-PFS |
| NCT02777710 (MEDIPLEX) | I | Durvalumab + pexidartinib | DLT, ORR |
| NCT03555149 (Morpheus-CRC) | I/II | Atezolizumab + bevacizumab or regorafenib combinations | ORR |
| NCT03170960 | I/II | Atezolizumab + cabozantinib | MTD, ORR |
| NCT03539822 | I/II | Durvalumab + cabozantinib | MTD, ORR |
| NCT05485909 | II | Toripalimab + regorafenib + RFA | ORR |
| NCT04110093 | I/II | Nivolumab or camrelizumab or sintilimab or toripalimab + regorafenib | ORR, PFS |
| NCT04866862 | II | Camrelizumab + fruquitinib | ORR |
| NCT04695470 | II | Sintilimab + fruquitinib | PFS |
| NCT04194359 | III | Xelox + bevacizumab + sintilimab vs. PBO | PFS |
| NCT04764006 | II | Sintilimab + surufatinib | ORR |
| NCT05438108 | II | SBRT + Xelox + sintilimab + bevacizumab | ORR, AEs |
| NCT04271813 (APICAL-CR) | II | Sintilimab + anlotinib | ORR |
| NCT04745130 | II | Sintilimab + regorafenib + cetuximab | ORR |
| NCT05524155 | II | Sintilimab + regorafenib + HAIC | ORR, AEs |
| NCT05292417 | II | Sintilimab + fruquitinib + GM-CSF | PFS |
| NCT04948034 (RIFLE) | II | SABR+ tislelizumab + fruquitinib | ORR |
| NCT05314101 | II | Tislelizumab + bevacizumab + TAS-102 | PFS |
| NCT04924179 | II | Tislelizumab + fruquitinib + SBRT | PFS |
| NCT04777162 | II | Tislelizumab + anlotinib | ORR |
| NCT05435313 | II | Tislelizumab + fruquitinib + HAIC | ORR |
| NCT04577963 | I/II | Tislelizumab + fruquitinib | RP2D, AEs, ORR |
| NCT04579757 | I/II | Tislelizumab + surufatinib | DLT, ORR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiorano, B.A.; Parisi, A.; Maiello, E.; Ciardiello, D. The Interplay between Anti-Angiogenics and Immunotherapy in Colorectal Cancer. Life 2022, 12, 1552. https://doi.org/10.3390/life12101552
Maiorano BA, Parisi A, Maiello E, Ciardiello D. The Interplay between Anti-Angiogenics and Immunotherapy in Colorectal Cancer. Life. 2022; 12(10):1552. https://doi.org/10.3390/life12101552
Chicago/Turabian StyleMaiorano, Brigida Anna, Alessandro Parisi, Evaristo Maiello, and Davide Ciardiello. 2022. "The Interplay between Anti-Angiogenics and Immunotherapy in Colorectal Cancer" Life 12, no. 10: 1552. https://doi.org/10.3390/life12101552
APA StyleMaiorano, B. A., Parisi, A., Maiello, E., & Ciardiello, D. (2022). The Interplay between Anti-Angiogenics and Immunotherapy in Colorectal Cancer. Life, 12(10), 1552. https://doi.org/10.3390/life12101552






