Natural Compounds: Potential Therapeutics for the Inhibition of Cartilage Matrix Degradation in Osteoarthritis
Abstract
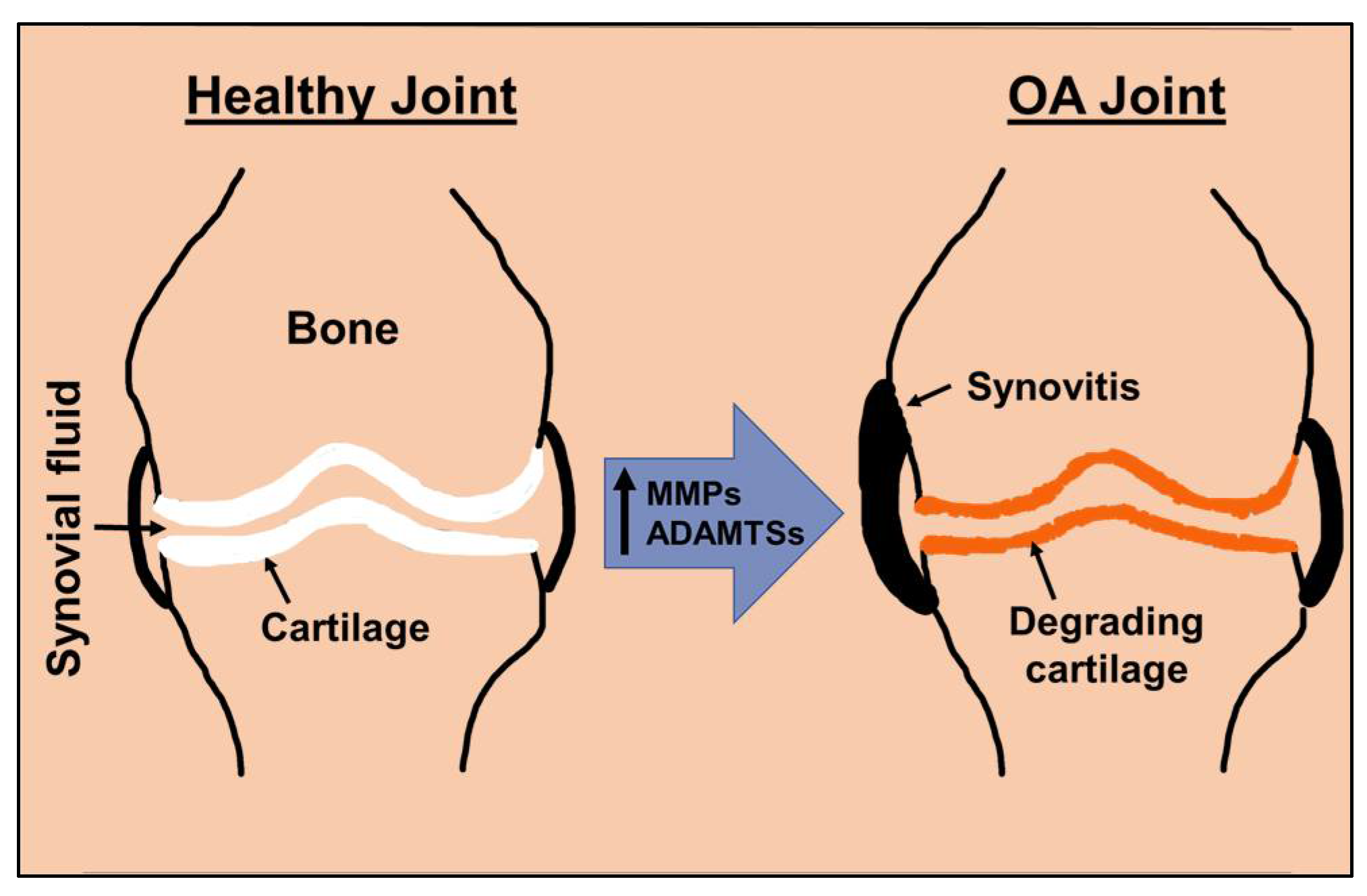
:1. Introduction
2. Matrix Degradation Is Central to Osteoarthritis
3. Signaling Pathways Regulating Matrix Degradation in Osteoarthritis
4. Natural Compounds, Suppressors of Cartilage Matrix Degradation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehana, E.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef]
- Horkay, F. Interactions of Cartilage Extracellular Matrix Macromolecules. J. Polym. Sci. B Polym. Phys. 2012, 50, 1699–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentili, C.; Cancedda, R. Cartilage and bone extracellular matrix. Curr. Pharm. Des. 2009, 15, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.A.; Flint, M.H.; Beaumont, B.W. Chondrons in cartilage: Ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J. Orthop. Res. 1987, 5, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R.; Kobayashi, M.; Yasuda, T.; Laverty, S.; Mwale, F.; Kojima, T.; Sakai, T.; Wahl, C.; El-Maadawy, S.; Webb, G.; et al. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann. Rheum. Dis. 2002, 61 (Suppl. S2), ii78–ii81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sport. Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, M.Y.; Ahmad, N.; Voleti, S.; Wase, S.J.; Novak, K.; Haqqi, T.M. Mitochondrial dysfunction triggers a catabolic response in chondrocytes via ROS-mediated activation of the JNK/AP1 pathway. J. Cell Sci. 2020, 133, jcs247353. [Google Scholar] [CrossRef]
- van den Berg, W.B. Osteoarthritis year 2010 in review: Pathomechanisms. Osteoarthr. Cartil. 2011, 19, 338–341. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Yuan, Y.; Min, J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res. Ther. 2017, 19, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Sampson, E.R.; Jin, H.; Li, J.; Ke, Q.H.; Im, H.J.; Chen, D. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res. Ther. 2013, 15, R5. [Google Scholar] [CrossRef] [Green Version]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Stone, A.V.; Loeser, R.F.; Vanderman, K.S.; Long, D.L.; Clark, S.C.; Ferguson, C.M. Pro-inflammatory stimulation of meniscus cells increases production of matrix metalloproteinases and additional catabolic factors involved in osteoarthritis pathogenesis. Osteoarthr. Cartil. 2014, 22, 264–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuhold, L.A.; Killar, L.; Zhao, W.; Sung, M.L.; Warner, L.; Kulik, J.; Turner, J.; Wu, W.; Billinghurst, C.; Meijers, T.; et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J. Clin. Invest. 2001, 107, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baragi, V.M.; Becher, G.; Bendele, A.M.; Biesinger, R.; Bluhm, H.; Boer, J.; Deng, H.; Dodd, R.; Essers, M.; Feuerstein, T.; et al. A new class of potent matrix metalloproteinase 13 inhibitors for potential treatment of osteoarthritis: Evidence of histologic and clinical efficacy without musculoskeletal toxicity in rat models. Arthritis Rheum. 2009, 60, 2008–2018. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, J.; Zhao, S.; Wu, J.; Jin, Y.; Yu, L.; Wu, N.; Wu, Z.; Wang, Y.; Lin, M. ADAMTS5 in Osteoarthritis: Biological Functions, Regulatory Network, and Potential Targeting Therapies. Front. Mol. Biosci. 2021, 8, 703110. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Dalal, K. ADAMTS-4 and ADAMTS-5: Key enzymes in osteoarthritis. J. Cell. Biochem. 2011, 112, 3507–3514. [Google Scholar] [CrossRef]
- Glasson, S.S.; Askew, R.; Sheppard, B.; Carito, B.A.; Blanchet, T.; Ma, H.L.; Flannery, C.R.; Kanki, K.; Wang, E.; Peluso, D.; et al. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 2004, 50, 2547–2558. [Google Scholar] [CrossRef]
- Majumdar, M.K.; Askew, R.; Schelling, S.; Stedman, N.; Blanchet, T.; Hopkins, B.; Morris, E.A.; Glasson, S.S. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 2007, 56, 3670–3674. [Google Scholar] [CrossRef]
- Glasson, S.S.; Askew, R.; Sheppard, B.; Carito, B.; Blanchet, T.; Ma, H.L.; Flannery, C.R.; Peluso, D.; Kanki, K.; Yang, Z.; et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 2005, 434, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Yan, X.; Zhang, M.; Chang, X.; Bai, Z.; He, Y.; Yuan, Z. Aggrecanases in the human synovial fluid at different stages of osteoarthritis. Clin. Rheumatol. 2013, 32, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Chiusaroli, R.; Visentini, M.; Galimberti, C.; Casseler, C.; Mennuni, L.; Covaceuszach, S.; Lanza, M.; Ugolini, G.; Caselli, G.; Rovati, L.C.; et al. Targeting of ADAMTS5’s ancillary domain with the recombinant mAb CRB0017 ameliorates disease progression in a spontaneous murine model of osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1807–1810. [Google Scholar] [CrossRef] [Green Version]
- Larkin, J.; Lohr, T.A.; Elefante, L.; Shearin, J.; Matico, R.; Su, J.L.; Xue, Y.; Liu, F.; Genell, C.; Miller, R.E.; et al. Translational development of an ADAMTS-5 antibody for osteoarthritis disease modification. Osteoarthr. Cartil. 2015, 23, 1254–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santamaria, S.; Yamamoto, K.; Botkjaer, K.; Tape, C.; Dyson, M.R.; McCafferty, J.; Murphy, G.; Nagase, H. Antibody-based exosite inhibitors of ADAMTS-5 (aggrecanase-2). Biochem. J. 2015, 471, 391–401. [Google Scholar] [CrossRef] [Green Version]
- Siebuhr, A.S.; Werkmann, D.; Bay-Jensen, A.C.; Thudium, C.S.; Karsdal, M.A.; Serruys, B.; Ladel, C.; Michaelis, M.; Lindemann, S. The Anti-ADAMTS-5 Nanobody((R)) M6495 Protects Cartilage Degradation Ex Vivo. Int. J. Mol. Sci. 2020, 21, 5992. [Google Scholar] [CrossRef]
- Brebion, F.; Gosmini, R.; Deprez, P.; Varin, M.; Peixoto, C.; Alvey, L.; Jary, H.; Bienvenu, N.; Triballeau, N.; Blanque, R.; et al. Discovery of GLPG1972/S201086, a Potent, Selective, and Orally Bioavailable ADAMTS-5 Inhibitor for the Treatment of Osteoarthritis. J. Med. Chem. 2021, 64, 2937–2952. [Google Scholar] [CrossRef]
- Chockalingam, P.S.; Sun, W.; Rivera-Bermudez, M.A.; Zeng, W.; Dufield, D.R.; Larsson, S.; Lohmander, L.S.; Flannery, C.R.; Glasson, S.S.; Georgiadis, K.E.; et al. Elevated aggrecanase activity in a rat model of joint injury is attenuated by an aggrecanase specific inhibitor. Osteoarthr. Cartil. 2011, 19, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; O’Keefe, H.; Davie, C.P.; Lind, K.E.; Acharya, R.A.; Franklin, G.J.; Larkin, J.; Matico, R.; Neeb, M.; Thompson, M.M.; et al. Discovery of highly potent and selective small molecule ADAMTS-5 inhibitors that inhibit human cartilage degradation via encoded library technology (ELT). J. Med. Chem. 2012, 55, 7061–7079. [Google Scholar] [CrossRef]
- Durham, T.B.; Marimuthu, J.; Toth, J.L.; Liu, C.; Adams, L.; Mudra, D.R.; Swearingen, C.; Lin, C.; Chambers, M.G.; Thirunavukkarasu, K.; et al. A Highly Selective Hydantoin Inhibitor of Aggrecanase-1 and Aggrecanase-2 with a Low Projected Human Dose. J. Med. Chem. 2017, 60, 5933–5939. [Google Scholar] [CrossRef]
- Ben-Aderet, L.; Merquiol, E.; Fahham, D.; Kumar, A.; Reich, E.; Ben-Nun, Y.; Kandel, L.; Haze, A.; Liebergall, M.; Kosinska, M.K.; et al. Detecting cathepsin activity in human osteoarthritis via activity-based probes. Arthritis Res. Ther. 2015, 17, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mort, J.S.; Magny, M.C.; Lee, E.R. Cathepsin B: An alternative protease for the generation of an aggrecan ‘metalloproteinase’ cleavage neoepitope. Biochem. J. 1998, 335 Pt 3, 491–494. [Google Scholar] [CrossRef] [Green Version]
- Morko, J.P.; Soderstrom, M.; Saamanen, A.M.; Salminen, H.J.; Vuorio, E.I. Up regulation of cathepsin K expression in articular chondrocytes in a transgenic mouse model for osteoarthritis. Ann. Rheum. Dis. 2004, 63, 649–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen-Mankonen, H.J.; Morko, J.; Vuorio, E. Role of cathepsin K in normal joints and in the development of arthritis. Curr. Drug Targets 2007, 8, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Ginnetti, A.T.; Paone, D.V.; Nanda, K.K.; Li, J.; Busuek, M.; Johnson, S.A.; Lu, J.; Soisson, S.M.; Robinson, R.; Fisher, J.; et al. Lead optimization of cathepsin K inhibitors for the treatment of Osteoarthritis. Bioorg. Med. Chem. Lett. 2022, 74, 128927. [Google Scholar] [CrossRef]
- Lindstrom, E.; Rizoska, B.; Tunblad, K.; Edenius, C.; Bendele, A.M.; Maul, D.; Larson, M.; Shah, N.; Yoder Otto, V.; Jerome, C.; et al. The selective cathepsin K inhibitor MIV-711 attenuates joint pathology in experimental animal models of osteoarthritis. J. Transl. Med. 2018, 16, 56. [Google Scholar] [CrossRef] [Green Version]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Mueller, M.B.; Tuan, R.S. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: Identifying molecular targets. PMR J. Inj. Funct. Rehabil. 2011, 3, S3–S11. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharm. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Haqqi, T.M. Interleukin-1beta induced Stress Granules Sequester COX-2 mRNA and Regulates its Stability and Translation in Human OA Chondrocytes. Sci. Rep. 2016, 6, 27611. [Google Scholar] [CrossRef]
- Daheshia, M.; Yao, J.Q. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J. Rheumatol. 2008, 35, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado, M.; Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. BioMed. Res. Int. 2013, 2013, 284873. [Google Scholar] [CrossRef] [Green Version]
- Min, S.; Wang, C.; Lu, W.; Xu, Z.; Shi, D.; Chen, D.; Teng, H.; Jiang, Q. Serum levels of the bone turnover markers dickkopf-1, osteoprotegerin, and TNF-alpha in knee osteoarthritis patients. Clin. Rheumatol. 2017, 36, 2351–2358. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Beier, F. Mouse models of osteoarthritis: Modelling risk factors and assessing outcomes. Nat. Rev. Rheumatol. 2014, 10, 413–421. [Google Scholar] [CrossRef]
- Favero, M.; Belluzzi, E.; Trisolino, G.; Goldring, M.B.; Goldring, S.R.; Cigolotti, A.; Pozzuoli, A.; Ruggieri, P.; Ramonda, R.; Grigolo, B.; et al. Inflammatory molecules produced by meniscus and synovium in early and end-stage osteoarthritis: A coculture study. J. Cell. Physiol. 2019, 234, 11176–11187. [Google Scholar] [CrossRef]
- Nam, J.; Aguda, B.D.; Rath, B.; Agarwal, S. Biomechanical thresholds regulate inflammation through the NF-kappaB pathway: Experiments and modeling. PloS ONE 2009, 4, e5262. [Google Scholar] [CrossRef]
- Rigoglou, S.; Papavassiliou, A.G. The NF-kappaB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.Y.; Chin, K.Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Huang, Z.; Zhang, H.; Lu, J.; Tian, Y.; Wei, Y.; Yang, Y.; Bai, L. P2X7 Receptor Induces Pyroptotic Inflammation and Cartilage Degradation in Osteoarthritis via NF-kappaB/NLRP3 Crosstalk. Oxid. Med. Cell. Longev. 2021, 2021, 8868361. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, X.; Xing, L.; Tian, F. Wnt signaling: A promising target for osteoarthritis therapy. Cell Commun. Signal. 2019, 17, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, X.; Boker, K.O.; Taheri, S.; Hawellek, T.; Lehmann, W.; Schilling, A.F. The Interaction between microRNAs and the Wnt/beta-Catenin Signaling Pathway in Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 9887. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, R.; Ohta, Y.; Yuasa, T.; Kondo, N.; Hoang, T.; Addya, S.; Fortina, P.; Pacifici, M.; Iwamoto, M.; Enomoto-Iwamoto, M. Roles of beta-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab. Invest. 2011, 91, 1739–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Wang, T.; Hamilton, J.L.; Chen, D. Wnt/beta-catenin Signaling in Osteoarthritis and in Other Forms of Arthritis. Curr. Rheumatol. Rep. 2017, 19, 53. [Google Scholar] [CrossRef] [Green Version]
- Lietman, C.; Wu, B.; Lechner, S.; Shinar, A.; Sehgal, M.; Rossomacha, E.; Datta, P.; Sharma, A.; Gandhi, R.; Kapoor, M.; et al. Inhibition of Wnt/beta-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Li, Z.; Dai, A.; Yang, M.; Chen, S.; Deng, Z.; Li, L. p38MAPK Signaling Pathway in Osteoarthritis: Pathological and Therapeutic Aspects. J. Inflamm. Res. 2022, 15, 723–734. [Google Scholar] [CrossRef]
- Sondergaard, B.C.; Schultz, N.; Madsen, S.H.; Bay-Jensen, A.C.; Kassem, M.; Karsdal, M.A. MAPKs are essential upstream signaling pathways in proteolytic cartilage degradation—divergence in pathways leading to aggrecanase and MMP-mediated articular cartilage degradation. Osteoarthr. Cartil. 2010, 18, 279–288. [Google Scholar] [CrossRef]
- Liu, J.; Han, L.; Li, B.; Yang, J.; Huen, M.S.; Pan, X.; Tsao, S.W.; Cheung, A.L. F-box only protein 31 (FBXO31) negatively regulates p38 mitogen-activated protein kinase (MAPK) signaling by mediating lysine 48-linked ubiquitination and degradation of mitogen-activated protein kinase kinase 6 (MKK6). J. Biol. Chem. 2014, 289, 21508–21518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, C.N.; Cai, W.J.; Shi, J.; Yi, Z.J. MAPK inhibitors protect against earlystage osteoarthritis by activating autophagy. Mol. Med. Rep. 2021, 24, 829. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, C.; Yi, Z.; Lan, C. Explore the variation of MMP3, JNK, p38 MAPKs, and autophagy at the early stage of osteoarthritis. IUBMB Life 2016, 68, 293–302. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Butein Activates Autophagy Through AMPK/TSC2/ULK1/mTOR Pathway to Inhibit IL-6 Expression in IL-1beta Stimulated Human Chondrocytes. Cell. Physiol. Biochem. 2018, 49, 932–946. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Khan, N.M.; Haqqi, T.M. A standardized extract of Butea monosperma (Lam.) flowers suppresses the IL-1beta-induced expression of IL-6 and matrix-metalloproteases by activating autophagy in human osteoarthritis chondrocytes. Biomed. Pharm. 2017, 96, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Smith, B.J.; Lo, D.F.; Chyu, M.C.; Dunn, D.M.; Chen, C.H.; Kwun, I.S. Dietary polyphenols and mechanisms of osteoarthritis. J. Nutr. Biochem. 2012, 23, 1367–1377. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, L. The protective activity of genistein against bone and cartilage diseases. Front. Pharm. 2022, 13, 1016981. [Google Scholar] [CrossRef]
- Carvalho, A.M.S.; Heimfarth, L.; Santos, K.A.; Guimaraes, A.G.; Picot, L.; Almeida, J.; Quintans, J.S.S.; Quintans-Junior, L.J. Terpenes as possible drugs for the mitigation of arthritic symptoms—A systematic review. Phytomedicine 2019, 57, 137–147. [Google Scholar] [CrossRef]
- Lei, M.; Guo, C.; Hua, L.; Xue, S.; Yu, D.; Zhang, C.; Wang, D. Crocin Attenuates Joint Pain and Muscle Dysfunction in Osteoarthritis Rat. Inflammation 2017, 40, 2086–2093. [Google Scholar] [CrossRef]
- Ying, X.; Chen, X.; Cheng, S.; Shen, Y.; Peng, L.; Xu, H.Z. Piperine inhibits IL-beta induced expression of inflammatory mediators in human osteoarthritis chondrocyte. Int. Immunopharmacol. 2013, 17, 293–299. [Google Scholar] [CrossRef]
- Ahmed, S.; Wang, N.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J. Pharmacol. Exp. Ther. 2004, 308, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.T.; Cheng, T.L.; Ho, C.J.; Huang, H.H.; Lu, C.C.; Chuang, S.C.; Li, J.Y.; Lee, T.C.; Chen, S.T.; Lin, Y.S.; et al. Intra-Articular Injection of (-)-Epigallocatechin 3-Gallate to Attenuate Articular Cartilage Degeneration by Enhancing Autophagy in a Post-Traumatic Osteoarthritis Rat Model. Antioxidants 2020, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.T.; Cheng, T.L.; Yang, C.D.; Chang, C.F.; Ho, C.J.; Chuang, S.C.; Li, J.Y.; Huang, S.H.; Lin, Y.S.; Shen, H.Y.; et al. Intra-Articular Injection of (-)-Epigallocatechin 3-Gallate (EGCG) Ameliorates Cartilage Degeneration in Guinea Pigs with Spontaneous Osteoarthritis. Antioxidants 2021, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.J.; Choudhury, M.; Hanstein, R.; Hirsh, D.M.; Kim, S.J.; Majeska, R.J.; Schaffler, M.B.; Hardin, J.A.; Spray, D.C.; Goldring, M.B.; et al. Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse post-traumatic osteoarthritis model. Arthritis Res. Ther. 2014, 16, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natarajan, V.; Madhan, B.; Tiku, M.L. Intra-Articular Injections of Polyphenols Protect Articular Cartilage from Inflammation-Induced Degradation: Suggesting a Potential Role in Cartilage Therapeutics. PloS ONE 2015, 10, e0127165. [Google Scholar] [CrossRef] [PubMed]
- Mevel, E.; Merceron, C.; Vinatier, C.; Krisa, S.; Richard, T.; Masson, M.; Lesoeur, J.; Hivernaud, V.; Gauthier, O.; Abadie, J.; et al. Olive and grape seed extract prevents post-traumatic osteoarthritis damages and exhibits in vitro anti IL-1beta activities before and after oral consumption. Sci. Rep. 2016, 6, 33527. [Google Scholar] [CrossRef] [Green Version]
- Scotece, M.; Conde, J.; Abella, V.; Lopez, V.; Francisco, V.; Ruiz, C.; Campos, V.; Lago, F.; Gomez, R.; Pino, J.; et al. Oleocanthal Inhibits Catabolic and Inflammatory Mediators in LPS-Activated Human Primary Osteoarthritis (OA) Chondrocytes Through MAPKs/NF-kappaB Pathways. Cell. Physiol. Biochem. 2018, 49, 2414–2426. [Google Scholar] [CrossRef]
- Feng, Z.; Li, X.; Lin, J.; Zheng, W.; Hu, Z.; Xuan, J.; Ni, W.; Pan, X. Oleuropein inhibits the IL-1beta-induced expression of inflammatory mediators by suppressing the activation of NF-kappaB and MAPKs in human osteoarthritis chondrocytes. Food Funct. 2017, 8, 3737–3744. [Google Scholar] [CrossRef]
- Horcajada, M.N.; Beaumont, M.; Sauvageot, N.; Poquet, L.; Saboundjian, M.; Costes, B.; Verdonk, P.; Brands, G.; Brasseur, J.; Urbin-Choffray, D.; et al. An oleuropein-based dietary supplement may improve joint functional capacity in older people with high knee joint pain: Findings from a multicentre-RCT and post hoc analysis. Adv. Musculoskelet. Dis 2022, 14, 1759720X211070205. [Google Scholar] [CrossRef]
- Horcajada, M.N.; Sanchez, C.; Membrez Scalfo, F.; Drion, P.; Comblain, F.; Taralla, S.; Donneau, A.F.; Offord, E.A.; Henrotin, Y. Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig. Osteoarthr. Cartil. 2015, 23, 94–102. [Google Scholar] [CrossRef]
- Clutterbuck, A.L.; Allaway, D.; Harris, P.; Mobasheri, A. Curcumin reduces prostaglandin E2, matrix metalloproteinase-3 and proteoglycan release in the secretome of interleukin 1beta-treated articular cartilage. F1000Res 2013, 2, 147. [Google Scholar] [CrossRef] [PubMed]
- Clutterbuck, A.L.; Mobasheri, A.; Shakibaei, M.; Allaway, D.; Harris, P. Interleukin-1beta-induced extracellular matrix degradation and glycosaminoglycan release is inhibited by curcumin in an explant model of cartilage inflammation. Ann. New York Acad. Sci. 2009, 1171, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Mathy-Hartert, M.; Jacquemond-Collet, I.; Priem, F.; Sanchez, C.; Lambert, C.; Henrotin, Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2009, 58, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Nicoliche, T.; Maldonado, D.C.; Faber, J.; Silva, M. Evaluation of the articular cartilage in the knees of rats with induced arthritis treated with curcumin. PloS ONE 2020, 15, e0230228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Ma, J.; Gu, J.H.; Wang, F.Y.; Shang, X.S.; Tao, H.R.; Wang, X. Regulation of type II collagen, matrix metalloproteinase-13 and cell proliferation by interleukin-1beta is mediated by curcumin via inhibition of NF-kappaB signaling in rat chondrocytes. Mol. Med. Rep. 2017, 16, 1837–1845. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 128. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Tao, Z.; Cai, L.; Chen, C.; Zhang, C.; Wang, Q.; Ying, X.; Hu, W.; Chen, H. Chrysin Attenuates IL-1beta-Induced Expression of Inflammatory Mediators by Suppressing NF-kappaB in Human Osteoarthritis Chondrocytes. Inflammation 2017, 40, 1143–1154. [Google Scholar] [CrossRef]
- Zheng, W.; Feng, Z.; You, S.; Zhang, H.; Tao, Z.; Wang, Q.; Chen, H.; Wu, Y. Fisetin inhibits IL-1beta-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. Int. Immunopharmacol. 2017, 45, 135–147. [Google Scholar] [CrossRef]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin inhibits IL-1beta-induced in fl ammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharm. 2019, 109, 1586–1592. [Google Scholar] [CrossRef]
- Gu, H.; Li, K.; Li, X.; Yu, X.; Wang, W.; Ding, L.; Liu, L. Oral Resveratrol Prevents Osteoarthritis Progression in C57BL/6J Mice Fed a High-Fat Diet. Nutrients 2016, 8, 233. [Google Scholar] [CrossRef]
- Kang, D.G.; Lee, H.J.; Lee, C.J.; Park, J.S. Inhibition of the Expression of Matrix Metalloproteinases in Articular Chondrocytes by Resveratrol through Affecting Nuclear Factor-Kappa B Signaling Pathway. Biomol. Ther. 2018, 26, 560–567. [Google Scholar] [CrossRef]
- Li, W.; Cai, L.; Zhang, Y.; Cui, L.; Shen, G. Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating SIRT1 and thereby silencing HIF-2alpha. J. Orthop. Res. 2015, 33, 1061–1070. [Google Scholar] [CrossRef]
- Qin, N.; Wei, L.; Li, W.; Yang, W.; Cai, L.; Qian, Z.; Wu, S. Local intra-articular injection of resveratrol delays cartilage degeneration in C57BL/6 mice by inducing autophagy via AMPK/mTOR pathway. J. Pharmacol. Sci. 2017, 134, 166–174. [Google Scholar] [CrossRef]
- Ruangsuriya, J.; Budprom, P.; Viriyakhasem, N.; Kongdang, P.; Chokchaitaweesuk, C.; Sirikaew, N.; Chomdej, S.; Nganvongpanit, K.; Ongchai, S. Suppression of Cartilage Degradation by Zingerone Involving the p38 and JNK MAPK Signaling Pathway. Planta Med. 2017, 83, 268–276. [Google Scholar] [CrossRef]
- Huang, X.; Pan, Q.; Mao, Z.; Wang, P.; Zhang, R.; Ma, X.; Chen, J.; You, H. Kaempferol inhibits interleukin1beta stimulated matrix metalloproteinases by suppressing the MAPKassociated ERK and P38 signaling pathways. Mol. Med. Rep. 2018, 18, 2697–2704. [Google Scholar] [CrossRef] [Green Version]
- Panahi, Y.; Alishiri, G.H.; Bayat, N.; Hosseini, S.M.; Sahebkar, A. Efficacy of Elaeagnus Angustifolia extract in the treatment of knee osteoarthritis: A randomized controlled trial. EXCLI J. 2016, 15, 203–210. [Google Scholar] [CrossRef]
- Ding, Q.H.; Ye, C.Y.; Chen, E.M.; Zhang, W.; Wang, X.H. Emodin ameliorates cartilage degradation in osteoarthritis by inhibiting NF-kappaB and Wnt/beta-catenin signaling in-vitro and in-vivo. Int. Immunopharmacol. 2018, 61, 222–230. [Google Scholar] [CrossRef]
- Liu, Z.; Lang, Y.; Li, L.; Liang, Z.; Deng, Y.; Fang, R.; Meng, Q. Effect of emodin on chondrocyte viability in an in vitro model of osteoarthritis. Exp. Med. 2018, 16, 5384–5389. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.; Horcajada, M.N.; Membrez Scalfo, F.; Ameye, L.; Offord, E.; Henrotin, Y. Carnosol Inhibits Pro-Inflammatory and Catabolic Mediators of Cartilage Breakdown in Human Osteoarthritic Chondrocytes and Mediates Cross-Talk between Subchondral Bone Osteoblasts and Chondrocytes. PloS ONE 2015, 10, e0136118. [Google Scholar] [CrossRef]
- Chen, M.P.; Yang, S.H.; Chou, C.H.; Yang, K.C.; Wu, C.C.; Cheng, Y.H.; Lin, F.H. The chondroprotective effects of ferulic acid on hydrogen peroxide-stimulated chondrocytes: Inhibition of hydrogen peroxide-induced pro-inflammatory cytokines and metalloproteinase gene expression at the mRNA level. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2010, 59, 587–595. [Google Scholar] [CrossRef]
- Du, K.; Fang, X.; Li, Z. Ferulic acid suppresses interleukin-1beta-induced degeneration of chondrocytes isolated from patients with osteoarthritis through the SIRT1/AMPK/PGC-1alpha signaling pathway. Immun. Inflamm. Dis. 2021, 9, 710–720. [Google Scholar] [CrossRef]
- Shang, L.; Qin, J.; Chen, L.B.; Liu, B.X.; Jacques, M.; Wang, H. Effects of sodium ferulate on human osteoarthritic chondrocytes and osteoarthritis in rats. Clin. Exp. Pharmacol. Physiol. 2009, 36, 912–918. [Google Scholar] [CrossRef]
- Chen, W.P.; Tang, J.L.; Bao, J.P.; Hu, P.F.; Shi, Z.L.; Wu, L.D. Anti-arthritic effects of chlorogenic acid in interleukin-1beta-induced rabbit chondrocytes and a rabbit osteoarthritis model. Int. Immunopharmacol. 2011, 11, 23–28. [Google Scholar] [CrossRef]
- Huh, J.E.; Seo, B.K.; Baek, Y.H.; Lee, S.; Lee, J.D.; Choi, D.Y.; Park, D.S. Standardized butanol fraction of WIN-34B suppresses cartilage destruction via inhibited production of matrix metalloproteinase and inflammatory mediator in osteoarthritis human cartilage explants culture and chondrocytes. BMC Complement. Altern. Med. 2012, 12, 256. [Google Scholar] [CrossRef] [Green Version]
- Britti, D.; Crupi, R.; Impellizzeri, D.; Gugliandolo, E.; Fusco, R.; Schievano, C.; Morittu, V.M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models. BMC Vet. Res. 2017, 13, 229. [Google Scholar] [CrossRef]
- Kanzaki, N.; Saito, K.; Maeda, A.; Kitagawa, Y.; Kiso, Y.; Watanabe, K.; Tomonaga, A.; Nagaoka, I.; Yamaguchi, H. Effect of a dietary supplement containing glucosamine hydrochloride, chondroitin sulfate and quercetin glycosides on symptomatic knee osteoarthritis: A randomized, double-blind, placebo-controlled study. J. Sci. Food Agric. 2012, 92, 862–869. [Google Scholar] [CrossRef]
- Mok, S.W.; Fu, S.C.; Cheuk, Y.C.; Chu, I.M.; Chan, K.M.; Qin, L.; Yung, S.H.; Kevin Ho, K.W. Intra-Articular Delivery of Quercetin Using Thermosensitive Hydrogel Attenuate Cartilage Degradation in an Osteoarthritis Rat Model. Cartilage 2018, 11, 1947603518796550. [Google Scholar] [CrossRef]
- Chen, W.P.; Hu, P.F.; Bao, J.P.; Wu, L.D. Morin exerts antiosteoarthritic properties: An in vitro and in vivo study. Exp. Biol. Med. 2012, 237, 380–386. [Google Scholar] [CrossRef]
- Blain, E.J.; Ali, A.Y.; Duance, V.C. Boswellia frereana (frankincense) suppresses cytokine-induced matrix metalloproteinase expression and production of pro-inflammatory molecules in articular cartilage. Phytother. Res. PTR 2010, 24, 905–912. [Google Scholar] [CrossRef]
- Jessberger, S.; Hogger, P.; Genest, F.; Salter, D.M.; Seefried, L. Cellular pharmacodynamic effects of Pycnogenol(R) in patients with severe osteoarthritis: A randomized controlled pilot study. BMC Complement. Altern. Med. 2017, 17, 537. [Google Scholar] [CrossRef]
- Mulek, M.; Seefried, L.; Genest, F.; Hogger, P. Distribution of Constituents and Metabolites of Maritime Pine Bark Extract (Pycnogenol®) into Serum, Blood Cells, and Synovial Fluid of Patients with Severe Osteoarthritis: A Randomized Controlled Trial. Nutrients 2017, 9, 443. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Kim, D.K.; Shin, H.D.; Lee, H.J.; Jo, H.S.; Jeong, J.H.; Choi, Y.L.; Lee, C.J.; Hwang, S.C. Apigenin Regulates Interleukin-1beta-Induced Production of Matrix Metalloproteinase Both in the Knee Joint of Rat and in Primary Cultured Articular Chondrocytes. Biomol. Ther. 2016, 24, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhang, Y.; Huang, Y. Icariin Reduces Cartilage Degeneration in a Mouse Model of Osteoarthritis and is Associated with the Changes in Expression of Indian Hedgehog and Parathyroid Hormone-Related Protein. Med. Sci. Monit. 2018, 24, 6695–6706. [Google Scholar] [CrossRef]
- Zhang, W.; Li, R.; Wang, S.; Mu, F.; Jia, P. Effect of Chinese traditional herb Epimedium grandiflorum C. Morren and its extract Icariin on osteoarthritis via suppressing NF-kappaB pathway. Indian J. Exp. Biol. 2013, 51, 313–321. [Google Scholar]
- Zhao, C.F.; Li, Z.H.; Li, S.J.; Li, J.A.; Hou, T.T.; Wang, Y. PLGA scaffold carrying icariin to inhibit the progression of osteoarthritis in rabbits. R Soc. Open Sci. 2019, 6, 181877. [Google Scholar] [CrossRef] [Green Version]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharm. 2015, 750, 141–150. [Google Scholar] [CrossRef]
- Ding, Q.; Zhong, H.; Qi, Y.; Cheng, Y.; Li, W.; Yan, S.; Wang, X. Anti-arthritic effects of crocin in interleukin-1beta-treated articular chondrocytes and cartilage in a rabbit osteoarthritic model. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2013, 62, 17–25. [Google Scholar] [CrossRef]
- Galluccio, F.; Barskova, T.; Cerinic, M.M. Short-term effect of the combination of hyaluronic acid, chondroitin sulfate, and keratin matrix on early symptomatic knee osteoarthritis. Eur. J. Rheumatol. 2015, 2, 106–108. [Google Scholar] [CrossRef]
- Ma, T.W.; Wen, Y.J.; Song, X.P.; Hu, H.L.; Li, Y.; Bai, H.; Zhao, M.C.; Gao, L. Puerarin inhibits the development of osteoarthritis through antiinflammatory and antimatrix-degrading pathways in osteoarthritis-induced rat model. Phytother. Res. PTR 2020, 35, 2579–2593. [Google Scholar] [CrossRef]
- Bokhari, R.A.; Tantowi, N.; Lau, S.F.; Mohamed, S. Java Tea (Orthosiphon stamineus) protected against osteoarthritis by mitigating inflammation and cartilage degradation: A preclinical study. Inflammopharmacology 2018, 26, 939–949. [Google Scholar] [CrossRef]
- Chin, K.Y.; Pang, K.L. Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside. Nutrients 2017, 9, 1060. [Google Scholar] [CrossRef]
- Gong, D.; Geng, C.; Jiang, L.; Wang, L.; Yoshimuram, H.; Zhong, L. Olive leaf extract facilitates healing of experimental cartilaginous injuries in rabbits. J. Med. Food 2011, 14, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: An in vivo and in vitro study on lubricin expression. J. Nutr. Biochem. 2013, 24, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, F.; Mevel, E.; Krisa, S.; Richard, T.; Valls, J.; Hornedo-Ortega, R.; Granel, H.; Boutin-Wittrant, L.; Urban, N.; Berger, J.; et al. Chondroprotective Properties of Human-Enriched Serum Following Polyphenol Extract Absorption: Results from an Exploratory Clinical Trial. Nutrients 2019, 11, 3071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Frost, B.; Liu, J. Oleuropein, unexpected benefits! Oncotarget 2017, 8, 17409. [Google Scholar] [CrossRef]
- Varela-Eirin, M.; Carpintero-Fernandez, P.; Sanchez-Temprano, A.; Varela-Vazquez, A.; Paino, C.L.; Casado-Diaz, A.; Calanas-Continente, A.; Mato, V.; Fonseca, E.; Kandouz, M.; et al. Senolytic activity of small molecular polyphenols from olive restores chondrocyte redifferentiation and promotes a pro-regenerative environment in osteoarthritis. Aging 2020, 12, 15882–15905. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y. The spice for joint inflammation: Anti-inflammatory role of curcumin in treating osteoarthritis. Drug Des. Dev. Ther. 2016, 10, 3029–3042. [Google Scholar] [CrossRef] [Green Version]
- Henrotin, Y.; Clutterbuck, A.L.; Allaway, D.; Lodwig, E.M.; Harris, P.; Mathy-Hartert, M.; Shakibaei, M.; Mobasheri, A. Biological actions of curcumin on articular chondrocytes. Osteoarthr. Cartil. 2010, 18, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, A.F.; Lien, Y.C.; Tzeng, I.S.; Liu, C.T.; Chou, S.H.; Horng, Y.S. The efficacy of high- and low-dose curcumin in knee osteoarthritis: A systematic review and meta-analysis. Complement. Ther. Med. 2021, 63, 102775. [Google Scholar] [CrossRef]
- Paultre, K.; Cade, W.; Hernandez, D.; Reynolds, J.; Greif, D.; Best, T.M. Therapeutic effects of turmeric or curcumin extract on pain and function for individuals with knee osteoarthritis: A systematic review. BMJ Open Sport Exerc. Med. 2021, 7, e000935. [Google Scholar] [CrossRef]
- Henrotin, Y.; Gharbi, M.; Dierckxsens, Y.; Priem, F.; Marty, M.; Seidel, L.; Albert, A.; Heuse, E.; Bonnet, V.; Castermans, C. Decrease of a specific biomarker of collagen degradation in osteoarthritis, Coll2-1, by treatment with highly bioavailable curcumin during an exploratory clinical trial. BMC Complement. Altern. Med. 2014, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Armenta, C.; Camacho-Rea, M.C.; Martinez-Nava, G.A.; Espinosa-Velazquez, R.; Pineda, C.; Gomez-Quiroz, L.E.; Lopez-Reyes, A. Therapeutic Potential of Bioactive Compounds in Honey for Treating Osteoarthritis. Front. Pharm. 2021, 12, 642836. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Yu, W.; Huang, C.; Ding, Q.; Liang, C.; Wang, L.; Hou, Z.; Zhang, Z. Chrysin protects human osteoarthritis chondrocytes by inhibiting inflammatory mediator expression via HMGB1 suppression. Mol. Med. Rep. 2019, 19, 1222–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Sun, M.; Zhang, X. Protective Effect of Resveratrol on Knee Osteoarthritis and its Molecular Mechanisms: A Recent Review in Preclinical and Clinical Trials. Front. Pharm. 2022, 13, 921003. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, D.; Tang, J. Identification of the Resveratrol Potential Targets in the Treatment of Osteoarthritis. Evid. Based Complement. Altern. Med. 2021, 2021, 9911286. [Google Scholar] [CrossRef]
- Wang, J.; Gao, J.S.; Chen, J.W.; Li, F.; Tian, J. Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit. Rheumatol. Int. 2012, 32, 1541–1548. [Google Scholar] [CrossRef]
- Liu, F.C.; Hung, L.F.; Wu, W.L.; Chang, D.M.; Huang, C.Y.; Lai, J.H.; Ho, L.J. Chondroprotective effects and mechanisms of resveratrol in advanced glycation end products-stimulated chondrocytes. Arthritis Res. Ther. 2010, 12, R167. [Google Scholar] [CrossRef] [Green Version]
- Ebrahim, H.A.; Alzamil, N.M.; Al-Ani, B.; Haidara, M.A.; Kamar, S.S.; Dawood, A.F. Suppression of knee joint osteoarthritis induced secondary to type 2 diabetes mellitus in rats by resveratrol: Role of glycated haemoglobin and hyperlipidaemia and biomarkers of inflammation and oxidative stress. Arch. Physiol. Biochem. 2022, 128, 1375–1382. [Google Scholar] [CrossRef]
- Hussain, S.A.; Marouf, B.H.; Ali, Z.S.; Ahmmad, R.S. Efficacy and safety of co-administration of resveratrol with meloxicam in patients with knee osteoarthritis: A pilot interventional study. Clin. Interv. Aging 2018, 13, 1621–1630. [Google Scholar] [CrossRef] [Green Version]
- Marouf, B.H.; Hussain, S.A.; Ali, Z.S. Correlation between serum pro inflammatory cytokines and clinical scores of knee osteoarthritic patients using resveratrol as a supplementary therapy with meloxicam. Indian J. Pharm. 2021, 53, 270–277. [Google Scholar] [CrossRef]
- Rondanelli, M.; Fossari, F.; Vecchio, V.; Gasparri, C.; Peroni, G.; Spadaccini, D.; Riva, A.; Petrangolini, G.; Iannello, G.; Nichetti, M.; et al. Clinical trials on pain lowering effect of ginger: A narrative review. Phytother. Res. PTR 2020, 34, 2843–2856. [Google Scholar] [CrossRef] [PubMed]
- Amorndoljai, P.; Taneepanichskul, S.; Niempoog, S.; Nimmannit, U. A Comparative of Ginger Extract in Nanostructure Lipid Carrier (NLC) and 1% Diclofenac Gel for Treatment of Knee Osteoarthritis (OA). J. Med. Assoc. Thail. Chotmaihet Thangphaet 2017, 100, 447–456. [Google Scholar]
- Bartels, E.M.; Folmer, V.N.; Bliddal, H.; Altman, R.D.; Juhl, C.; Tarp, S.; Zhang, W.; Christensen, R. Efficacy and safety of ginger in osteoarthritis patients: A meta-analysis of randomized placebo-controlled trials. Osteoarthr. Cartil. 2015, 23, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Mozaffari-Khosravi, H.; Naderi, Z.; Dehghan, A.; Nadjarzadeh, A.; Fallah Huseini, H. Effect of Ginger Supplementation on Proinflammatory Cytokines in Older Patients with Osteoarthritis: Outcomes of a Randomized Controlled Clinical Trial. J. Nutr. Gerontol. Geriatr. 2016, 35, 209–218. [Google Scholar] [CrossRef]
- Naderi, Z.; Mozaffari-Khosravi, H.; Dehghan, A.; Nadjarzadeh, A.; Huseini, H.F. Effect of ginger powder supplementation on nitric oxide and C-reactive protein in elderly knee osteoarthritis patients: A 12-week double-blind randomized placebo-controlled clinical trial. J. Tradit. Complement. Med. 2016, 6, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Tosun, B.; Unal, N.; Yigit, D.; Can, N.; Aslan, O.; Tunay, S. Effects of Self-Knee Massage with Ginger Oil in Patients with Osteoarthritis: An Experimental Study. Res. Theory Nurs. Pract. 2017, 31, 379–392. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, L.; Zheng, Y.; Liu, W.; Xu, Y. Kaempferol attenuates the effects of XIST/miR-130a/STAT3 on inflammation and extracellular matrix degradation in osteoarthritis. Future Med. Chem. 2021, 13, 1451–1464. [Google Scholar] [CrossRef]
- Zhuang, Z.; Ye, G.; Huang, B. Kaempferol Alleviates the Interleukin-1beta-Induced Inflammation in Rat Osteoarthritis Chondrocytes via Suppression of NF-kappaB. Med. Sci. Monit. 2017, 23, 3925–3931. [Google Scholar] [CrossRef] [Green Version]
- Shrimali, D.; Shanmugam, M.K.; Kumar, A.P.; Zhang, J.; Tan, B.K.; Ahn, K.S.; Sethi, G. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013, 341, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018, 98, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Moon, S.M.; Han, S.H.; Hwang, E.J.; Park, B.R.; Kim, J.S.; Kim, D.K.; Kim, C.S. Chondroprotective effects of aqueous extract of Anthriscus sylvestris leaves on osteoarthritis in vitro and in vivo through MAPKs and NF-kappaB signaling inhibition. Biomed. Pharm. 2018, 103, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, Y.C.; Fu, S.C.; Mok, S.W.; Ho, K.K.; Hung, L.K.; Chan, K.M. Intra-articular injection of an antioxidant formulation did not improve structural degeneration in a rat model of post-traumatic osteoarthritis. J. Orthop. Transl. 2017, 8, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, K.; Kolla, J.N.; Krishnaraju, A.V.; Yalamanchili, N.; Rao, C.V.; Golakoti, T.; Raychaudhuri, S.; Raychaudhuri, S.P. Cellular and molecular mechanisms of anti-inflammatory effect of Aflapin: A novel Boswellia serrata extract. Mol. Cell. Biochem. 2011, 354, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Al-Eid, F.; Wang, C. Efficacy of curcumin and Boswellia for knee osteoarthritis: Systematic review and meta-analysis. Semin. Arthritis Rheum. 2018, 48, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Rohdewald, P.J. Review on Sustained Relief of Osteoarthritis Symptoms with a Proprietary Extract from Pine Bark, Pycnogenol. J. Med. Food 2018, 21, 1–4. [Google Scholar] [CrossRef]
- Grimm, T.; Chovanova, Z.; Muchova, J.; Sumegova, K.; Liptakova, A.; Durackova, Z.; Hogger, P. Inhibition of NF-kappaB activation and MMP-9 secretion by plasma of human volunteers after ingestion of maritime pine bark extract (Pycnogenol). J. Inflamm. 2006, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Feragalli, B.; Dugall, M.; Luzzi, R.; Ledda, A.; Hosoi, M.; Belcaro, G.; Cesarone, M.R. Pycnogenol(R): Supplementary management of symptomatic osteoarthritis with a patch. An observational registry study. Minerva Endocrinol. 2019, 44, 97–101. [Google Scholar] [CrossRef]
- Cisar, P.; Jany, R.; Waczulikova, I.; Sumegova, K.; Muchova, J.; Vojtassak, J.; Durackova, Z.; Lisy, M.; Rohdewald, P. Effect of pine bark extract (Pycnogenol) on symptoms of knee osteoarthritis. Phytother. Res. PTR 2008, 22, 1087–1092. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Errichi, S.; Zulli, C.; Errichi, B.M.; Vinciguerra, G.; Ledda, A.; Di Renzo, A.; Stuard, S.; Dugall, M.; et al. Variations in C-reactive protein, plasma free radicals and fibrinogen values in patients with osteoarthritis treated with Pycnogenol. Redox Rep. 2008, 13, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Durigova, M.; Roughley, P.J.; Mort, J.S. Mechanism of proteoglycan aggregate degradation in cartilage stimulated with oncostatin M. Osteoarthr. Cartil. 2008, 16, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Fan, F.; Liu, A.; Zhang, C.; Li, Q.; Zhang, C.; He, F.; Shang, M. Icariin: A Potential Molecule for Treatment of Knee Osteoarthritis. Front. Pharm. 2022, 13, 811808. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Meng, Q.; Wang, W.; Zhang, S.; Xiong, X.; Qin, S.; Zhang, J.; Li, A.; Liu, Z. Icariin inhibits the inflammation through down-regulating NF-kappaB/HIF-2alpha signal pathways in chondrocytes. Biosci. Rep. 2020, 40, BSR20203107. [Google Scholar] [CrossRef]
- Wang, L.; Shan, H.; Wang, B.; Wang, N.; Zhou, Z.; Pan, C.; Wang, F. Puerarin Attenuates Osteoarthritis via Upregulating AMP-Activated Protein Kinase/Proliferator-Activated Receptor-gamma Coactivator-1 Signaling Pathway in Osteoarthritis Rats. Pharmacology 2018, 102, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, C.; Sun, H.; Hong, H.; Jin, J.; Bei, C.; Lu, Z.; Zhang, X. Puerarin suppresses inflammation and ECM degradation through Nrf2/HO-1 axis in chondrocytes and alleviates pain symptom in osteoarthritic mice. Food Funct. 2021, 12, 2075–2089. [Google Scholar] [CrossRef]
- Liu, F.C.; Wang, C.C.; Lu, J.W.; Lee, C.H.; Chen, S.C.; Ho, Y.J.; Peng, Y.J. Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation. Nutrients 2019, 11, 1180. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Xu, P. The articular cartilage preservative effects of genistein in an experimental model of knees osteoarthritis. Appl. Physiol. Nutr. Metab. 2021, 46, 1331–1336. [Google Scholar] [CrossRef]

| Compound | Model | Target/Action | Reference |
|---|---|---|---|
| Butein | Human chondrocyte | Suppression of MMP-3, MMP-9, MMP-13 | [64,65] |
| EGCG | AGEs stimulated human chondrocyte, MIA rat, DMM mouse, guinea pig | Suppression of MMP-1, MMP-13, and ADAMTS5 | [71,72,73,74,75] |
| Olive oil | |||
| Hydroxytyrosol | Surgically induced rabbit and mouse | Suppression of MMP-13 and protection of aggrecan via antioxidant activity | [76] |
| Oleocanthal | LPS-induced human chondrocyte | Significant suppression in MMP-13, and ADAMTS5 via MAPK/ NFκB inhibition | [77] |
| Oleuropein | Human chondrocyte, guinea pig | Suppression of MMP-1, MMP-13, ADAMTS5, and increased deposition of Col2A1/proteoglycan via MAPK/NFκB inhibition | [78,79,80] |
| Curcumin | Human cartilage explant, rat primary chondrocyte, zymosan mouse, DMM mouse, equine cartilage explant | Suppression of MMP-3, MMP-8, MMP-13, ADAMTS5 via NFκB inhibition and increased expression of type II collagen and CITED2. | [81,82,83,84,85,86] |
| Honey | |||
| Chrysin | Human chondrocyte | Suppression of MMP-1, MMP-3, MMP-13, ADAMTS5 via NFκB inhibition and reduced HMGB-1 activity | [87] |
| Fisetin | Human chondrocyte, rat | Decreased MMP-3, MMP-13, ADAMTS5 expression | [88,89] |
| Resveratrol | Human chondrocyte, ACLT rabbit, DMM mouse, porcine cartilage explant | Suppression of MMP-13 via JNK/ERK-AP-1 inhibition, suppression of MMP-1, MMP-3, MMP-13, ADAMTS4, ADAMTS5 via NFκB inhibition, increased expression of type II collagen and aggrecan via AMPK/mTOR signaling, activation of SIRT1 and inhibition of HIF-2α | [90,91,92,93] |
| Zingerone | Human cartilage explant | Suppression of MMP-13 via p38/JNK-MAPK pathway | [94] |
| Kaempferol | Human chondrocyte, rat chondrocyte | Suppression of MMP-1, MMP-3, MMP-13, ADAMTS4, ADAMTS5 via p38/ERK-MAPK inhibition, suppression of STAT3, inhibition of type II collagen degradation | [95,96] |
| Emodin | Human chondrocyte, rat chondrocyte, ACLT rat | Suppression of MMP-3, MMP-13, ADAMTS4, ADAMTS5 via NFκB and Wnt/B-catenin inhibition, preservation of aggrecan and type II collagen | [97,98] |
| Carnosol | Human chondrocyte | Suppression of MMP-3, ADAMTS4, ADAMTS5, increased expression of type II collagen and aggrecan | [99] |
| Ferulic acid | Human chondrocyte, papain rat | Suppression of MMP-1, MMP-3, MMP-13 via SIRT1/AMPK/PGC-1α inhibition, restoration of SOX9, upregulation of TIMP-1 | [100,101,102] |
| Chlorogenic acid | Human chondrocyte, human cartilage explant, rabbit chondrocyte, ACLT rabbit, rat chondrocyte | Suppression of MMP-1, MMP-3, MMP-13, ADAMTS4, ADAMTS5 via NFκB inhibition, increased expression of type II collagen and aggrecan | [103,104] |
| Quercetin | Rat chondrocyte | Suppression of MMP-1, MMP-3, MMP-9 | [105,106,107] |
| Morin | Human chondrocyte, ACLT rat | Suppression of MMP-3, MMP-13 and upregulation of TIMP-1 | [108] |
| B serrata | Human chondrocyte, human cartilage explant | Suppression of MMP-9, MMP-13 | [109] |
| Pycnogenol | Human chondrocyte | Suppression of MMP-3, MMP-9, MMP-13, ADAMTS5 via NFκB inhibition | [110,111] |
| Apigenin | Human cartilage explant, rabbit chondrocyte, rat chondrocyte | Blocking IL-1β, TNF-α, and suppression of MMP-1, MMP-3, MMP-13, ADAMTS4, ADAMTS5 | [112] |
| Icariin | Rabbit chondrocytes and OA model, mouse OA model | Suppression of Mmp-13 and increased Col2a1 levels by targeting Indian Hedgehog and NFκB pathway | [113,114,115] |
| Terpenes | |||
| Myrcene/ Limonene | Human chondrocyte | Reduced nitric oxide production, induced TIMP-1 and TIMP-3 expression, suppressed MMP-1 and MMP-13 expression via NFκB, JNK, and p38 inhibition | [116] |
| Crocin | ACLT mouse | Suppression of MMP-1, MMP-3, MMP-13 gene expression via NFκB inhibition | [117] |
| Alkaloids | |||
| Piperine | Human chondrocyte | Suppression of gene expression of MMP-3, MMP-13, nitric oxide, COX-2 | [70,118] |
| Isoflavones | |||
| Puerarin | ACLT rat, MIA rat, human chondrocyte, | Suppression of MMP-3, MMP-13, ADAMTS5 protein expression, reducing levels of IL-1β, IL-6, TNF-α, and reversing type II collagen degradation via Nrf2/HO-1 and NFκB inhibition | [119] |
| Genistein | Human chondrocyte, MIA rat | Suppression of MMP-1, MMP-2, MMP-3, MMP-13 protein expression via Nrf-2 mediated NFκB inhibition |
| Reference | Sample Size | Supplementation | Results |
|---|---|---|---|
| [124] | n = 20 | 8 caps, 400 mg each, of grapeseed and olive extract (hydroxytyrosol and procyanidins content) | Venous blood collected post-ingestion showed peak metabolic concentration at 100 min with reduction in IL-1β and inflammatory cytokines |
| [79] | n = 124 (62 control, 62 treatment) | One capsule of 50 mg oleuropein twice a day | Knee injury and Osteoarthritis Outcome Score (KOOS) determined significantly reduced walking pain in subjects |
| [131] | n = 22 | 6 caps, 42 mg each, of bio-optimized curcumin per day | Significant reduction in Coll2-1 and insignificant pain alleviation |
| [140] | n = 110 (55 control, 55 treatment) | 15 mg meloxicam + 500 mg resveratrol once daily | Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores indicated significant improvement in pain, stiffness, and physical function |
| [141] | n = 82 (32 control, 50 treatment) | 15 mg meloxicam + 500 mg resveratrol once daily | WOMAC scoring indicated no significant clinical relief with minimal difference in IL-1β, TNF-α, and IL-6 serum level |
| [145] | n = 120 (60 control, 60 treatment) | One capsule of 500 mg powdered ginger twice daily | At 3 months, there was a significant reduction in IL-1β and TNF-α concentrations in the treatment group |
| [146] | n = 120 (60 control, 60 treatment) | One capsule of 500 mg powdered ginger twice daily | At 3 months, there was a significant reduction in serum nitric oxide and hs-C reactive protein levels in the treatment group |
| [147] | n = 68 (34 control, 34 treatment) | Knee massage with ginger oil twice a week | WOMAC scoring and visual analog scale (VAS) determined significant clinical relief in pain, stiffness, and function in treatment group |
| [96] | n = 99 (33 control, 33 low-dose, 33 high-dose) | Low-dose: 300 mg of Elaeagnus angustifolia extract with kaempferol administered as syrup in two doses per day High-dose: 600 mg of Elaeagnus Angustifolia extract with kaempferol administered as syrup in two doses per day | WOMAC, VAS, and Leguesne’s Pain-Function Index (LPFI), and Patient’s Global Assessment (PGA) all indicated improvement for both dosages after 7 weeks. Low and high dosages exhibited significant reduction in pain and stiffness while only high dose exhibited improvement in physical function |
| [106] | n = 40 (20 control, 20 treatment) | 6 tablets of 1200 mg glucosamine hydrochloride, 60 mg chondroitin sulfate and 45 mg quercetin glycosides per day | After 16 weeks, treatment group experienced pain alleviation with walking and ascending/descending the stairs, per Japan Orthopaedic Association (JOA) criteria. Type II collagen levels were preserved, although not significant |
| [158] | n = 67 (34 control, 33 treatment) | Pycnogenol patch was applied to affected joint | Treatment group experienced reduced dependence on non-steroidal anti-inflammatory drugs, improved OA symptoms, and significant reduction in C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) |
| [159] | n = 100 (50 control, 50 treatment) | 150 mg Pycnogenol per day with meals | WOMAC and VAS criteria determined the treatment group experienced significant reduction in pain by the first month, while maximum effect was seen by the second month |
| [111] | n = 33 (17 control, 16 treatment) | 2 capsules of Pycnogenol, 50 mg each, twice daily | Supplementation was well tolerated and distributed into the synovial fluid of OA patients |
| [110] | N = 33 (17 control, 16 treatment) | 100 mg Pycnogenol twice a day | Treatment group saw reduced expression of IL-1β, MMP-3, MMP-13, and ADAMTS5 levels in the serum after 3 weeks |
| [160] | n = 55 (26 control, 29 treatment) | 2 tablets, 50 mg Pycnogenol each, per day | After 3 weeks, the treatment group experienced significant reduction in CRP levels and plasma free radicals |
| [118] | n = 40 | Oral formulation including piperine given over one month span | After 2 months, participants experienced significant reduction in pain via WOMAC scoring and no side effects/good tolerability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashruf, O.S.; Ansari, M.Y. Natural Compounds: Potential Therapeutics for the Inhibition of Cartilage Matrix Degradation in Osteoarthritis. Life 2023, 13, 102. https://doi.org/10.3390/life13010102
Ashruf OS, Ansari MY. Natural Compounds: Potential Therapeutics for the Inhibition of Cartilage Matrix Degradation in Osteoarthritis. Life. 2023; 13(1):102. https://doi.org/10.3390/life13010102
Chicago/Turabian StyleAshruf, Omer S., and Mohammad Yunus Ansari. 2023. "Natural Compounds: Potential Therapeutics for the Inhibition of Cartilage Matrix Degradation in Osteoarthritis" Life 13, no. 1: 102. https://doi.org/10.3390/life13010102
APA StyleAshruf, O. S., & Ansari, M. Y. (2023). Natural Compounds: Potential Therapeutics for the Inhibition of Cartilage Matrix Degradation in Osteoarthritis. Life, 13(1), 102. https://doi.org/10.3390/life13010102






