Genomic Analysis of LEA Genes in Carica papaya and Insight into Lineage-Specific Family Evolution in Brassicales
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Retrieval and Identification of LEA Genes in Papaya, Horseradish Tree, and Spider Flower
2.2. Synteny Analysis and Gene Expansion Patterns
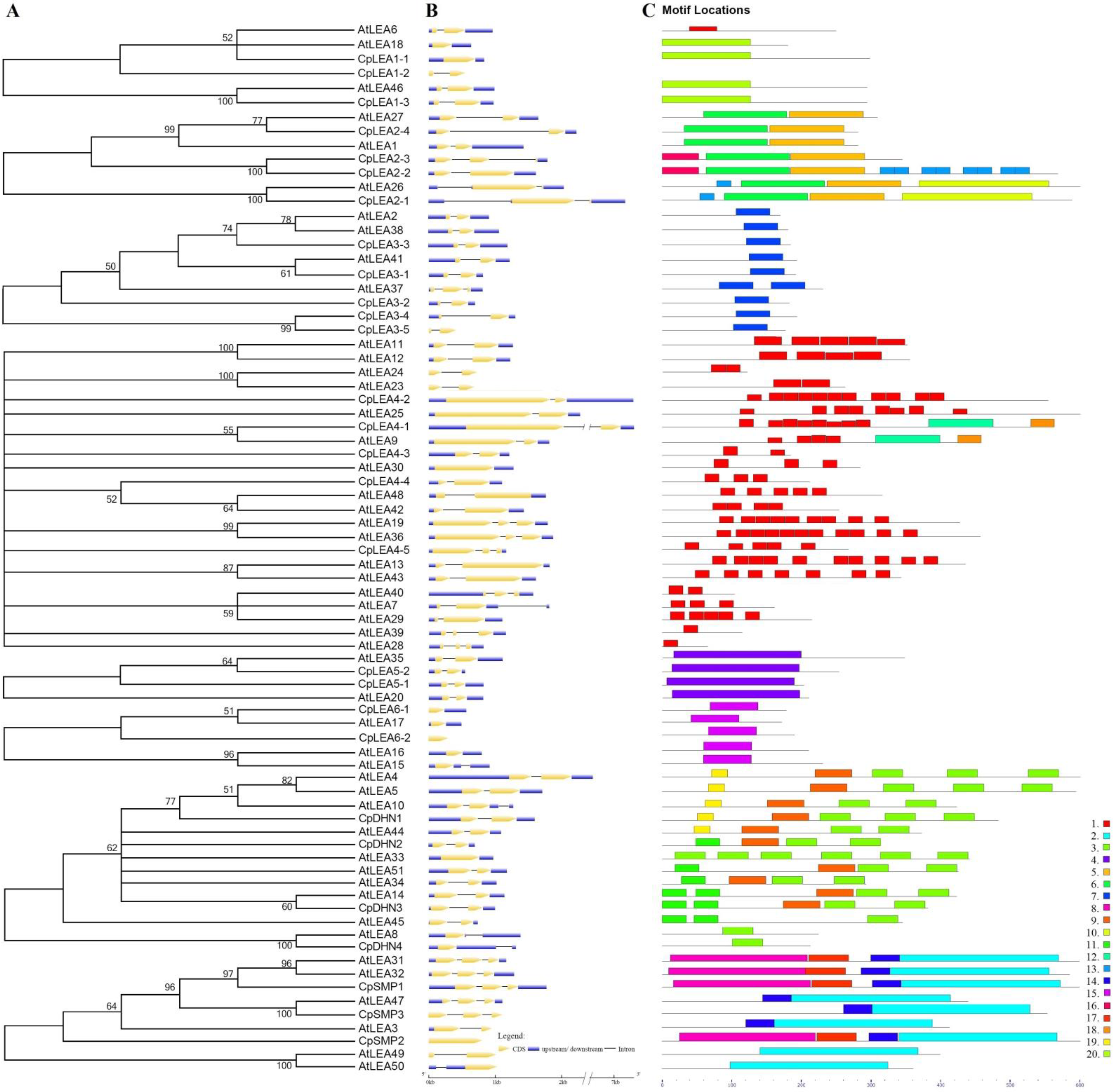
2.3. Exon-Intron Structure, Phylogenetic Analysis, and Structural Characterization
2.4. Promoter Analysis
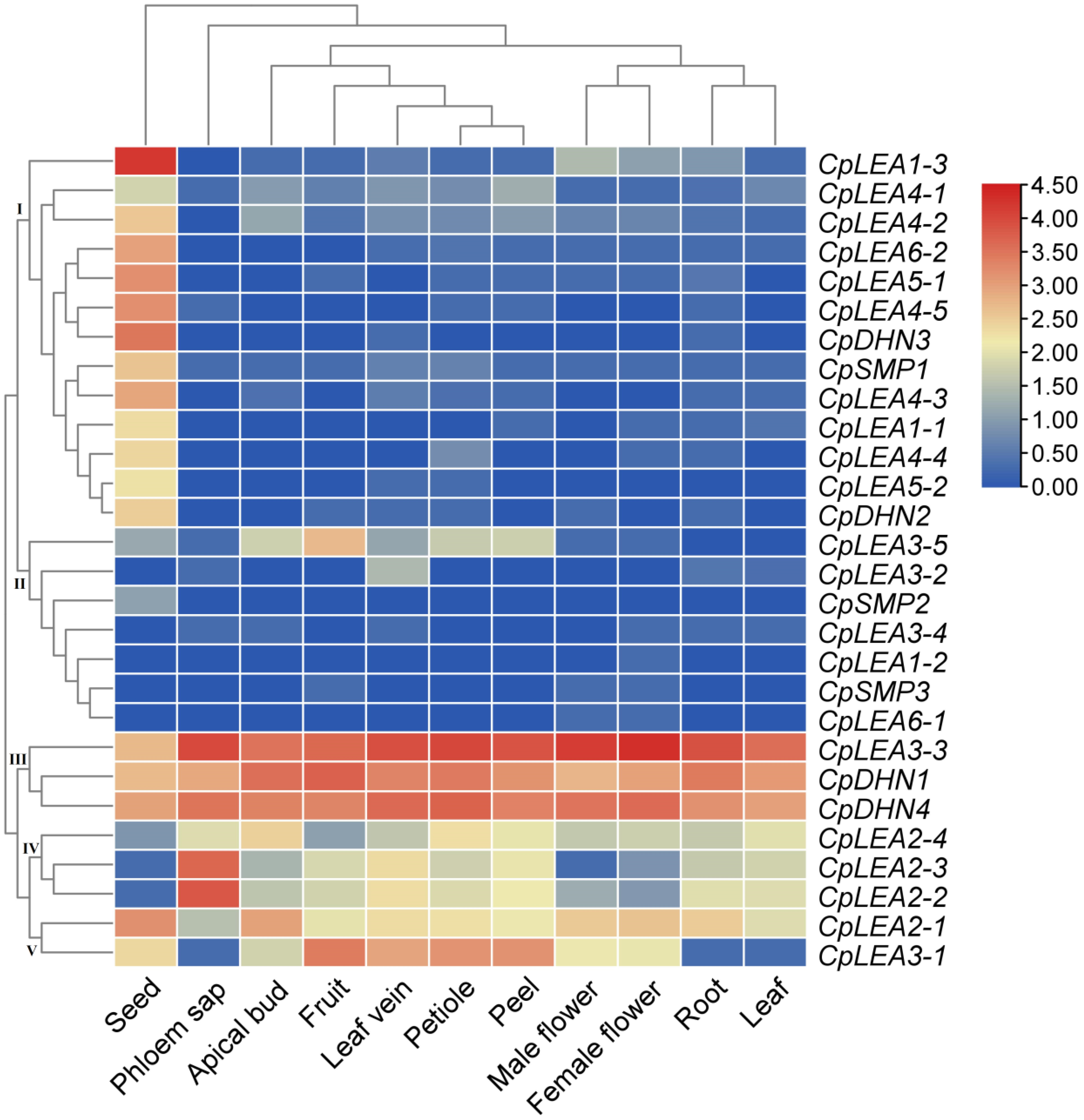
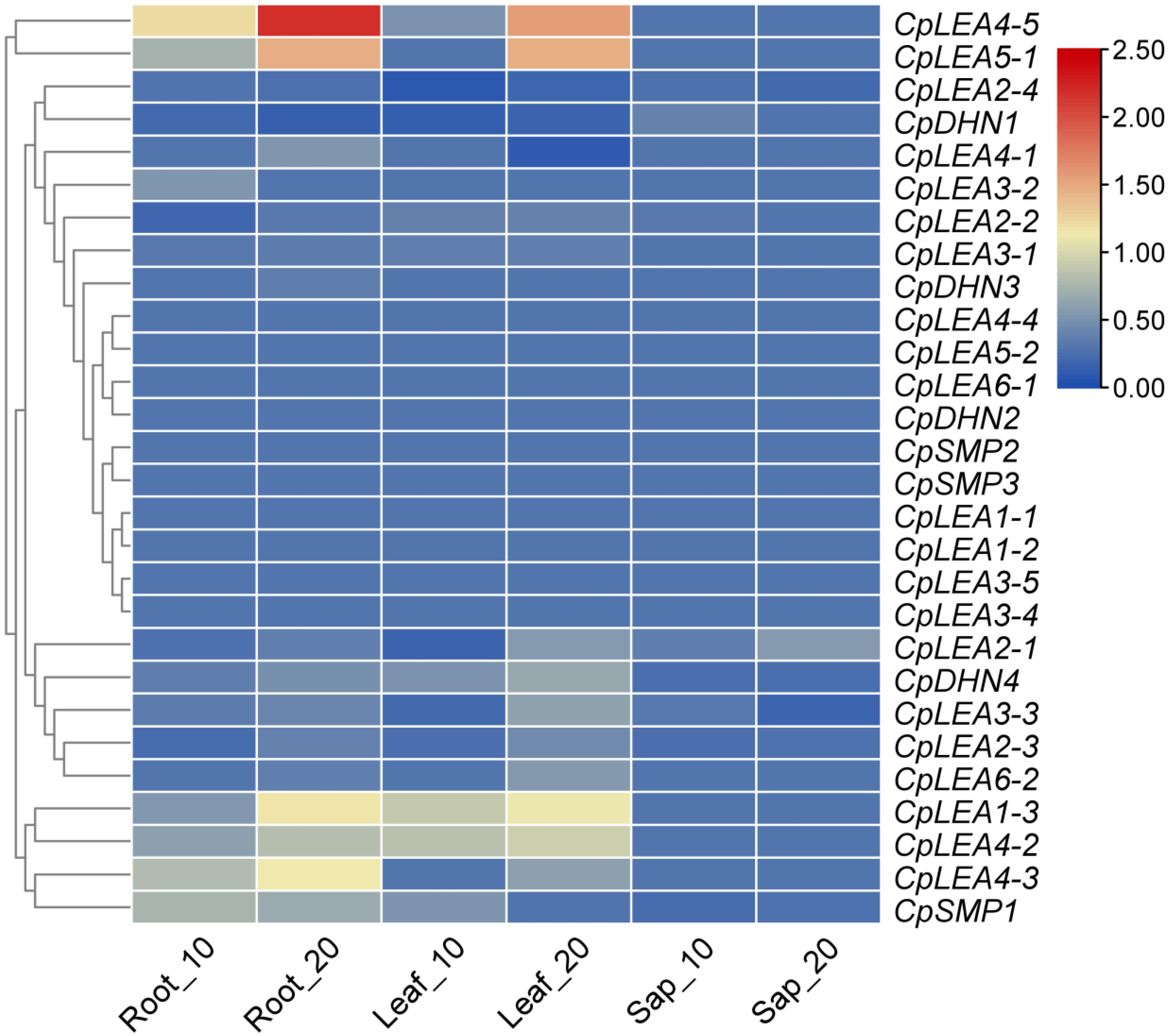
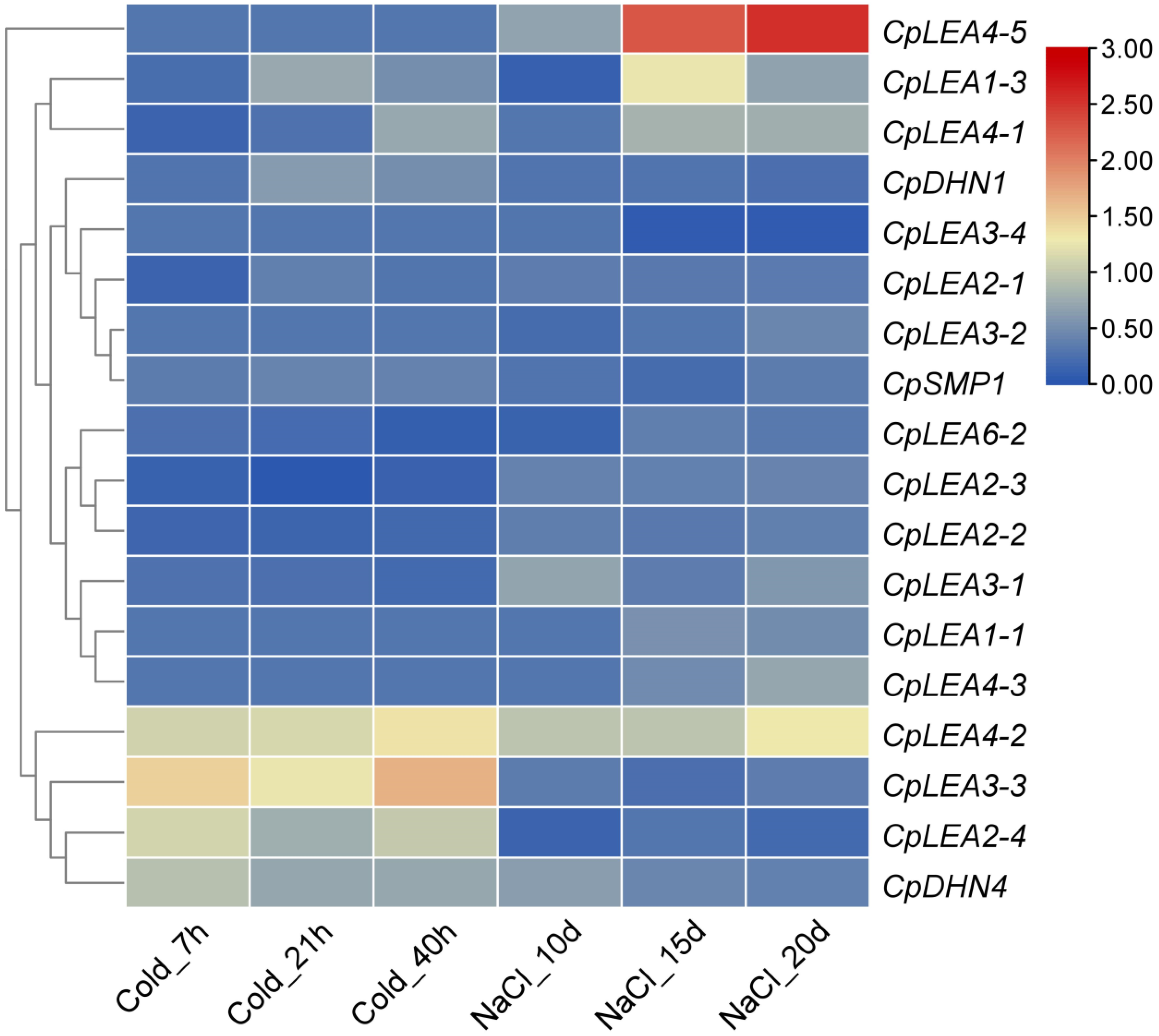
2.5. Plant Materials, RNA-seq, and Gene Expression Analysis
3. Results
3.1. Identification, Chromosome Localization, and Synteny Analysis of 28 LEA Genes in Papaya
3.2. Identification of LEA Genes in Horseradish Tree and Spider Flower and Definition of Orthogroups
3.3. Exon-Intron Structure, Phylogenetic Analysis, and Structural Characterization
3.3.1. LEA_1
3.3.2. LEA_2
3.3.3. LEA_3
3.3.4. LEA_4
3.3.5. LEA_5
3.3.6. LEA_6
3.3.7. DHN
3.3.8. SMP
3.4. ABRE and LTRE cis-Acting Elements Present in the Promoter Region of CpLEA Genes
3.5. Tissue-Specific Expression Profiles of CpLEA Genes
3.6. Expression Patterns of CpLEA Genes during Fruit Development
3.7. Expression Patterns of CpLEA Genes under Drought, Cold and Salt Stresses
4. Discussion
4.1. Small Number but High Diversity of LEA Genes in Papaya
4.2. Comparative Genomics Analysis Reveals Lineage-Specific Evolution of the LEA Superfamily in Brassicales
4.3. Diverse Expression Patterns of CpLEA Genes and a Role in Fruit Development and Abiotic Stress Responses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Battaglia, M.; Olvera-Carrillo, Y.; Garciarrubio, A.; Campos, F.; Covarrubias, A.A. The enigmatic LEA proteins and other hydrophilins. Plant. Physiol. 2008, 148, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Bies-Etheve, N.; Gaubier-Comella, P.; Debures, A.; Lasserre, E.; Jobet, E.; Raynal, M.; Cooke, R.; Delseny, M. Inventory, evolution and expression profiling diversity of the LEA (late embryogenesis abundant) protein gene family in Arabidopsis thaliana. Plant. Mol. Biol. 2008, 67, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Hundertmark, M.; Hincha, D.K. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Dure, L.; Chlan, C. Developmental biochemistry of cottonseed embryogenesis and germination. XII. Purification and properties of principal storage proteins. Plant. Physiol. 1981, 68, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Dure, L.; Galau, G.A. Developmental biochemistry of cottonseed embryogenesis and germination. XIII. Regulation of biosynthesis of principal storage proteins. Plant. Physiol. 1981, 68, 187–194. [Google Scholar] [CrossRef]
- Dure, L.; Greenway, S.C.; Galau, G.A. Developmental biochemistry of cottonseed embryogenesis and germination: Changing messenger ribonucleic acid populations as shown by in vitro and in vivo protein synthesis. Biochemistry 1981, 20, 4162–4168. [Google Scholar] [CrossRef]
- Hand, S.C.; Menze, M.A.; Toner, M.; Boswell, L.; Moore, D. LEA proteins during water stress: Not just for plants anymore. Annu. Rev. Physiol. 2011, 73, 115–134. [Google Scholar] [CrossRef]
- Zou, Z.; Huang, Q.X.; An, F. Genome-wide identification, classification and phylogenetic analysis of LEA gene family in castor bean (Ricinus communis L.). Chin. J. Oil Crop. Sci. 2013, 35, 637–643. [Google Scholar]
- Artur, M.A.S.; Zhao, T.; Ligterink, W.; Schranz, E.; Hilhorst, H.W. Dissecting the genomic diversification of late embryogenesis abundant (LEA) protein gene families in plants. Genome Biol. Evol. 2019, 11, 459–471. [Google Scholar] [CrossRef]
- Raynal, M.; Guilleminot, J.; Gueguen, C.; Cooke, R.; Delseny, M.; Gruber, V. Structure, organization and expression of two closely related novel Lea (late-embryogenesis abundant) genes in Arabidopsis thaliana. Plant. Mol. Biol. 1999, 40, 153–165. [Google Scholar] [CrossRef]
- Singh, S.; Cornilescu, C.C.; Tyler, R.C.; Cornilescu, G.; Tonelli, M.; Lee, M.S.; Markley, J.L. Solution structure of a late embryogenesis abundant protein (LEA14) from Arabidopsis thaliana, a cellular stress-related protein. Protein Sci. 2005, 14, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Zhu, H.B.; Jin, G.L.; Liu, H.L.; Wu, W.R.; Zhu, J. Genome-scale identification and analysis of LEA genes in rice (Oryza sativa L.). Plant. Sci. 2007, 172, 414–420. [Google Scholar] [CrossRef]
- Wu, C.; Hu, W.; Yan, Y.; Tie, W.; Ding, Z.; Guo, J.; He, G. The late embryogenesis abundant protein family in cassava (Manihot esculenta Crantz): Genome-wide characterization and expression during abiotic stress. Molecules 2018, 23, 1196. [Google Scholar] [CrossRef]
- Salleh, F.M.; Evans, K.; Goodall, B.; Machin, H.; Mowla, S.B.; Mur, L.A.; Runions, J.; Theodoulou, F.L.; Foyer, C.H.; Rogers, H.J. A novel function for a redox-related LEA protein (SAG21/AtLEA5) in root development and biotic stress responses. Plant. Cell Environ. 2012, 35, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.X.; Popova, A.V.; Hundertmark, M.; Hincha, D.K. Functional characterization of selected LEA proteins from Arabidopsis thaliana in yeast and in vitro. Planta 2014, 240, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, S.; Jiang, C.; Wang, Y.; Lv, B.; Shen, J.; Ming, F. RcLEA, a late embryogenesis abundant protein gene isolated from Rosa chinensis, confers tolerance to Escherichia coli and Arabidopsis thaliana and stabilizes enzyme activity under diverse stresses. Plant. Mol. Biol. 2014, 85, 333–347. [Google Scholar] [CrossRef]
- Xiang, D.J.; Man, L.L.; Zhang, C.L.; Li, Z.G.; Zheng, G.C. A new Em-like protein from Lactuca sativa, LsEm1, enhances drought and salt stress tolerance in Escherichia coli and rice. Protoplasma 2018, 255, 1089–1106. [Google Scholar] [CrossRef]
- Hernández-Sánchez, I.E.; Maruri-López, I.; Molphe-Balch, E.P.; Becerra-Flora, A.; Jaimes-Miranda, F.; Jiménez-Bremont, J.F. Evidence for in vivo interactions between dehydrins and the aquaporin AtPIP2B. Biochem. Bioph. Res. Commun. 2019, 510, 545–550. [Google Scholar] [CrossRef]
- Bowers, J.E.; Chapman, B.A.; Rong, J.; Paterson, A.H. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 2003, 422, 433–438. [Google Scholar] [CrossRef]
- Ming, R.; Hou, S.; Feng, Y.; Yu, Q.; Dionne-Laporte, A.; Saw, J.H.; Senin, P.; Wang, W.; Ly, B.V.; Lewis, K.L.; et al. The draft genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus). Nature 2008, 452, 991–996. [Google Scholar] [CrossRef]
- Cheng, S.; van den Bergh, E.; Zeng, P.; Zhong, X.; Xu, J.; Liu, X.; Hofberger, J.; de Bruijn, S.; Bhide, A.S.; Kuelahoglu, C.; et al. The Tarenaya hassleriana genome provides insight into reproductive trait and genome evolution of crucifers. Plant. Cell 2013, 25, 2813–2830. [Google Scholar] [CrossRef] [PubMed]
- Shyamli, P.S.; Pradhan, S.; Panda, M.; Parida, A. De novo whole-genome assembly of Moringa oleifera helps identify genes regulating drought stress tolerance. Front. Plant. Sci. 2021, 12, 766999. [Google Scholar] [CrossRef] [PubMed]
- Ming, R.; Moore, P. Genetics and Genomics of Papaya. Plant Genetics and Genomics: Crops and Models; Springer: Cham, Switzerland, 2014; Volume 10. [Google Scholar] [CrossRef]
- Allan, P. Carica papaya responses under cool subtropical growth conditions. Acta Hortic. 2002, 575, 757–763. [Google Scholar] [CrossRef]
- Mahouachi, J.; Socorro, A.; Talon, M. Responses of papaya seedlings (Carica papaya L.) to water stress and re-hydration: Growth, photosynthesis and mineral nutrient imbalance. Plant. Soil. 2006, 281, 137–146. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Zou, Z.; Yang, L.; Gong, J.; Mo, Y.; Wang, J.; Cao, J.; An, F.; Xie, G. Genome-wide identification of Jatropha curcas aquaporin genes and the comparative analysis provides insights into the gene family expansion and evolution in Hevea brasiliensis. Front. Plant. Sci. 2016, 7, 395. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization-diploidization cycles in plants. Genome Biol. 2019, 20, 38. [Google Scholar] [CrossRef]
- Moreno-Hagelsieb, G.; Latimer, K. Choosing BLAST options for better detection of orthologs as reciprocal best hits. Bioinformatics 2008, 24, 319–324. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Gong, J.; An, F.; Xie, G.; Wang, J.; Mo, Y.; Yang, L. Genome-wide identification of rubber tree (Hevea brasiliensis Muell. Arg.) aquaporin genes and their response to ethephon stimulation in the laticifer, a rubber-producing tissue. BMC Genom. 2015, 16, 1001. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Yang, J.H.; Zhang, X.C. Insights into genes encoding respiratory burst oxidase homologs (RBOHs) in rubber tree (Hevea brasiliensis Muell. Arg.). Ind. Crop. Prod. 2019, 128, 126–139. [Google Scholar] [CrossRef]
- Gamboa-Tuz, S.D.; Pereira-Santana, A.; Zamora-Briseño, J.A.; Castano, E.; Espadas-Gil, F.; Ayala-Sumuano, J.T.; Keb-Llanes, M.Á.; Sanchez-Teyer, F.; Rodríguez-Zapata, L.C. Transcriptomics and co-expression networks reveal tissue-specific responses and regulatory hubs under mild and severe drought in papaya (Carica papaya L.). Sci. Rep. 2018, 8, 14539. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Yue, J.; VanBuren, R.; Liu, J.; Fang, J.; Zhang, X.; Liao, Z.; Wai, C.M.; Xu, X.; Chen, S.; Zhang, S.; et al. SunUp and Sunset genomes revealed impact of particle bombardment mediated transformation and domestication history in papaya. Nat. Genet. 2022, 54, 715–724. [Google Scholar] [CrossRef]
- Dure, L. A repeating 11-mer amino acid motif and plant desiccation. Plant. J. 1993, 3, 363–369. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, X.; Paterson, A.H. Different patterns of gene structure divergence following gene duplication in Arabidopsis. BMC Genom. 2013, 14, 652. [Google Scholar] [CrossRef] [PubMed]
- Colmenero-Flores, J.M.; Moreno, L.P.; Smith, C.E.; Covarrubias, A.A. Pvlea18, a member of a new late-embryogenesis-abundant protein family that accumulates during water stress and in the growing region of well-irrigated bean seedlings. Plant. Physiol. 1999, 120, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zhao, Y.; Zhang, L.; Xiao, Y.; Guo, A. Analysis of Cyperus esculentus SMP family genes reveals lineage-specific evolution and seed desiccation-like transcript accumulation during tuber maturation. Ind. Crop. Prod. 2022, 187, 115382. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant. Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant. Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Nylander, M.; Svensson, J.; Palva, E.T.; Welin, B.V. Stress-induced accumulation and tissue-specific localization of dehydrins in Arabidopsis thaliana. Plant. Mol. Biol. 2001, 45, 263–279. [Google Scholar] [CrossRef]
- Alsheikh, M.K.; Svensson, J.T.; Randall, S.K. Phosphorylation regulated ion-binding is a property shared by the acidic subclass dehydrins. Plant. Cell Environ. 2005, 28, 1114–1122. [Google Scholar] [CrossRef]
- Candat, A.; Paszkiewicz, G.; Neveu, M.; Gautier, R.; Logan, D.C.; Avelange-Macherel, M.H.; Macherel, D. The ubiquitous distribution of late embryogenesis abundant proteins across cell compartments in Arabidopsis offers tailored protection against abiotic stress. Plant. Cell 2014, 26, 3148–3166. [Google Scholar] [CrossRef]
- Lü, P.; Yu, S.; Zhu, N.; Chen, Y.R.; Zhou, B.; Pan, Y.; Tzeng, D.; Fabi, J.P.; Argyris, J.; Garcia-Mas, J.; et al. Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nat. Plants. 2018, 4, 784–791. [Google Scholar] [CrossRef]
- Rensing, S.A.; Lang, D.; Zimmer, A.D.; Terry, A.; Salamov, A.; Shapiro, H.; Nishiyama, T.; Perroud, P.F.; Lindquist, E.A.; Kamisugi, Y.; et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 2008, 319, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Van de Peer, Y.; Fawcett, J.A.; Proost, S.; Sterck, L.; Vandepoele, K. The flowering world: A tale of duplications. Trends Plant. Sci. 2009, 14, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Leebens-Mack, J.; Ayyampalayam, S.; Bowers, J.E.; McKain, M.R.; McNeal, J.; Rolf, M.; Ruzicka, D.R.; Wafula, E.; Wickett, N.J.; et al. A genome triplication associated with early diversification of the core eudicots. Genome Biol. 2012, 13, R3. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, K.; Baele, G.; Maere, S.; Van de Peer, Y. Analysis of 41 plant genomes supports a wave of successful genome duplications in association with the Cretaceous-Paleogene boundary. Genome Res. 2014, 24, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.P.; Crabtree, J.; Zhao, Q.; Lorenzi, H.; Orvis, J.; Puiu, D.; Melake-Berhan, A.; Jones, K.M.; Redman, J.; Chen, G. Draft genome sequence of the oilseed species Ricinus communis. Nat. Biotechnol. 2010, 28, 951–956. [Google Scholar] [CrossRef]
- Koonin, E.V. Orthologs, paralogs, and evolutionary genomics. Annu. Rev. Genet. 2005, 39, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.A.; Renner, S.S. The Phylogeny of the Caricaceae. In Genetics and Genomics of Papaya. Plant Genetics and Genomics: Crops and Models; Ming, R., Moore, P., Eds.; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Luo, M.C.; You, F.M.; Li, P.; Wang, J.R.; Zhu, T.; Dandekar, A.M.; Leslie, C.A.; Aradhya, M.; McGuire, P.E.; Dvorak, J. Synteny analysis in Rosids with a walnut physical map reveals slow genome evolution in long-lived woody perennials. BMC Genom. 2015, 16, 707. [Google Scholar] [CrossRef]




| Family | Gene Name | Locus | AS | Deduced Protein | |||||
|---|---|---|---|---|---|---|---|---|---|
| Sunset | ASGPBv0.4 | AA | MW (kDa) | pI | GRAVY | Loc | |||
| LEA_1 | CpLEA1-1 | sunset05G0006380 | evm.TU.supercontig_18.65 | - | 160 | 17.01 | 9.65 | −0.755 | Nucl |
| CpLEA1-2 | sunset05G0013060 | evm.TU.supercontig_41.41 | - | 102 | 11.41 | 7.03 | −0.908 | Mito | |
| CpLEA1-3 | sunset08G0019430 | evm.TU.supercontig_85.72 | Yes | 158 | 16.07 | 8.83 | −0.878 | Mito | |
| LEA_2 | CpLEA2-1 | sunset05G0003590 | evm.TU.supercontig_9.242 | Yes | 316 | 35.12 | 4.69 | −0.384 | Cyto |
| CpLEA2-2 | sunset05G0009060 | evm.TU.supercontig_11.66 | Yes | 305 | 34.10 | 5.38 | −0.243 | Chlo | |
| CpLEA2-3 | sunset05G0009070 | evm.TU.supercontig_11.68 | Yes | 185 | 20.23 | 5.65 | −0.056 | Chlo | |
| CpLEA2-4 | sunset05G0009080 | evm.TU.supercontig_11.69 | Yes | 151 | 16.16 | 4.75 | 0.094 | Cyto | |
| LEA_3 | CpLEA3-1 | sunset03G0023320 | evm.TU.supercontig_16.192 | - | 103 | 11.21 | 10.07 | −0.472 | Chlo |
| CpLEA3-2 | sunset04G0017920 | evm.TU.supercontig_25.184 | Yes | 98 | 10.94 | 9.52 | −0.526 | Chlo | |
| CpLEA3-3 | sunset05G0003680 | evm.TU.supercontig_9.251 | Yes | 99 | 10.61 | 9.89 | −0.531 | Cyto | |
| CpLEA3-4 | sunset05G0018090 | evm.TU.supercontig_2471.1 | Yes | 95 | 10.62 | 9.66 | −0.997 | Mito | |
| CpLEA3-5 | sunset06G0002130 | evm.TU.supercontig_200.7 | - | 104 | 11.78 | 9.69 | −0.839 | Cyto | |
| LEA_4 | CpLEA4-1 | sunset01G0016400 | evm.TU.supercontig_66.6 | - | 590 | 66.20 | 8.91 | −0.515 | Extr |
| CpLEA4-2 | sunset03G0025310 | evm.TU.supercontig_209.19 | - | 581 | 61.45 | 5.20 | −0.864 | Nucl | |
| CpLEA4-3 | sunset05G0000220 | evm.TU.supercontig_146.20 | - | 193 | 21.63 | 5.21 | −1.053 | Extr | |
| CpLEA4-4 | sunset07G0004690 | evm.TU.supercontig_464.2 | - | 222 | 24.57 | 8.95 | −1.333 | Chlo | |
| CpLEA4-5 | sunset08G0016230 | evm.TU.supercontig_5.110 | Yes | 280 | 30.34 | 6.17 | −1.360 | Nucl | |
| LEA_5 | CpLEA5-1 | sunset02G0011780 | evm.TU.supercontig_19.160 | - | 89 | 9.64 | 5.51 | −1.319 | Cyto |
| CpLEA5-2 | sunset08G0009640 | evm.TU.supercontig_2485.2 | - | 111 | 12.10 | 5.51 | −1.338 | Nucl | |
| LEA_6 | CpLEA6-1 | sunset01G0017510 | evm.TU.supercontig_88.61 | - | 97 | 10.42 | 5.56 | −0.705 | Nucl |
| CpLEA6-2 | sunset04G0003310 | evm.TU.supercontig_6.54 | - | 78 | 8.77 | 5.22 | −1.573 | Nucl | |
| DHN | CpDHN1 | sunset01G0014930 | evm.TU.supercontig_26.225 | Yes | 211 | 24.10 | 5.05 | −1.584 | Nucl |
| CpDHN2 | sunset04G0004410 | evm.TU.supercontig_6.176 | - | 137 | 14.76 | 9.45 | −1.222 | Nucl | |
| CpDHN3 | sunset06G0003520 | evm.TU.supercontig_106.3 | Yes | 167 | 17.93 | 5.94 | −1.265 | Nucl | |
| CpDHN4 | sunset06G0021280 | evm.TU.supercontig_161.14 | Yes | 93 | 10.50 | 6.62 | −1.984 | Nucl | |
| SMP | CpSMP1 | sunset03G0005590 | evm.TU.supercontig_58.99 | - | 262 | 26.70 | 4.70 | −0.270 | Chlo |
| CpSMP2 | sunset03G0027120 | evm.TU.supercontig_487.3 | - | 267 | 27.97 | 4.56 | −0.246 | Cyto | |
| CpSMP3 | sunset06G0024460 | evm.TU.contig_34050.2 | - | 244 | 25.13 | 6.44 | −0.359 | Nucl | |
| Family | Orthogroup | Papaya | Horseradish Tree | Spider Flower | Castor Been | Arabidopsis |
|---|---|---|---|---|---|---|
| LEA_1 | LEA1a | CpLEA1-1 | MoLEA1-1 | ThLEA1-1 ThLEA1-2 | RcLEA1-2 | AtLEA6 AtLEA18 |
| LEA1b | CpLEA1-2 | MoLEA1-2 | - | RcLEA1-1 | - | |
| LEA1c | CpLEA1-3 | MoLEA1-3 | ThLEA1-3 ThLEA1-4 | RcLEA1-3 | AtLEA46 | |
| LEA_2 | LEA2a | CpLEA2-1 | MoLEA2-1 | ThLEA2-1 ThLEA2-2 | RcLEA2-2 | AtLEA26 |
| LEA2b | CpLEA2-2 CpLEA2-3 | - | ThLEA2-3 ThLEA2-4 ThLEA2-5 ThLEA2-6 | - | - | |
| LEA2c | CpLEA2-4 | MoLEA2-2 | ThLEA2-7 | RcLEA2-1 | AtLEA1 AtLEA27 | |
| LEA_3 | LEA3a | CpLEA3-1 | MoLEA3-1 | ThLEA3-1 | RcLEA3-5 | AtLEA41 |
| LEA3b | CpLEA3-2 | MoLEA3-2 | ThLEA3-2 | RcLEA3-4 | AtLEA37 | |
| LEA3c | CpLEA3-3 | MoLEA3-3 | ThLEA3-3 ThLEA3-4 | RcLEA3-1 | AtLEA2 AtLEA38 | |
| LEA3d | CpLEA3-4 | MoLEA3-4 | ThLEA3-5 | RcLEA3-2 | - | |
| LEA3e | CpLEA3-5 | MoLEA3-5 | - | RcLEA3-3 | - | |
| LEA_4 | LEA4a | CpLEA4-1 | MoLEA4-1 | ThLEA4-1 | - | AtLEA9 |
| LEA4b | CpLEA4-2 | MoLEA4-2 | ThLEA4-2 | RcLEA4-2 | AtLEA25 | |
| LEA4c | CpLEA4-3 | MoLEA4-3 | - | RcLEA4-4 | AtLEA30 | |
| LEA4d | CpLEA4-4 | MoLEA4-4 | ThLEA4-3 | RcLEA4-3 | AtLEA42 AtLEA48 | |
| LEA4e | CpLEA4-5 | MoLEA4-5 | ThLEA4-4 | RcLEA4-5 | AtLEA19 AtLEA36 | |
| LEA4f | - | MoLEA4-6 | ThLEA4-5 ThLEA4-6 | RcLEA4-1 | AtLEA13 AtLEA43 | |
| LEA_5 | LEA5a | CpLEA5-1 | MoLEA5-1 | ThLEA5-1 | RcLEA5-1 | AtLEA20 |
| LEA5b | CpLEA5-2 | MoLEA5-2 MoLEA5-3 | ThLEA5-2 | RcLEA5-2 | AtLEA35 | |
| LEA_6 | LEA6a | CpLEA6-1 | MoLEA6-1 | ThLEA6-1 | RcLEA6-1 | AtLEA17 |
| LEA6b | CpLEA6-2 | MoLEA6-2 | ThLEA6-2 | RcLEA6-2 | AtLEA15 AtLEA16 | |
| DHN | DHNa | CpDHN1 | MoDHN1 | ThDHN1 ThDHN2 | RcDHN1 | AtLEA4 AtLEA5 AtLEA10 |
| DHNb | CpDHN2 | MoDHN2 | ThDHN3 | RcDHN2 RcDHN3 | AtLEA33 AtLEA34 AtLEA51 | |
| DHNc | CpDHN3 | MoDHN3 | ThDHN4 ThDHN5 | RcDHN4 | AtLEA14 AtLEA45 | |
| DHNd | CpDHN4 | MoDHN4 | ThDHN6 ThDHN7 ThDHN8 | RcDHN5 | AtLEA8 | |
| DHNe | - | MoDHN5 | ThDHN9 | - | - | |
| SMP | SMPa | CpSMP1 | MoSMP1 | ThSMP1 | RcSMP3 | AtLEA31 AtLEA32 |
| SMPb | CpSMP2 | MoSMP2 | ThSMP2 | RcSMP1 RcSMP2 | AtLEA3 | |
| SMPc | CpSMP3 | MoSMP3 | ThSMP3 ThSMP4 | RcSMP4 | AtLEA47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Z.; Guo, J.; Zheng, Y.; Xiao, Y.; Guo, A. Genomic Analysis of LEA Genes in Carica papaya and Insight into Lineage-Specific Family Evolution in Brassicales. Life 2022, 12, 1453. https://doi.org/10.3390/life12091453
Zou Z, Guo J, Zheng Y, Xiao Y, Guo A. Genomic Analysis of LEA Genes in Carica papaya and Insight into Lineage-Specific Family Evolution in Brassicales. Life. 2022; 12(9):1453. https://doi.org/10.3390/life12091453
Chicago/Turabian StyleZou, Zhi, Jingyuan Guo, Yujiao Zheng, Yanhua Xiao, and Anping Guo. 2022. "Genomic Analysis of LEA Genes in Carica papaya and Insight into Lineage-Specific Family Evolution in Brassicales" Life 12, no. 9: 1453. https://doi.org/10.3390/life12091453
APA StyleZou, Z., Guo, J., Zheng, Y., Xiao, Y., & Guo, A. (2022). Genomic Analysis of LEA Genes in Carica papaya and Insight into Lineage-Specific Family Evolution in Brassicales. Life, 12(9), 1453. https://doi.org/10.3390/life12091453








