COVID-19 Smell Impairment and Crosstalk with Hypoxia Physiology
Abstract
:1. Introduction
2. Materials and Methods
3. Results
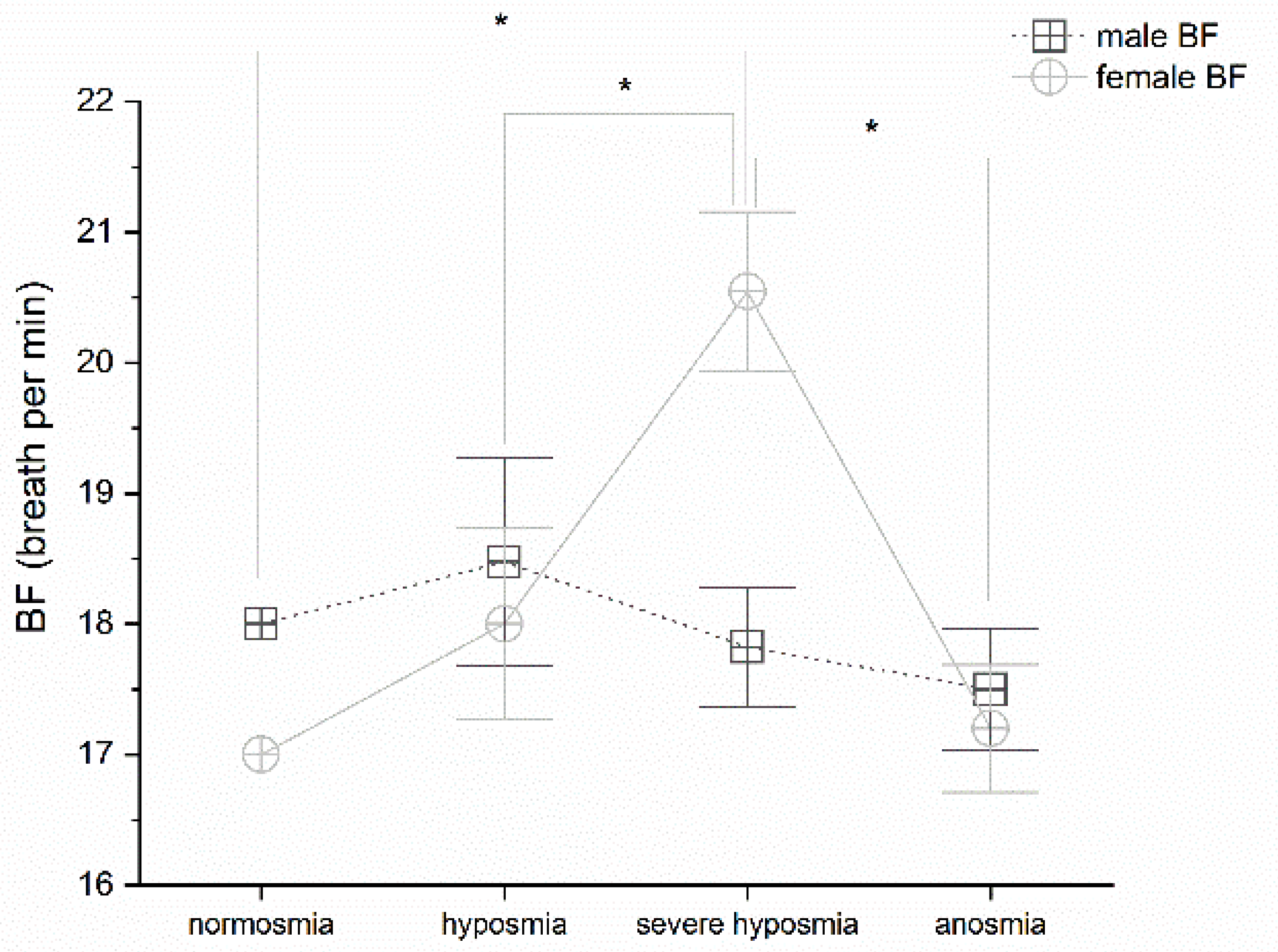
3.1. Olfactory Threshold vs. Breath Frequency
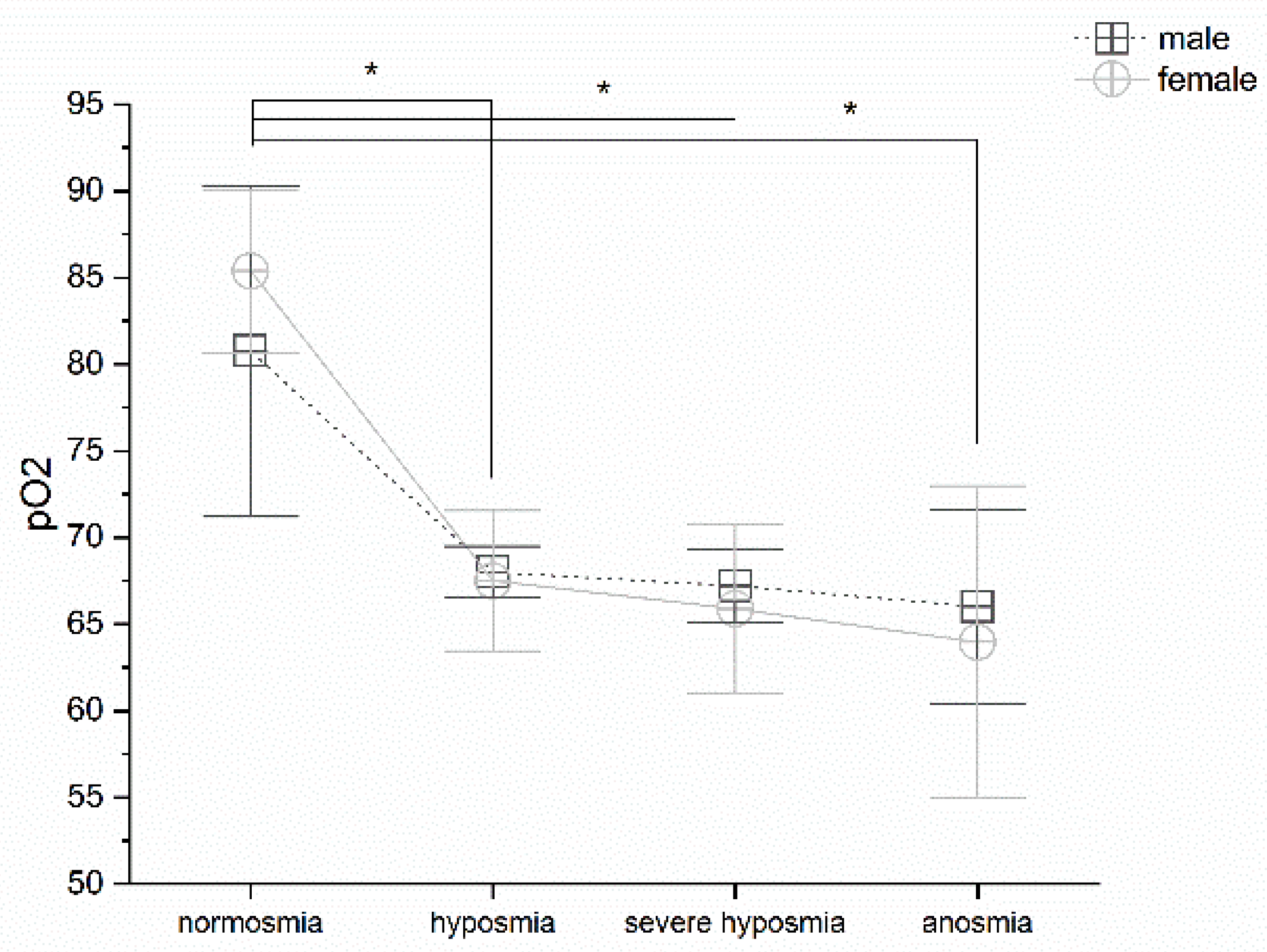
3.2. Olfactory Threshold vs. pO2
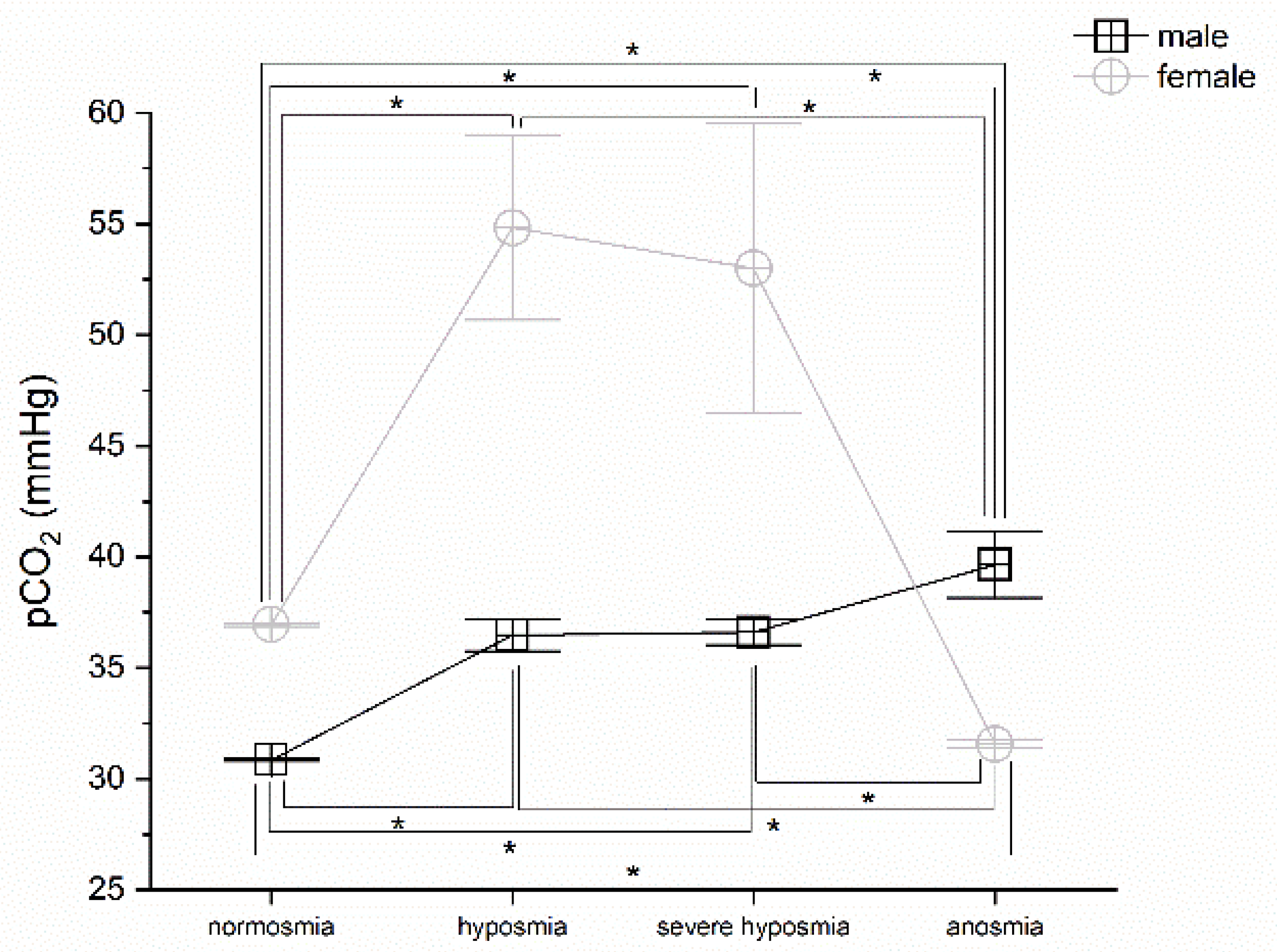
3.3. Olfactory Threshold vs. pCO2
3.4. Olfactory Threshold vs. Hemoglobin Levels
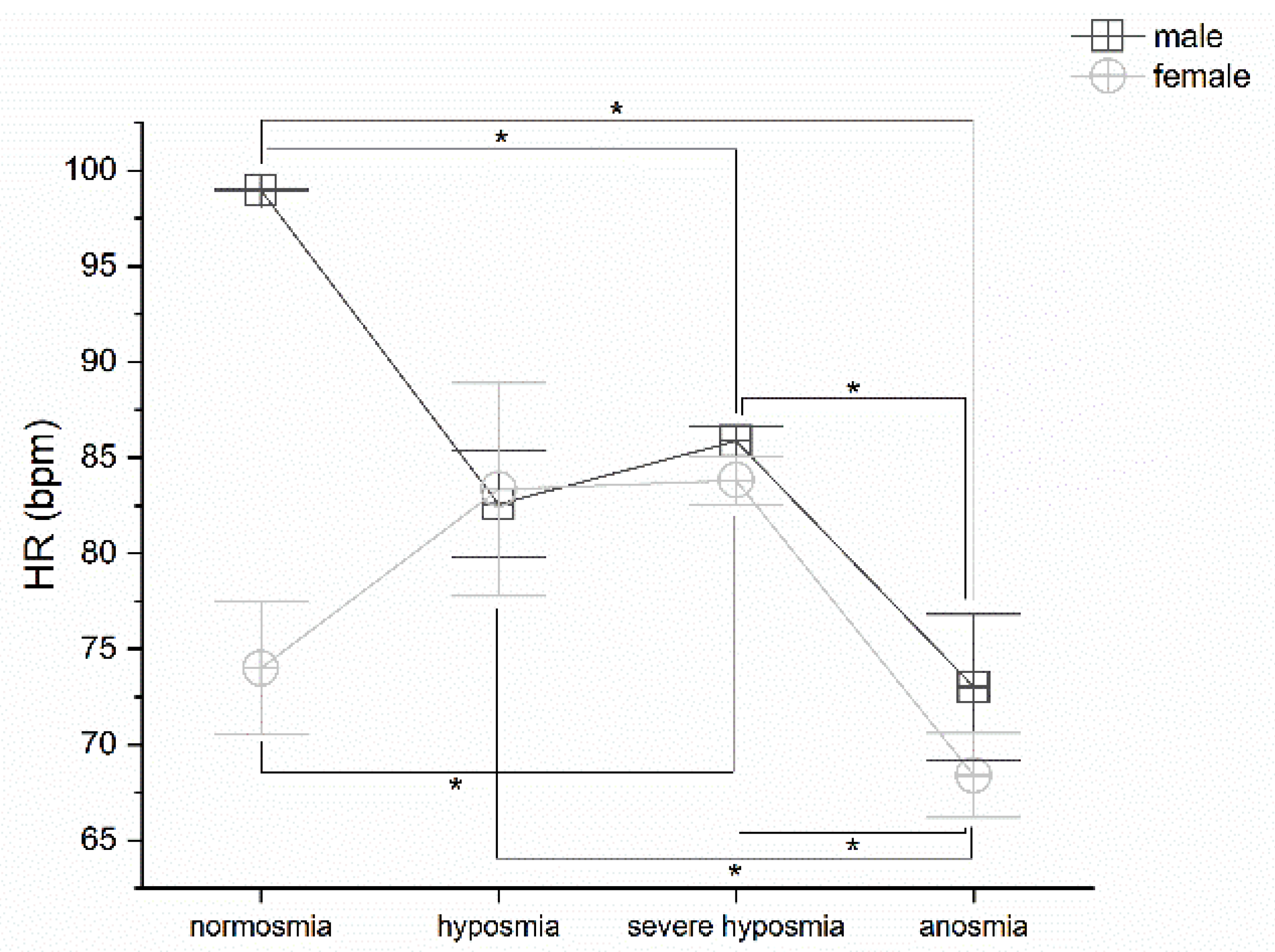
3.5. Olfactory Threshold vs. HR
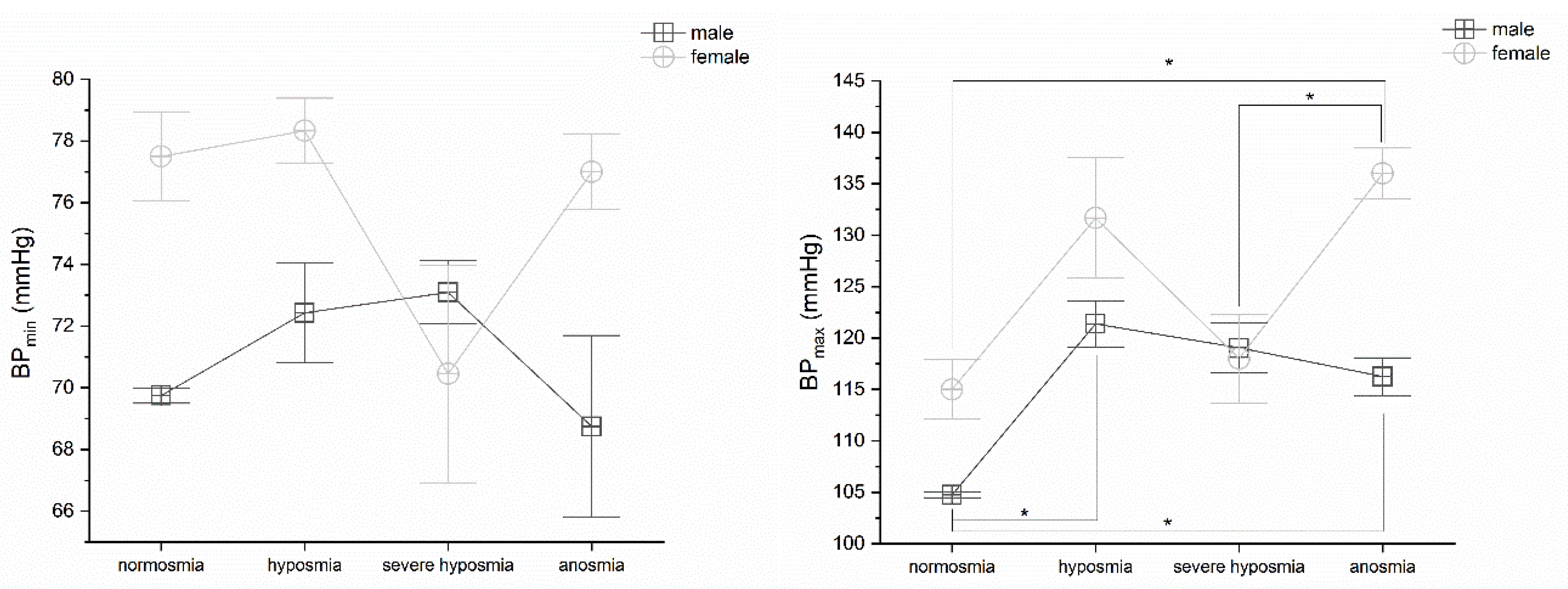
3.6. Olfactory Threshold vs. BP
4. Discussion
4.1. Olfactory Threshold, Breath Frequency, pO2, pCO2, and Hemoglobin Levels
4.2. Olfactory Threshold vs. HR and BP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanche, S.; Lin, Y.T.; Xu, C.; Romero-Severson, E.; Hengartner, N.; Ke, R. High Contagiousness and Rapid Spread of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg. Infect. Dis. 2020, 26, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Lovato, A.; de Filippis, C. Clinical Presentation of COVID-19: A Systematic Review Focusing on Upper Airway Symptoms. Ear Nose Throat J. 2020, 99, 569–576. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-remarksat-the-media-briefing-on-2019-ncov-on-11-february-2020 (accessed on 11 February 2020).
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nagata, N.; Shirato, K.; Kawase, M.; Takeda, M.; Taguchi, F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 2010, 84, 12658–12664. [Google Scholar] [CrossRef] [PubMed]
- Glowacka, I.; Bertram, S.; Muller, M.A.; Allen, P.; Soilleux, E.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; HCA Lung Biological Network. SARS-CoV-2 Entry Genes Are Most Highly Expressed in Nasal Goblet and Ciliated Cells within Human Airways. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Chalmers, S.; Khawaja, A.; Wieruszewski, P.M.; Gajic, O.; Odeyemi, Y. Diagnosis and treatment of acute pulmonary inflammation in critically ill patients: The role of inflammatory biomarkers. World J. Crit. Care Med. 2019, 8, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, R.; He, G. Hematological findings in coronavirus disease 2019: Indications of progression of disease. Ann. Hematol. 2020, 99, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R.; Adhikari, S.; Pulgarin, C.; Troxel, A.B.; Iturrate, E.; Johnson, S.B.; Hausvater, A.; Newman, J.D.; Berger, J.S.; Bangalore, S.; et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of COVID-19. N. Engl. J. Med. 2020, 382, 2441–2448. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Koralnik, I.J.; Tyler, K.L. COVID-19: A Global Threat to the Nervous System. Ann. Neurol. 2020, 88, 1–11. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Harthi, A.; Hussein, J.; Bouchama, A.; Johani, S.; Hajeer, A.H.; Saeed, B.T.; Wahbi, A.; Saedy, A.; AlDabbagh, T.; et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection 2015, 43, 495–501. [Google Scholar] [CrossRef]
- Kim, J.E.; Heo, J.H.; Kim, H.O.; Song, S.H.; Park, S.S.; Park, T.H.; Ahn, J.Y.; Kim, M.K.; Choi, J.P. Neurological Complications during Treatment of Middle East Respiratory Syndrome. J. Clin. Neurol. 2017, 13, 227–233. [Google Scholar] [CrossRef]
- Lau, K.K.; Yu, W.C.; Chu, C.M.; Lau, S.T.; Sheng, B.; Yuen, K.Y. Possible central nervous system infection by SARS coronavirus. Emerg. Infect. Dis. 2004, 10, 342–344. [Google Scholar] [CrossRef]
- Tsai, L.K.; Hsieh, S.T.; Chao, C.C.; Chen, Y.C.; Lin, Y.H.; Chang, S.C.; Chang, Y.C. Neuromuscular disorders in severe acute respiratory syndrome. Arch. Neurol. 2004, 61, 1669–1673. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhong, S.; Liu, J.; Li, L.; Li, Y.; Wu, X.; Li, Z.; Deng, P.; Zhang, J.; Zhong, N.; et al. Detection of severe acute respiratory syndrome coronavirus in the brain: Potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005, 41, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Fotuhi, M.; Mianc, A.; Meysamid, S.; Rajic, C.A. Neurobiology of COVID-19. J. Alzh. Dis. 2020, 76, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Surda, P.; Kumar, N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology 2020, 58, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Xydakis, M.S.; Dehgani-Mobaraki, P.; Holbrook, E.H.; Geisthoff, U.W.; Bauer, C.; Hautefort, C.; Herman, P.; Manley, G.T.; Lyon, D.M.; Hopkins, C. Smell and taste dysfunction in patients with COVID-19. Lancet Infect. Dis. 2020, 20, 1015–1016. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Neri, G.; D’Ardes, D.; De Luca, C.; Marinari, S.; Porreca, E.; Cipollone, F.; Vecchiet, J.; Falcicchia, C.; Panichi, V.; et al. Smell and Taste in Severe COVID-19: Self-Reported vs. Testing. Front. Med. 2020, 7, 589409. [Google Scholar] [CrossRef]
- Li, Y.C.; Bai, W.Z.; Hashikawa, T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 92, 552. [Google Scholar] [CrossRef]
- Smith, J.; Ellenberger, H.; Ballanyi, K.; Richter, D.; Feldman, J. PreBötzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science 1991, 254, 726–729. [Google Scholar] [CrossRef]
- Burgold, T.; Voituron, N.; Caganova, M.; Tripathi, P.P.; Menuet, C.; Tusi, B.K.; Spreafico, F.; Bévengut, M.; Gestreau, C.; Buontempo, S.; et al. The H3K27 demethylase JMJD3 is required for maintenance of the embryonic respiratory neuronal network, neonatal breathing, and survival. Cell Rep. 2012, 2, 1244–1258. [Google Scholar] [CrossRef]
- Whitcroft, K.L.; Hummel, T. Olfactory Dysfunction in COVID-19: Diagnosis and Management. JAMA 2020, 323, 2512–2514. [Google Scholar] [CrossRef]
- Leung, P.S. Novel roles of a local angiotensin-generating system in the carotid body. J. Physiol. 2006, 575, 1–4. [Google Scholar] [CrossRef]
- Gonzalez, C.; Almaraz, L.; Obeso, A.; Rigual, R. Carotid body chemoreceptors: From natural stimuli to sensory discharges. Physiol. Rev. 1994, 74, 829–898. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.P.; Schultz, H.D. Angiotensin peptides and nitric oxide in 479 cardiovascular disease. Antioxid Redox Signal 2013, 19, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Emmi, A.; Stocco, E.; Barbon, S.; Boscolo-Berto, R.; Macchi, V.; De Caro, R. The potential role of the Carotid Body in COVID-19. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2020, 319, L620–L626. [Google Scholar] [CrossRef] [PubMed]
- Soliz, J.; Schneider-Gasser, E.M.; Arias-Reyes, C.; Aliaga-Raduan, F.; Poma-Machicao, L.; Zubieta-Callejac, G.; Furuya, W.I.; Trevizan-Baú, E.; Dhingra, R.R.; Dutschmann, M. Coping with hypoxemia: Could erythropoietin (EPO) be an adjuvant treatment of COVID-19? Resp. Physiol. Neurobiol. 2020, 279, 103476. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Noor, S.; Tippairote, T.; Dadar, M.; Menzel, A.; Bjorklund, G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin. Immunol. 2020, 215, 108409. [Google Scholar] [CrossRef]
- Frisancho, A.R. Functional adaptation to high altitude hypoxia. Science 1975, 187, 313–319. [Google Scholar] [CrossRef]
- Joseph, V.; Soliz, J.; Pequignot, J.; Sempore, B.; Cottet-Emard, J.M.; Dalmaz, Y.; Favier, R.; Spielvogel, H.; Pequignot, J.M. Gender differentiation of the chemoreflex during growth at high altitude: Functional and neurochemical studies. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R806–R816. [Google Scholar] [CrossRef]
- Basnyat, B.; Murdoch, D.R. High-altitude illness. Lancet 2003, 361, 1967–1974. [Google Scholar] [CrossRef]
- Ruffini, R.; Di Giulio, C.; Verratti, V.; Pokorski, M.; Fanò-Illic, G.; Mazzatenta, A. Adaptation of olfactory threshold at high altitude. Adv. Exp. Med. Biol. 2015, 837, 19–22. [Google Scholar] [PubMed]
- Mazzatenta, A.; Cellerino, A.; Origlia, N.; Barloscio, D.; Sartucci, F.; Di Giulio, C.; Domenici, L. Olfactory phenotypic expression unveils human aging. Oncotarget 2016, 7, 19193–19200. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, M.; Hasegawa, S.; Nakano, Y. Examination and classification of human olfactory mucosa in patients with clinical olfactory disturbances. Arch. Otorhinolaryngol. 1988, 245, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Yoo, S.J.; Clijsters, M.; Backaert, W.; Vanstapel, A.; Speleman, K.; Van Gerven, L. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell 2021, 184, 5932–5949. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.J.; Tan, B.K.J.; Tan, X.Y.; Liu, H.T.; Teo, C.B.; See, A.; Xu, S.; Toh, S.T.; Kheok, S.W.; Charn, T.C.; et al. Neuroradiological Basis of COVID-19 Olfactory Dysfunction: A Systematic Review and Meta-Analysis. Laryngoscope 2022, 132, 1260–1274. [Google Scholar] [CrossRef] [PubMed]
- Xydakis, M.S.; Albers, M.W.; Holbrook, E.H.; Lyon, D.M.; Shih, R.Y.; Frasnelli, J.A.; Pagenstecher, A.; Kupke, A.; Enquist, L.W.; Perlman, S. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021, 20, 753–761. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Berardi, A.; Novarria, G.A.; Neri, G. Unmasking the ‘Asymptomatic’ COVID-19: A Nose Question. Life 2022, 12, 1248. [Google Scholar] [CrossRef]
- Izquierdo-Domínguez, A.; Rojas-Lechuga, M.J.; Chiesa-Estomba, C.; Calvo-Henríquez, C.; Ninchritz-Becerra, E.; Soriano-Reixach, M.; Poletti-Serafini, D.; Villarreal, I.M.; Maza-Solano, J.M.; Moreno-Luna, R.; et al. Smell and taste dysfunction in COVID-19 is associated with younger age in ambulatory settings: A multicenter cross-sectional study. J. Investig. Allergol. Clin. Immunol. 2020, 30, 346–357. [Google Scholar] [CrossRef]
- Hintschich, C.A.; Wenzel, J.J.; Hummel, T.; Hankir, M.K.; Kühnel, T.; Vielsmeier, V.; Bohr, C. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. Int. Forum. Allergy Rhinol. 2020, 10, 1105–1107. [Google Scholar] [CrossRef]
- Escanilla, O.D.; Victor, X.D.; Di Lorenzo, P.M. Odor-taste convergence in the nucleus of the solitary tract of the awake freely licking rat. J. Neurosci. 2015, 35, 6284–6297. [Google Scholar] [CrossRef]
- Doty, R.L.; Smith, R.; McKeown, D.A.; Raj, J. Tests of human olfactory function: Principal components analysis suggests that most measure a common source of variance. Percept. Psychophys. 1994, 56, 701–707. [Google Scholar] [CrossRef]
- Pepe, A.; Pietropaoli, S.; Vos, M.; Barba-Spaeth, G.; Zurzolo, C. Tunneling nanotubes provide a route for SARS-CoV-2 spreading. Sci. Adv. 2022, 8, eabo0171. [Google Scholar] [CrossRef]
- Dando, S.J.; Mackay-Sim, A.; Norton, R.; Currie, B.J.; St. John, J.A.; Ekberg, J.A.; Batzloff, M.; Ulett, G.C.; Beacham, I.R. Pathogens penetrating the central nervous system: Infection pathways and the cellular and molecular mechanisms of invasion. Clin. Microbiol. Rev. 2014, 27, 691–726. [Google Scholar] [CrossRef] [PubMed]
- Simonson, T.S.; Baker, T.L.; Banzett, R.B.; Bishop, T.; Dempsey, J.A.; Feldman, J.L.; Guyenet, P.G.; Hodson, E.J.; Mitchell, G.S.; Moya, E.A.; et al. Silent hypoxaemia in COVID-19 patients. J. Physiol. 2021, 599, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Marini, J.J.; Camporota, L. The Respiratory Drive: An Overlooked Tile of COVID-19 Pathophysiology. Am. J. Respir. Crit. Care Med. 2020, 202, 1079–1080. [Google Scholar] [CrossRef]
- Chen, N.S.; Zhou, M.; Dong, X.; Qu, J.M.; Gong, F.Y.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Kumar, P.; Prabhakar, N.R. Peripheral chemoreceptors: Function and plasticity of the carotid body. Compr. Physiol. 2012, 2, 141–219. [Google Scholar] [PubMed]
- De Lorenzo, A.; Kasal, D.A.; Tura, B.R.; Lamas, C.C.; Rey, H.C. Acute cardiac injury in patients with COVID-19. Am. J. Cardiovasc. Dis. 2020, 10, 28–33. [Google Scholar] [PubMed]
- Fung, M.L.; Lam, S.Y.; Dong, X.; Chen, Y.; Leung, P.S. Postnatal hypoxemia increases angiotensin II sensitivity and up-regulates AT1a angiotensin receptors in rat carotid body chemoreceptors. J. Endocrinol. 2002, 173, 305–313. [Google Scholar]
- Moein, S.T.; Hashemian, S.M.; Tabarsi, P.; Doty, R.L. Prevalence and reversibility of smell dysfunction measured psychophysically in a cohort of COVID-19 patients. Int. Forum. Allergy Rhinol. 2020, 10, 1127–1135. [Google Scholar] [CrossRef]
- Doty, R.L. Olfactory dysfunction in neurodegenerative diseases: Is there a common pathological substrate? Lancet Neurol. 2017, 16, 478–488. [Google Scholar] [CrossRef]
- Henry, J.; Smeyne, R.J.; Jang, H.; Miller, B.; Okun, M.S. Parkinsonism and neurological mani-festations of influenza throughout the 20th and 21st centuries. Parkinsonism Relat. Disord. 2010, 16, 566–571. [Google Scholar] [CrossRef]
- Lam, S.Y.; Fung, M.L.; Leung, P.S. Regulation of the angiotensin-converting enzyme activity by a time-course hypoxia in the carotid body. J. Appl. Physiol. 2004, 96, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Netland, J.; Meyerholz, D.K.; Moore, S.; Cassell, M.; Perlman, S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008, 82, 7264–7275. [Google Scholar] [CrossRef] [PubMed]
- Casez, O.; Willaume, G.; Grand, S.; Nemoz, B.; Lupo, J.; Kahane, P.; Brion, J.P. Teaching NeuroImages: SARS-CoV-2-Related Encephalitis: MRI Pattern of Olfactory Tract Involvement. Neurology 2021, 96, e645–e646. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.; Gad, K. Absent blood oxygen level-dependent functional magnetic resonance imaging activation of the orbitofrontal cortex in a patient with persistent cacosmia and cacogeusia after COVID-19 infection. JAMA Neurol. 2021, 78, 609–610. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Rizzo, P.; Menegaldo, A.; Fabbris, C.; Spinato, G.; Borsetto, D.; Vaira, L.A.; Calvanese, L.; Pettorelli, A.; Sonego, M.; Frezza, D.; et al. Six-Month Psychophysical Evaluation of Olfactory Dysfunction in Patients with COVID-19. Chem. Senses 2021, 46, bjab006. [Google Scholar] [CrossRef]
- Xydakis, M.S.; Belluscio, L. Detection of neurodegenerative disease using olfaction. Lancet Neurol. 2017, 16, 415–416. [Google Scholar] [CrossRef]
- Tan, B.K.J.; Han, R.; Zhao, J.J.; Tan, N.K.W.; Quah, E.S.H.; Tan, C.J.; Chan, Y.H.; Teo, N.W.Y.; Charn, T.C.; See, A.; et al. Prognosis and persistence of smell and taste dysfunction in patients with COVID-19: Meta-analysis with parametric cure modelling of recovery curves. BMJ 2022, 378, e069503. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzatenta, A.; Maffei, M.; Di Giulio, C.; Neri, G. COVID-19 Smell Impairment and Crosstalk with Hypoxia Physiology. Life 2022, 12, 1408. https://doi.org/10.3390/life12091408
Mazzatenta A, Maffei M, Di Giulio C, Neri G. COVID-19 Smell Impairment and Crosstalk with Hypoxia Physiology. Life. 2022; 12(9):1408. https://doi.org/10.3390/life12091408
Chicago/Turabian StyleMazzatenta, Andrea, Margherita Maffei, Camillo Di Giulio, and Giampiero Neri. 2022. "COVID-19 Smell Impairment and Crosstalk with Hypoxia Physiology" Life 12, no. 9: 1408. https://doi.org/10.3390/life12091408
APA StyleMazzatenta, A., Maffei, M., Di Giulio, C., & Neri, G. (2022). COVID-19 Smell Impairment and Crosstalk with Hypoxia Physiology. Life, 12(9), 1408. https://doi.org/10.3390/life12091408










