Transcriptomic Analysis Provides Novel Insights into the Heat Stress-Induced Response in Codonopsis tangshen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sample Collection
2.2. RNA Extraction and cDNA Library Preparation
2.3. Measurement of Physiological Properties
2.4. Bioinformatics Analysis
2.4.1. Processing and Assembling of Illumina Reads
2.4.2. Gene Functional Annotation
2.4.3. Differential Gene Expression Analysis
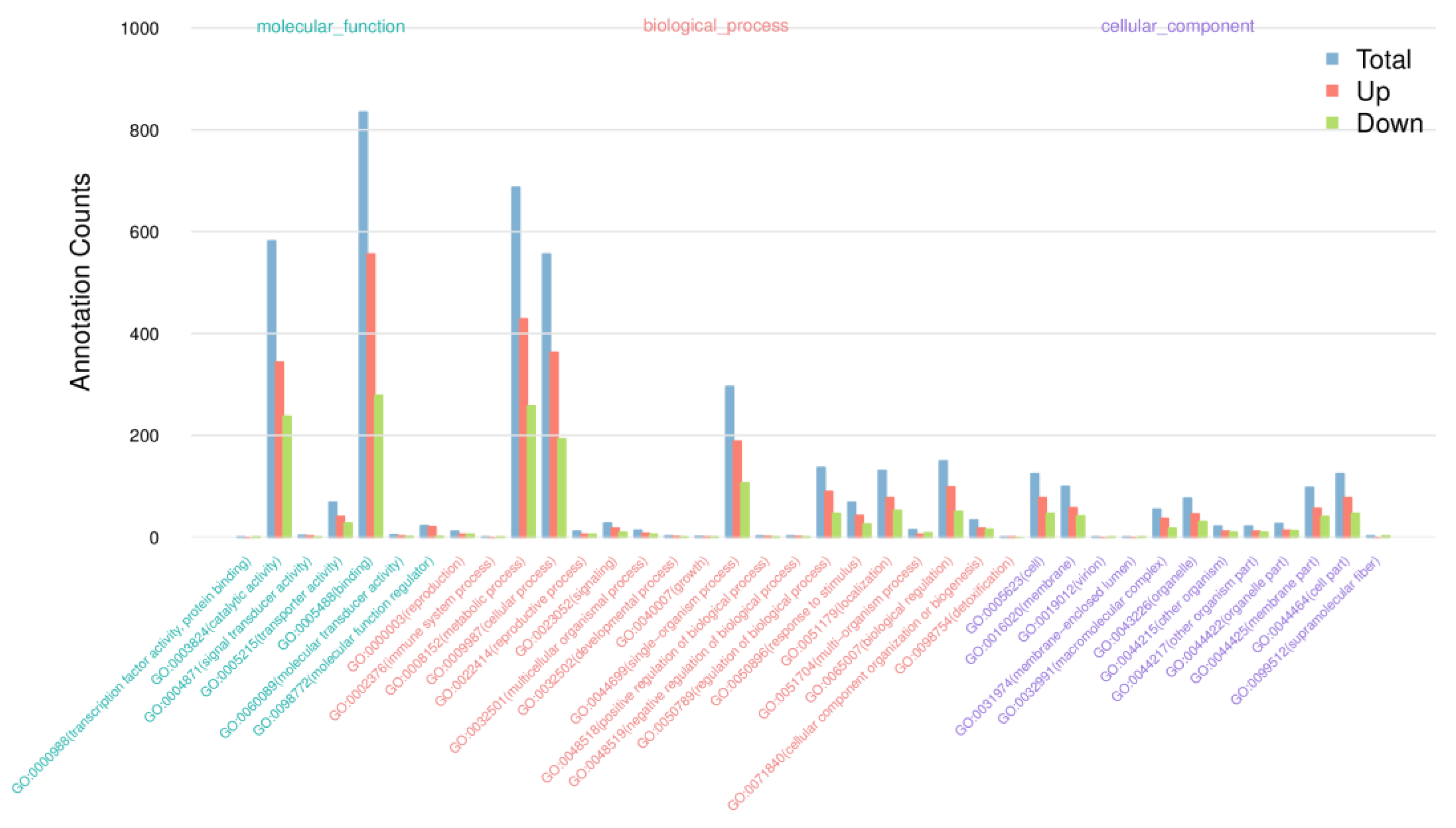
2.4.4. GO and KEGG Pathway Enrichment Analysis
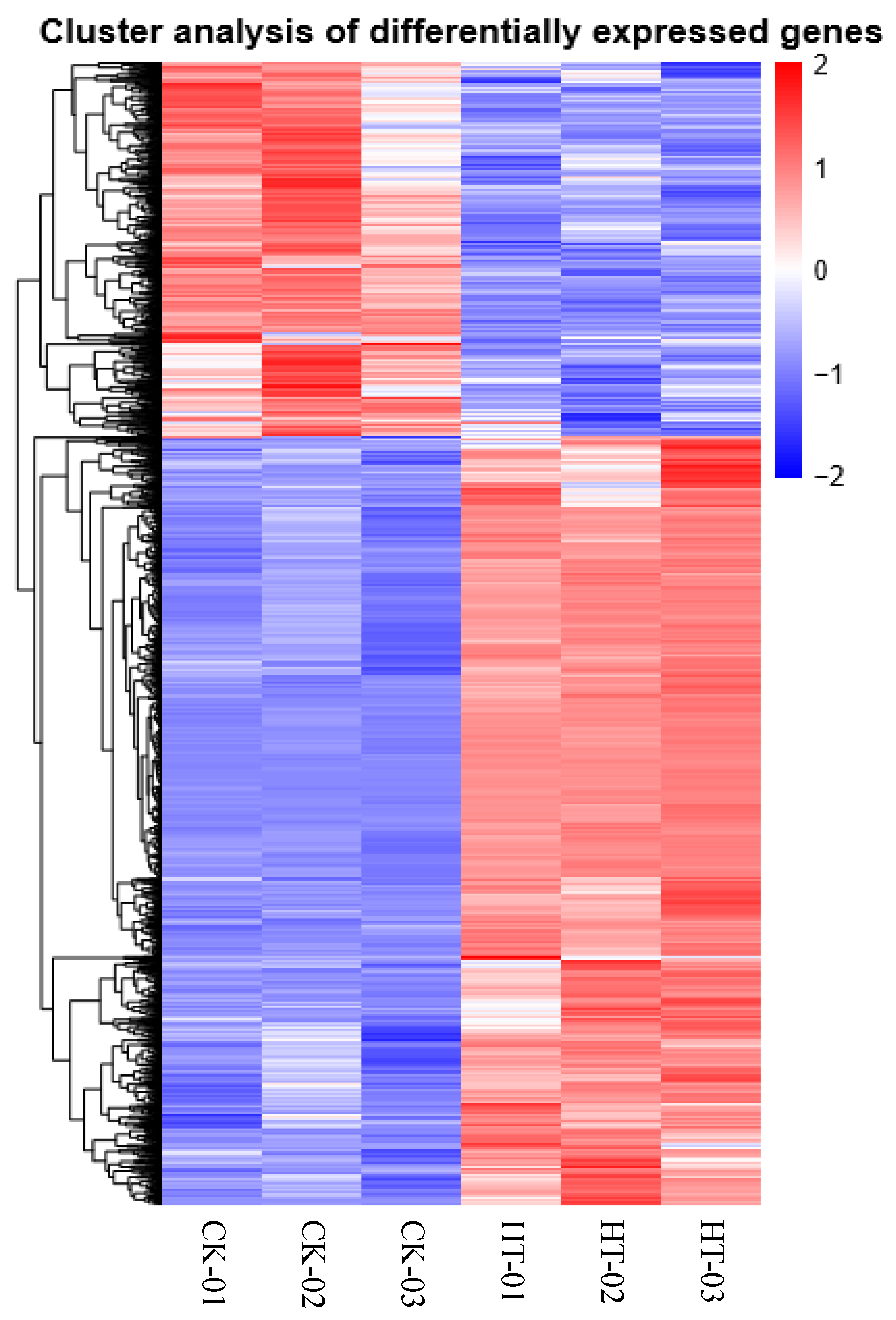
2.4.5. Clustering of the DEGs
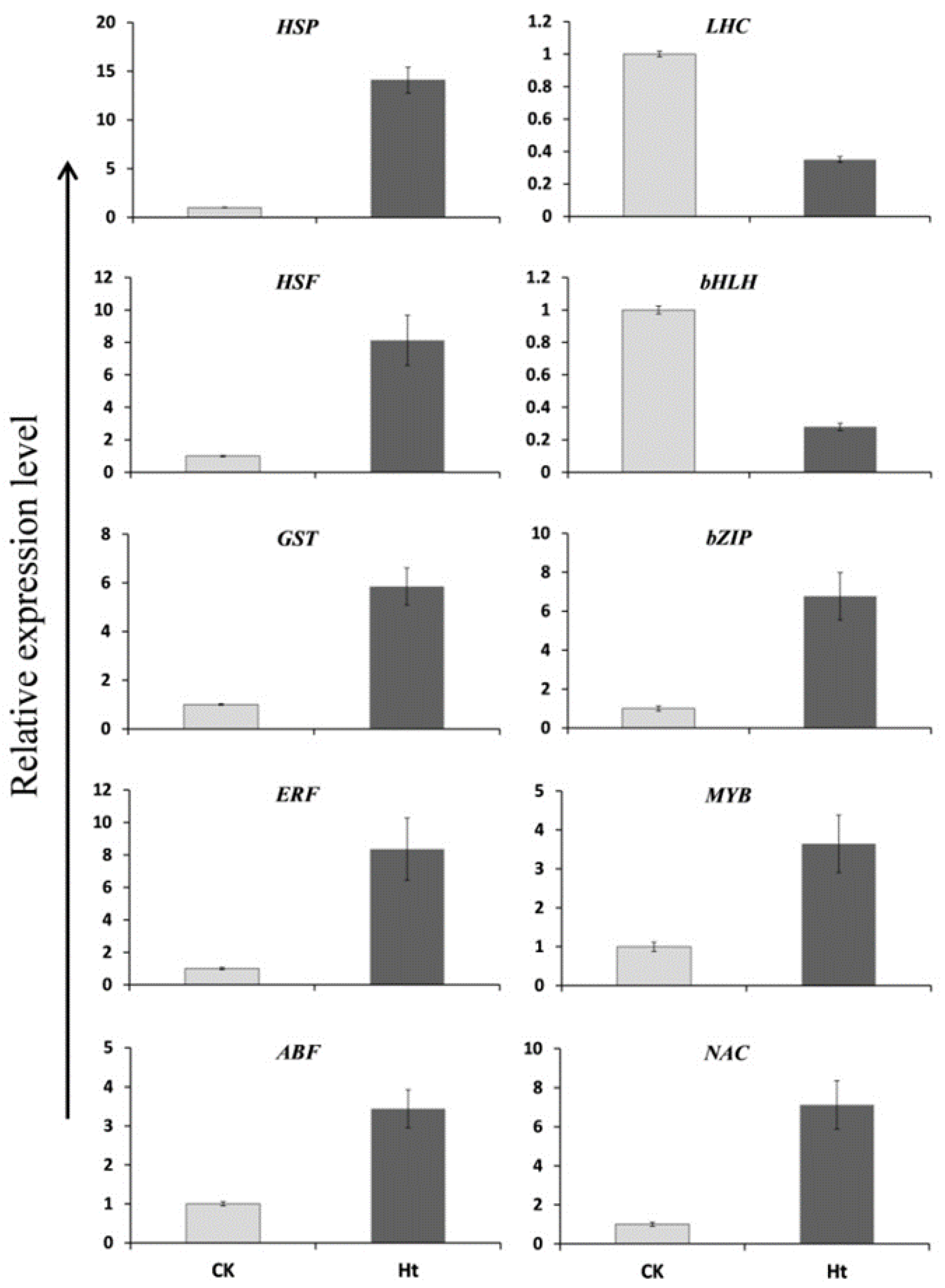
2.5. Validation of RNA-Seq Data by Real-Time Quantitative PCR
3. Results
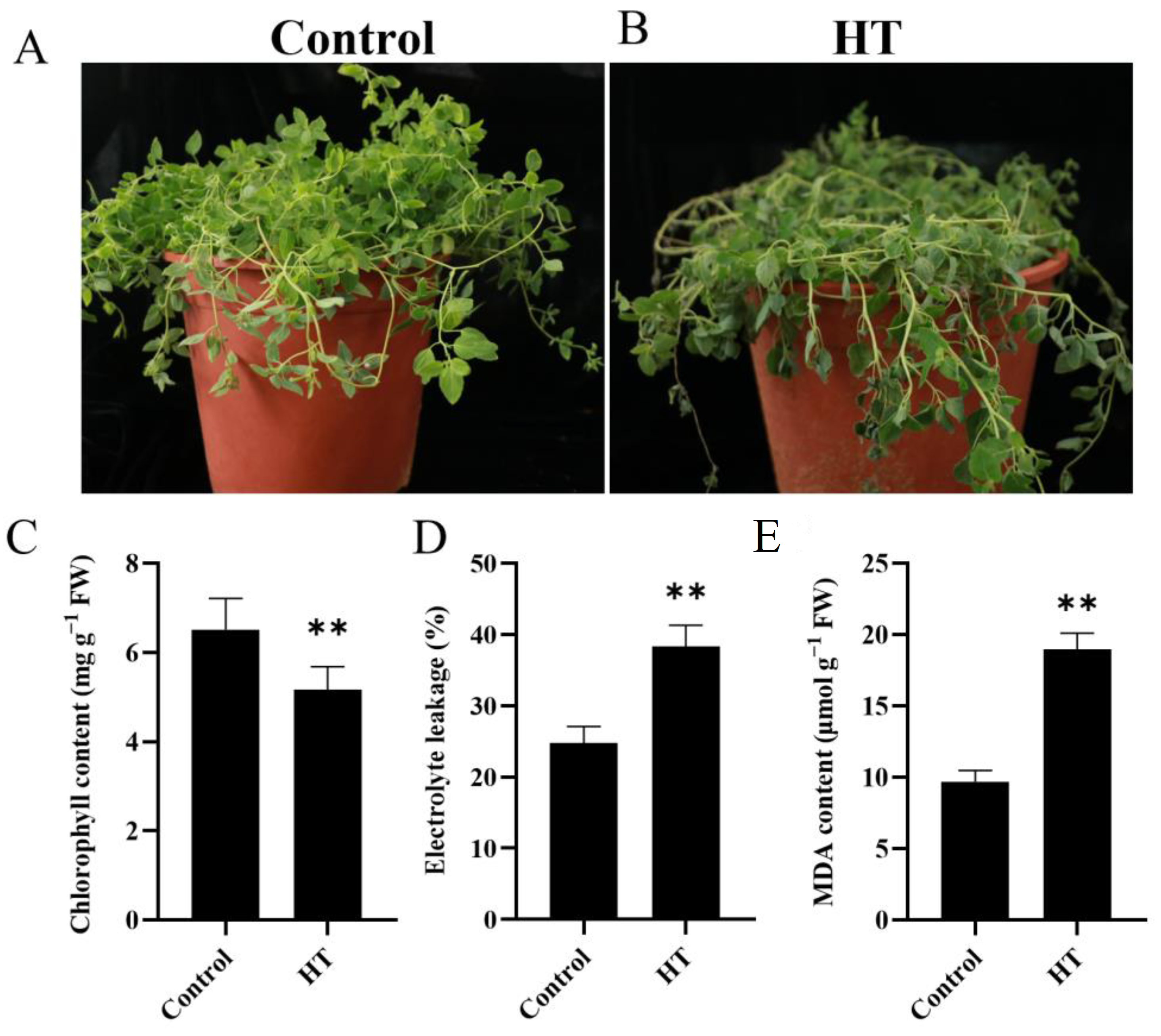
3.1. Effect of Heat Stress on Physiological and Biochemical Traits
3.2. Illumina Sequencing and De Novo Assembly
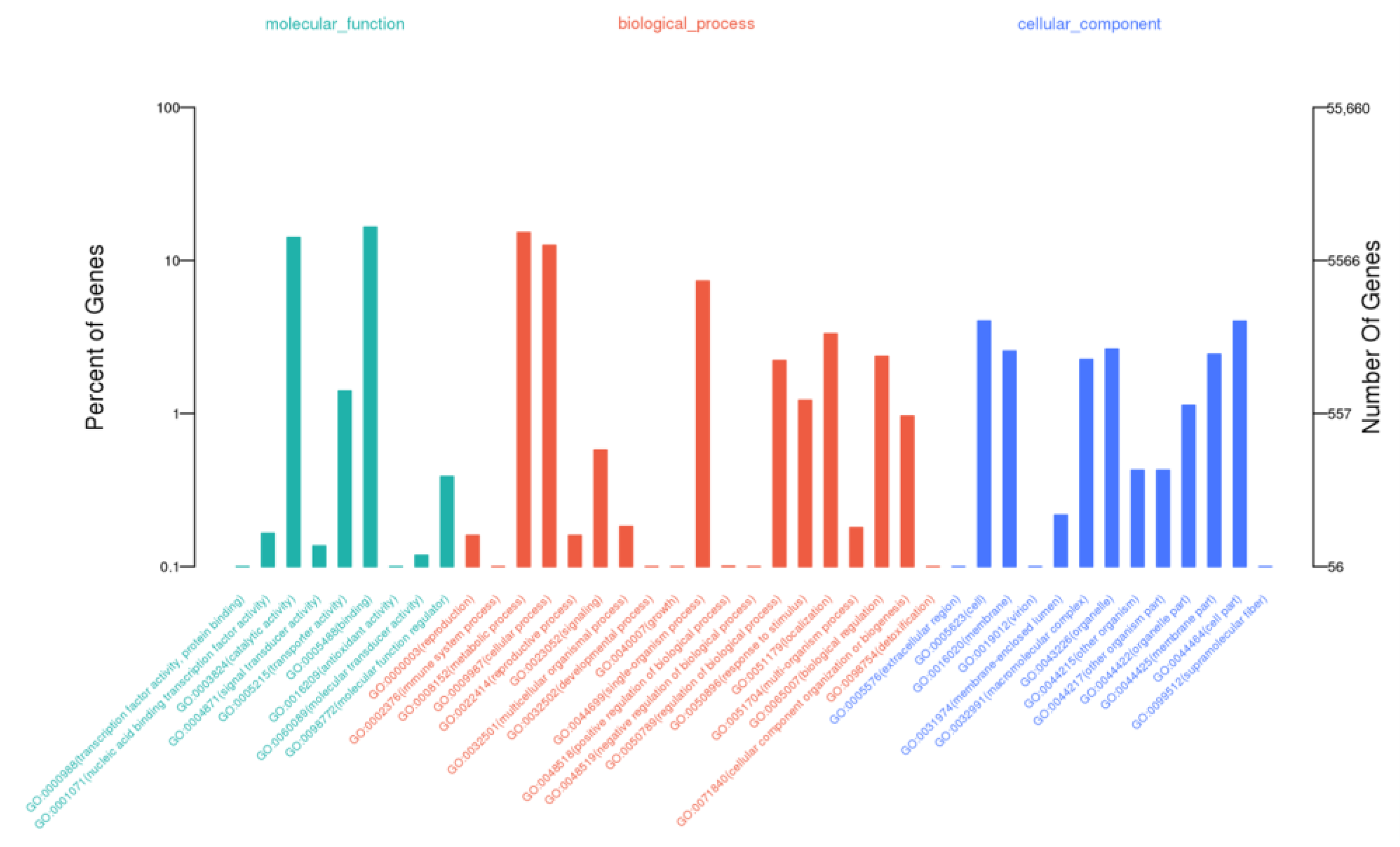
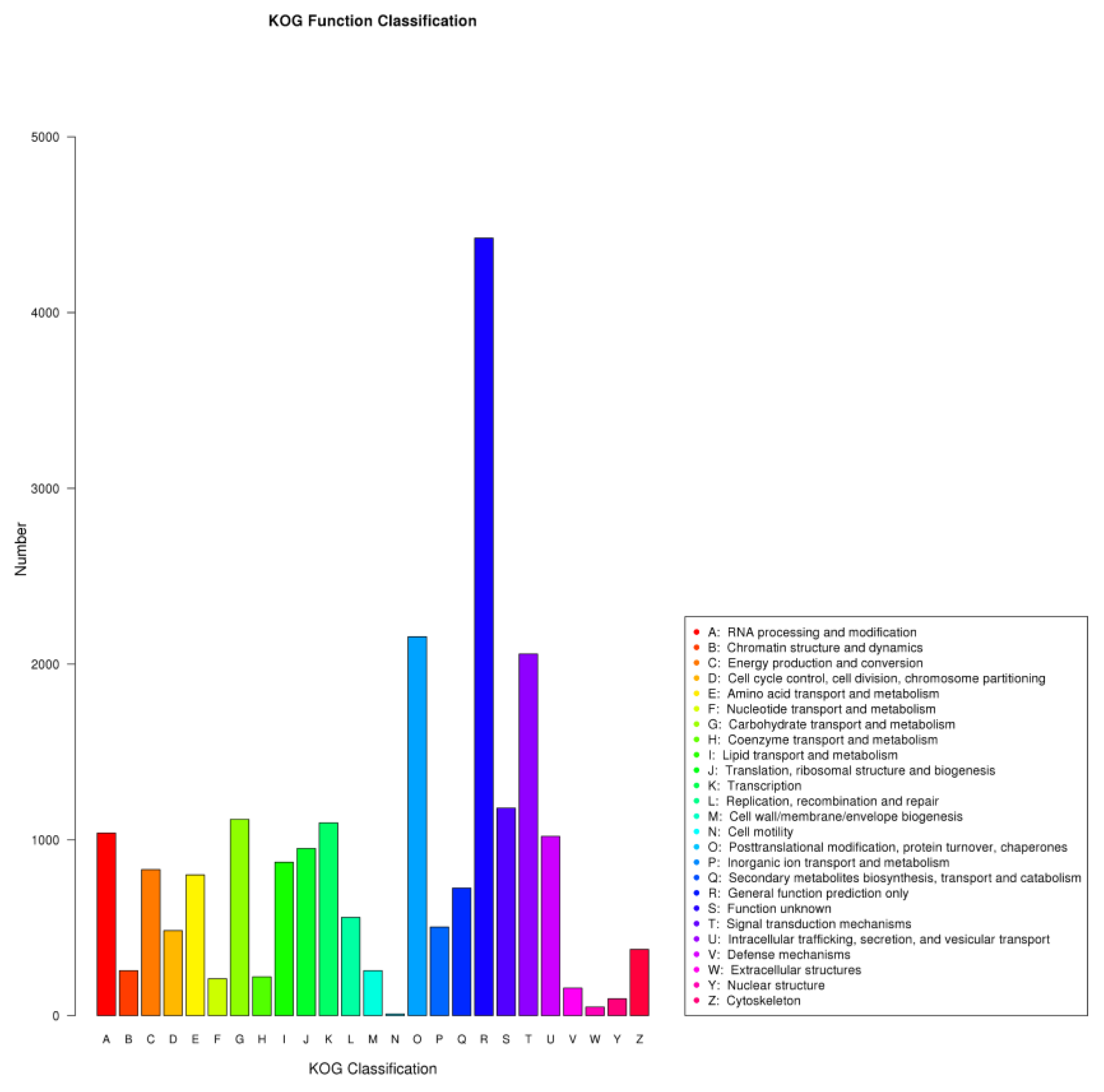
3.3. Functional Annotations of C. tangshen Unigenes
3.4. Analysis of DEGs in Response to Heat Stress in C. tangshen
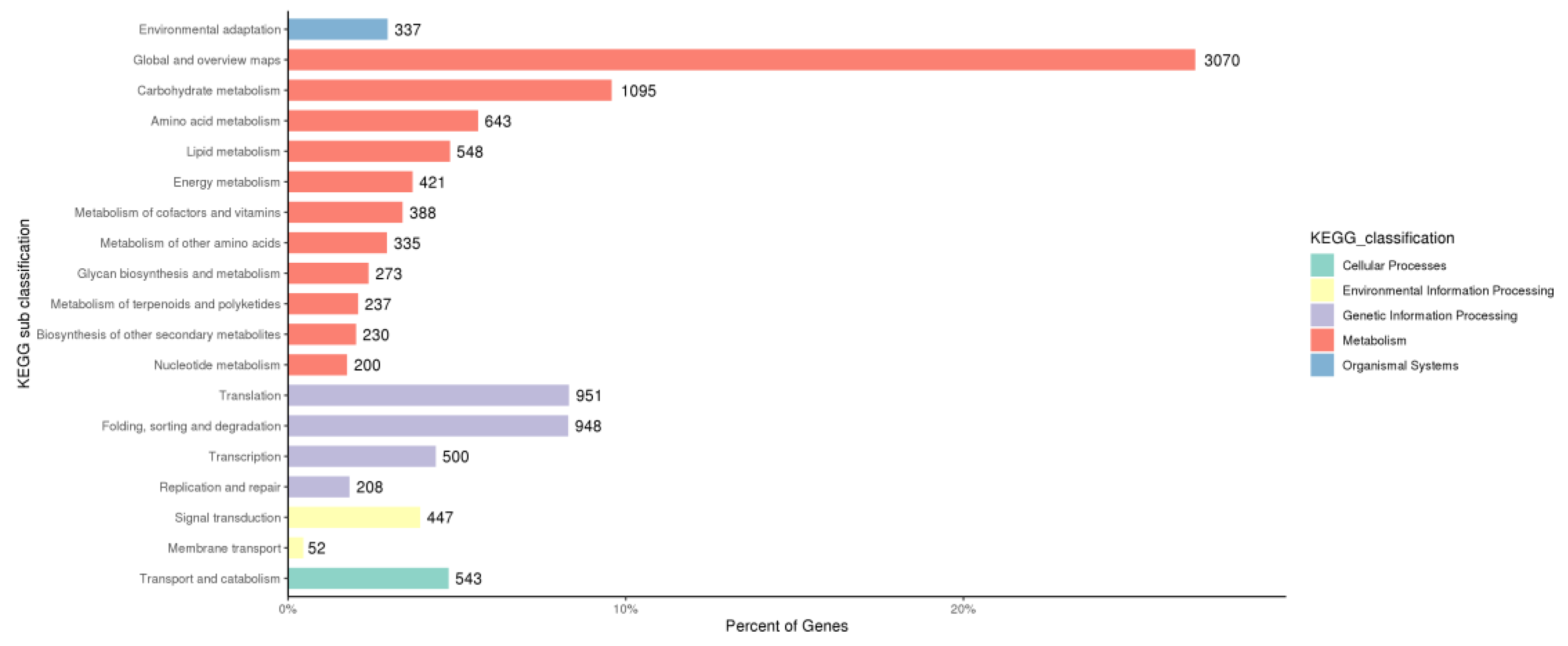
3.5. GO and KEGG Enrichment Analyses of DEGs
3.6. DEGs Related to Short-Term Heat Stress in C. tangshen
3.7. Validation of the DEGs by qRT-PCR
4. Discussion
4.1. Heat Stress Regulates Physiological and Biochemical Traits in C. tangshen
4.2. Transcriptomic Response of C. tangshen to Heat Stress
4.3. ROS–Scavenging Activity and HSF–HSP Network Involved in HSR
4.4. DEGs Related to Plant Hormone Signal Transduction
4.5. DEGs Related to Transcription Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.Y.; Xu, H.Y.; Shang, Y.; Zhu, R.; Hong, X.X.; Song, Z.H.; Yang, Z.P. Development of the general chapters of the Chinese Pharmacopoeia 2020 edition: A review. J. Pharm. Anal. 2021, 11, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.N.; Luo, M.; Yao, X.D.; Yu, L. Purification, structural characterization, and antioxidant activity of the COP-W1 polysaccharide from Codonopsis tangshen Oliv. Carbohyd. Polym. 2020, 236, 116020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zhou, N.; Tan, J.P. Preliminary analysis of the quality difference of Codonopsis pilosula from different producing areas. Asia-Pac. Tradit. Med. 2017, 13, 37–39. [Google Scholar]
- Zou, Y.F.; Zhang, Y.Y.; Paulsen, B.S.; Rise, F.; Chen, Z.L.; Jia, R.Y.; Li, L.X.; Song, X.; Feng, B.; Tang, H.Q.; et al. Structural features of pectic polysaccharides from stems of two species of Radix Codonopsis and their antioxidant activities. Int. J. Biol. Macromol. 2020, 159, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, J.; Li, E.T.; Fan, Q.; Wang, D.Y.; Li, P.; Li, X.P.; Chen, X.Y.; Qiu, S.L.; Gao, Z.Z.; et al. The comparison of antioxidative and hepatoprotective activities of Codonopsis pilosula polysaccharide (CP) and sulfated CP. Int. Immunopharmacol. 2015, 24, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xu, Y.J.; Chang, C.; Qiu, Z.P.; Hu, J.J.; Wu, Y.; Zhang, B.H.; Zheng, G.H. Extraction, characterization and anti-inflammatory activities of an inulin-type fructan from Codonopsis pilosula. Int. J. Biol. Macromol. 2020, 163, 1677–1686. [Google Scholar] [CrossRef]
- Yang, D.D.; Chen, Y.; Guo, F.X.; Huang, B.T.; Okyere, S.A.; Wang, H. Comparative analysis of chemical composition, antioxidant and antimicrobial activities of leaves, leaf tea and root from Codonopsis pilosula. Ind. Crop. Prod. 2019, 142, 111844. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Zou, X.M.; Sun, G.R.; Bao, Y.H. Codonopsis lanceolata polysaccharide CLPS inhibits melanoma metastasis via regulating integrin signaling. Int. J. Biol. Macromol. 2017, 103, 435–440. [Google Scholar] [CrossRef]
- Gao, Z.Z.; Zhang, C.; Jing, L.R.; Feng, M.; Li, R.; Yang, Y. The structural characterization and immune modulation activitives comparison of Codonopsis pilosula polysaccharide (CPPS) and selenizing CPPS (sCPPS) on mouse in vitro and vivo. Int. J. Biol. Macromol. 2020, 160, 814–822. [Google Scholar] [CrossRef]
- He, Y.S.; Zhang, M.D.; Zhou, W.X.; Ai, L.Q.; You, J.W.; Liu, H.H.; You, J.M.; Wang, H.; Wassie, M.; Wang, M.; et al. Transcriptome analysis reveals novel insights into the continuous cropping induced response in Codonopsis tangshen, a medicinal herb. Plant Physiol. Biochem. 2019, 141, 279–290. [Google Scholar] [CrossRef]
- Niu, Y.T.; Zhang, J.; Wang, H.Z.; Jin, L.; Du, T.; Song, P.S.; Zhao, W.L. Study on the suitability of Codonopsis pilosula. China J. Inf. Tradit. Chin. Med. 2021, 28, 13–16. [Google Scholar]
- Liao, C.L.; You, J.W. The Cultivation Technology of Medicinal Plants in Enshi, Hubei; Hubei Science and Technology Press: Enshi, China, 2006. [Google Scholar]
- Ashraf, M. Thermotolerance in plants: Potential physio-biochemical and molecular markers for crop improvement. Environ. Exp. Bot. 2021, 186, 104454. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Pisipati, S.R.; Mutava, R.N.; Tuinstra, M.R. Sensitivity of Grain Sorghum to High Temperature Stress during Reproductive Development. Crop. Sci. 2008, 48, 1911–1917. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.M.; Hou, L.J.; Lu, Y.Z.; Wu, B.J.; Xue, G.; Liu, M.S.; Wang, J.; Sun, Q.X.; Elizabeth, V.; Xu, S.B. Metabolic adaptation of wheat grain contributes to a stable filling rate under heat stress. J. Exp. Bot. 2018, 69, 5531–5545. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.J.; Zhang, M.D.; Xu, J.L.; Zhou, W.X.; Cao, L.W. Transcriptomic analysis of short-term heat stress response in Pinellia ternata provided novel insights into the improved thermotolerance by spermidine and melatonin. Ecotoxicol. Environ. Saf. 2020, 202, 110877. [Google Scholar] [CrossRef]
- Parrotta, L.; Aloisi, I.; Faleri, C.; Romi, M.; Del Duca, S.; Cai, G. Chronic heat stress affects the photosynthetic apparatus of Solanum lycopersicum L. cv Micro-Tom. Plant Physiol. Biochem. 2020, 154, 463–475. [Google Scholar] [CrossRef]
- Collado-González, J.; Piñero, M.C.; Otálora, G.; López-Marín, J.; del Amor, F.M. Exogenous spermidine modifies nutritional and bioactive constituents of cauliflower (Brassica oleracea var. botrytis L.) florets under heat stress. Sci. Hort-Amst. 2021, 277, 109818. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.; Dubey, R.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Baron, K.N.; Schroeder, D.F.; Stasolla, C. Transcriptional response of abscisic acid (ABA) metabolism and transport to cold and heat stress applied at the reproductive stage of development in Arabidopsis thaliana. Plant. Sci. 2012, 188–189, 48–59. [Google Scholar] [CrossRef]
- Sharma, E.; Borah, P.; Kaur, A.; Bhatnagar, A.; Mohapatra, T.; Kapoor, S.; Khurana, J.P. A comprehensive transcriptome analysis of contrasting rice cultivars highlights the role of auxin and ABA responsive genes in heat stress response. Genomics 2021, 113, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Iqbal, J.; Shaukat, M.; Naseer, S.; Mahmood, T. The epigenetic chromatin-based regulation of somatic heat stress memory in plants. Plant Gene 2021, 27, 100318. [Google Scholar] [CrossRef]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Heat Stress Response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef]
- Sarkar, J.; Chakraborty, U.; Chakraborty, B. High-temperature resilience in Bacillus safensis primed wheat plants: A study of dynamic response associated with modulation of antioxidant machinery, differential expression of HSPs and osmolyte biosynthesis. Environ. Exp. Bot. 2021, 182, 104315. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Dobrá, J.; Černý, M.; Štorchová, H.; Dobrev, P.; Skalák, J.; Jedelský, P.L.; Lukšanová, H.; Gaudinová, A.; Pešek, B.; Malbeck, J.; et al. The impact of heat stress targeting on the hormonal and transcriptomic response in Arabidopsis. Plant. Sci. 2015, 231, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Shahzad, R.; Khan, A.L.; Asaf, S.; Kim, Y.H.; Kang, S.M.; Bilal, S.; Hamayun, M.; Lee, I.J. Salvaging effect of triacontanol on plant growth, thermotolerance, macro-nutrient content, amino acid concentration and modulation of defense hormonal levels under heat stress. Plant Physiol. Biochem. 2016, 99, 118–125. [Google Scholar] [CrossRef]
- Qi, Y.X.; Liu, Y.B.; Rong, W.H. RNA-Seq and its applications: A new technology for transcriptomics. Hereditas 2011, 33, 1191–1202. [Google Scholar] [CrossRef]
- Liu, H.B.; Shi, J.H.; Wu, M.K.; Xu, D.L. The application and future prospect of RNA-Seq technology in Chinese medicinal plants. J. Appl. Res. Med. Aromat. 2021, 24, 100318. [Google Scholar] [CrossRef]
- Grabherr, M.; Haas, B.; Yassour, M.; Levin, J.; Thompson, D.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-Length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dewey, C. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wu, T.Z.; Hu, E.Q.; Xu, S.B.; Chen, M.J.; Guo, P.F.; Dai, Z.H.; Feng, T.Z.; Zhou, L.; Tang, W.L.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- de Hoon, M.J.L.; Imoto, S.; Nolan, J.; Miyano, S. Open Source Clustering Software. Bioinformatics 2004, 20, 1453–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saldanha, A.J. Java Treeview-extensible visualization of microarray data. Bioinformatics 2004, 20, 3246–3248. [Google Scholar] [CrossRef] [Green Version]
- Li, H.Y.; Hu, T.; Amombo, E.; Fu, J.M. Transcriptome profilings of two tall fescue (Festuca arundinacea) cultivars in response to lead (Pb) stress. BMC Genomics 2017, 18, 145. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pal, S.; Sharma, R. Transcription factors and chaperone proteins play a role in launching a faster response to heat stress and aggregation. Comput. Biol. Chem. 2021, 97, 107534. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, L.J.; Liu, J.C.; Du, X.X.; Asad, M.A.U.; Huang, F.D.; Pan, G.; Cheng, F.M. Relationship of ROS accumulation and superoxide dismutase isozymes in developing anther with floret fertility of rice under heat stress. Plant Physiol. Bioch. 2018, 122, 90–101. [Google Scholar] [CrossRef]
- Wan, B.L.; Lin, Y.J.; Mou, T.M. Expression of rice Ca2+-dependent protein kinases (CDPKs) genes under different environmental stresses. Febs. Lett. 2007, 581, 1179–1189. [Google Scholar] [CrossRef] [Green Version]
- Asgary, S.; Karimi, R.; Pour, P.M.; Heydarpour, F.; Mostafaei, S.; Farzaei, M.H.; Moradi, S.; Aneva, I.Y. Is consumption of pomegranate supplementation effective on oxidative stress biomarkers including MDA, ox-LDL, POX 1, GPX, TAC, and TBRAS? A systematic review and meta-analysis of randomized controlled trials. Curr. Prob. Cardiol. 2022, 101198, in press. [Google Scholar] [CrossRef] [PubMed]
- Wassie, M.; Zhang, W.H.; Zhang, Q.; Ji, K.; Cao, L.W.; Chen, L. Exogenous salicylic acid ameliorates heat stress-induced damages and improves growth and photosynthetic efficiency in alfalfa (Medicago sativa L.). Ecotox. Environ. Saf. 2020, 191, 110206. [Google Scholar] [CrossRef] [PubMed]
- Larkindale, J.; Mishkind, M.; Vierling, E. Plant Responses to High Temperature. Plant Abiotic. Stress 2005, 100, 100–144. [Google Scholar]
- Sarkar, S.; Islam, A.K.M.A.; Barma, N.C.D.; Ahmed, J.U. Tolerance mechanisms for breeding wheat against heat stress: A review. S. Afr. J. Bot. 2021, 138, 262–277. [Google Scholar] [CrossRef]
- Wen, F.; Wu, X.Z.; Li, T.J.; Jia, M.L.; Liu, X.S.; Li, P.; Zhou, X.J.; Ji, X.X.; Yue, X.M. Genome-wide survey of heat shock factors and heat shock protein 70s and their regulatory network under abiotic stresses in Brachypodium distachyon. PLoS ONE 2017, 12, e0180352. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Chopperla, R.; Shingote, P.; Chhapekar, S.S.; Deshmukh, R.; Khan, S.; Padaria, J.C.; Sharma, T.R.; Solanke, A.U. Overexpression of EcDREB2A transcription factor from finger millet in tobacco enhances tolerance to heat stress through ROS scavenging. J. Biotechnol. 2021, 336, 10–24. [Google Scholar] [CrossRef]
- Wei, S.W.; Zhang, L.; Huo, G.T.; Ge, G.J.; Luo, L.J.; Yang, Q.C.; Yang, X.; Long, P. Comparative transcriptomics and metabolomics analyses provide insights into thermal resistance in lettuce (Lactuca sativa L.). Sci. Hortic. 2021, 289, 110423. [Google Scholar] [CrossRef]
- Pucciariello, C.; Banti, V.; Perata, P. ROS signaling as common element in low oxygen and heat stresses. Plant Physiol. Bioch. 2012, 59, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Balla, K.; Bencze, S.; Janda, T.; Veisz, O. Analysis of heat stress tolerance in winter wheat. Acta Agron. Hung. 2009, 57, 437–444. [Google Scholar] [CrossRef]
- Gad, M.; Ron, M. Could Heat Shock Transcription Factors Function as Hydrogen Peroxide Sensors in Plants? Ann. Bot. 2006, 98, 279–288. [Google Scholar]
- Timperio, A.M.; Egidi, M.G.; Zolla, L. Proteomics applied on plant abiotic stresses: Role of heat shock proteins (HSP). J. Proteom. 2008, 71, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.R.; Yan, B.; Shi, M.; Zhou, W.; Zekria, D.; Wang, H.Z.; Kai, G.Y. Overexpression of a Brassica campestris HSP70 in tobacco confers enhanced tolerance to heat stress. Protoplasma 2016, 253, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Q.; Xia, X.; Su, J.H.; Wei, M.R.; Wu, Y.Q.; Tao, J. Overexpression of herbaceous peony HSP70 confers high temperature tolerance. BMC Genom. 2019, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Xuan, H.D.; Yang, C.F.; Guo, N.; Wang, H.T.; Zhao, J.M.; Xing, H. GmHsp90A2 is involved in soybean heat stress as a positive regulator. Plant Sci. 2019, 285, 26–33. [Google Scholar] [CrossRef]
- Dhakal, S.; Reiter, J.W.; Laroche, A.; Schultz, E.A. Leaf vein pattern response to heat and drought requires genes that influence PINFORMED1 localization and is mimicked by ABA treatment. Environ. Exp. Bot. 2021, 185, 104426. [Google Scholar] [CrossRef]
- Hays, D.B.; Do, J.H.; Mason, R.E.; Morgan, G.; Finlayson, S.A. Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Sci. 2007, 172, 1113–1123. [Google Scholar] [CrossRef]
- Larkindale, J.; Huang, B. Thermotolerance and antioxidant systems in Agrostis stolonifera: Involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J. Plant Physiol. 2004, 161, 405–413. [Google Scholar] [CrossRef]
- El-Bassiony, A.M.; Ghoname ElSayed, A.; el-awadi, M.; Fawzy, Z.; Gruda, N. Ameliorative Effects of Brassinosteroids on Growth and Productivity of Snap Beans Grown Under High Temperature. Gesunde Pflanz 2012, 64, 175–182. [Google Scholar] [CrossRef]
- Hu, Y.B.; Zhao, L.F.; Chong, K.; Wang, T. Overexpression of OsERF1, a novel rice ERF gene, up-regulates ethylene-responsive genes expression besides affects growth and development in Arabidopsis. J. Plant Physiol. 2008, 165, 1717–1725. [Google Scholar] [CrossRef]
- He, Y.H.; Xue, J.; Li, H.; Han, S.K.; Jiao, J.Q.; Rao, J.P. Ethylene response factors regulate ethylene biosynthesis and cell wall modification in persimmon (Diospyros kaki L.) fruit during ripening. Postharvest Biol. Tec. 2020, 168, 111255. [Google Scholar] [CrossRef]
- Zhang, H.N.; Pan, X.L.; Liu, S.H.; Lin, W.Q.; Li, Y.H.; Zhang, X.M. Genome-wide analysis of AP2/ERF transcription factors in pineapple reveals functional divergence during flowering induction mediated by ethylene and floral organ development. Genomics 2021, 113, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Klay, I.; Gouia, S.; Liu, M.; Mila, I.; Khoudi, H.; Bernadac, A.; Bouzayen, M.; Pirrello, J. Ethylene Response Factors (ERF) are differentially regulated by different abiotic stress types in tomato plants. Plant Sci. 2018, 274, 137–145. [Google Scholar] [CrossRef]
- Choi, H.I.; Hong, J.H.; Ha, J.O.; Kang, J.Y.; Kim, S.Y. ABFs, a Family of ABA-responsive Element Binding Factors*. J. Biol. Chem. 2000, 275, 1723–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Li, C.; Liu, W.J.; Lv, Y.D.; Yu, C.S.; Li, H.Y.; Zhao, T.; Liu, B. The OsABF1 transcription factor improves drought tolerance by activating the transcription of COR413-TM1 in rice. J. Exp. Bot. 2017, 68, 4695–4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.B.; Qiu, X.P.; Yang, Y.X.; Kim, H.S.; Jia, X.Y.; Yu, H.; Kwak, S.S. Sweetpotato bZIP Transcription Factor IbABF4 Confers Tolerance to Multiple Abiotic Stresses. Front. Plant Sci. 2019, 10, 630. [Google Scholar] [CrossRef] [Green Version]
- Li, J.B.; Zhao, S.; Yu, X.; Du, W.; Li, H.; Sun, Y.; Sun, H.; Ruan, C.J. Role of Xanthoceras sorbifolium MYB44 in tolerance to combined drought and heat stress via modulation of stomatal closure and ROS homeostasis. Plant Physiol. Bioch. 2021, 162, 410–420. [Google Scholar] [CrossRef]
- Guan, Q.M.; Yue, X.L.; Zeng, H.T.; Zhu, J.H. The Protein Phosphatase RCF2 and Its Interacting Partner NAC019 Are Critical for Heat Stress-Responsive Gene Regulation and Thermotolerance in Arabidopsis. Plant Cell 2014, 26, 438–453. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Chen, X.Q.; Han, J.; Lu, W.L.; Ren, Z.H. Genome-wide analysis of the WRKY gene family in the cucumber genome and transcriptome-wide identification of WRKY transcription factors that respond to biotic and abiotic stresses. BMC Plant Biol. 2020, 20, 443. [Google Scholar] [CrossRef]
- Koini, M.A.; Alvey, L.; Allen, T.; Tilley, C.A.; Harberd, N.P.; Whitelam, G.C.; Franklin, K.A. High Temperature-Mediated Adaptations in Plant Architecture Require the bHLH Transcription Factor PIF4. Curr. Biol. 2009, 19, 408–413. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.; Deng, Y.; Howell, S. Stress sensing in plants by an ER stress sensor/transducer, bZIP28. Front. Plant Sci. 2014, 5, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Li, T.; Liu, X.Y.; Yuan, G.Z.; Hou, H.Z.; Teng, N.J. A novel R2R3-MYB transcription factor LlMYB305 from Lilium longiflorum plays a positive role in thermotolerance via activating heat-protective genes. Environ. Exp. Bot. 2021, 184, 104399. [Google Scholar] [CrossRef]
- Fu, Q.T.; Yu, D. Q Expression profiles of AtWRKY25, AtWRKY26 and AtWRKY33 under abiotic stresses. Yi Chuan 2010, 32, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.D.; Wang, Y.; Kuzma, M.; Chalifoux, M.; Tremblay, L.; Yang, S.J.; Ying, J.F.; Sample, A.; Wang, H.M.; Griffiths, R.; et al. Activation tagging identifies Arabidopsis transcription factor AtMYB68 for heat and drought tolerance at yield determining reproductive stages. Plant J. 2020, 104, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zhang, H.; Li, X.H.; Xiao, J.H.; Xiong, L.Z. Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiol. 2012, 158, 1755–1768. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.; Ling, Q.Q.; Zhou, Y.; Liu, X.; Qian, Y.X. ZmNAC074, a maize stress-responsive NAC transcription factor, confers heat stress tolerance in transgenic Arabidopsis. Front. Plant Sci. 2022, 13, 986628. [Google Scholar] [CrossRef]
- Schulz, P.; Herde, M.; Romeis, T. Calcium-dependent protein kinases: Hubs in plant stress signaling and development. Plant Physiol. 2013, 163, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Tian, Y.S.; Peng, R.H.; Xiong, A.S.; Zhu, B.; Jin, X.F.; Gao, F.; Fu, X.Y.; Hou, X.L.; Yao, Q.H. AtCPK6, a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis. Planta 2010, 231, 1251–1260. [Google Scholar] [CrossRef]
- Saijo, Y.; Hata, S.; Kyozuka, J.; Shimamoto, K.; Izui, K. Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 2000, 23, 319–327. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Du, H.W.; Wang, Y.K.; Wang, H.L.; Yang, S.Y.; Li, C.H.; Chen, N.; Yang, H.; Zhang, Y.H.; Zhu, Y.; et al. The calcium-dependent protein kinase ZmCDPK7 functions in heat-stress tolerance in maize. J. Integr. Plant Biol. 2021, 63, 510–527. [Google Scholar] [CrossRef]


| Sample | Total Reads | Total Bases | Clean Reads | Clean Bases | Q20 Rate (%) | Q30 Rate (%) | GC (%) |
|---|---|---|---|---|---|---|---|
| CK-1 | 45,863,972 | 6,879,595,800 | 44,915,008 | 6,680,680,398 | 97.85 | 93.46 | 44.94 |
| CK-2 | 47,993,664 | 7,199,049,600 | 47,205,020 | 7,025,130,948 | 98.10 | 94.05 | 44.77 |
| CK-3 | 53,683,124 | 8,052,468,600 | 52,814,730 | 7,734,007,771 | 98.03 | 93.87 | 44.67 |
| average | 49,180,253 | 7,377,038,000 | 48,311,586 | 7,146,606,372 | 97.99 | 93.79 | 44.79 |
| Ht-1 | 51,810,464 | 7,771,569,600 | 50,974,272 | 7,534,318,727 | 98.02 | 93.85 | 45.00 |
| Ht-2 | 45,663,096 | 6,849,464,400 | 44,953,364 | 6,625,104,742 | 98.10 | 94.07 | 45.65 |
| Ht-3 | 50,863,920 | 7,629,588,000 | 49,951,766 | 7,400,820,966 | 98.22 | 94.44 | 45.24 |
| average | 49,445,827 | 7,416,874,000 | 48,626,467 | 7,186,748,145 | 98.11 | 94.12 | 45.30 |
| Database | KOG | KEGG | NR | Swiss-Prot | GO | Total Unigenes | Overall Annotated |
|---|---|---|---|---|---|---|---|
| Gene Number | 19,218 | 12,563 | 30,131 | 26,193 | 19,546 | 32,719 | 30,464 |
| Annotation Ratio | 0.5874 | 0.3840 | 0.9209 | 0.8005 | 0.5974 | - | 0.9311 |
| Gene ID | Log2 Ratio | p-Value | q Value | Gene Description |
|---|---|---|---|---|
| PB.23444.1 | 3.0345 | 0.00016 | 0.001745 | Calcium-dependent protein kinase 1 OS = Oryza sativa subsp. japonica OX = 39947 GN = CPK1 PE = 2 SV = 1 |
| PB.16020.1 | 10.482 | 5.72 × 10−18 | 2.04 × 10−16 | Calcium-dependent protein kinase 8 OS = Arabidopsis thaliana OX = 3702 GN = CPK8 PE = 1 SV = 1 |
| PB.1840.1 | 1.1687 | 0.007945 | 0.049257 | Calcium-dependent protein kinase 1 OS = Oryza sativa subsp. japonica OX = 39947 GN = CPK1 PE = 2 SV = 1 |
| PB.15694.1 | 7.6079 | 1.11 × 10−6 | 1.79 × 10−5 | Calcium-dependent protein kinase 8 OS = Arabidopsis thaliana OX = 3702 GN = CPK8 PE = 1 SV = 1 |
| PB.13451.1 | 3.0578 | 9.96 × 10−11 | 2.45 × 10−9 | Calcium-dependent protein kinase 1 OS = Arabidopsis thaliana OX = 3702 GN = CPK1 PE = 1 SV = 1 |
| PB.12258.1 | 1.0192 | 0.00019 | 0.002038 | Calcium-dependent protein kinase 30 OS = Arabidopsis thaliana OX = 3702 GN = CPK30 PE = 1 SV = 1 |
| PB.13498.1 | 1.0004 | 7.03 × 10−11 | 1.75 × 10−9 | Calcium-dependent protein kinase 8 OS = Arabidopsis thaliana OX = 3702 GN = CPK8 PE = 1 SV = 1 |
| PB.23444.1 | 3.0345 | 0.00016 | 0.001745 | Calcium-dependent protein kinase 1 OS = Oryza sativa subsp. japonica OX = 39947 GN = CPK1 PE = 2 SV = 1 |
| PB.16020.1 | 10.482 | 5.72 × 10−18 | 2.04 × 10−16 | Calcium-dependent protein kinase 8 OS = Arabidopsis thaliana OX = 3702 GN = CPK8 PE = 1 SV = 1 |
| PB.32683.1 | 2.4636 | 1.97 × 10−6 | 3.06 × 10−5 | Calcium-binding protein CML24 OS = Arabidopsis thaliana OX = 3702 GN = CML24 PE = 2 SV = 2 |
| PB.25136.1 | 1.7180 | 2.06 × 10−8 | 4.12 × 10−7 | Probable calcium-binding protein CML46 OS = Arabidopsis thaliana OX = 3702 GN = CML46 PE = 1 SV = 1 |
| PB.1840.1 | 1.1687 | 0.007945 | 0.049257 | Calcium-dependent protein kinase OS = Oryza sativa subsp. japonica OX = 39947 GN = CPK1 PE = 2 SV = 1 |
| PB.31428.1 | 5.0403 | 3.81 × 10−70 | 4.27 × 10−68 | Probable calcium-binding protein CML40 OS = Arabidopsis thaliana OX = 3702 GN = CML40 PE = 2 SV = 1 |
| PB.31509.1 | 8.2027 | 0 | 0 | Probable calcium-binding protein CML44 OS = Arabidopsis thaliana OX = 3702 GN = CML44 PE = 2 SV = 2 |
| PB.15694.1 | 7.6079 | 1.11 × 10−6 | 1.79 × 10−5 | Calcium-dependent protein kinase 8 OS = Arabidopsis thaliana OX = 3702 GN = CPK8 PE = 1 SV = 1 |
| PB.13451.1 | 3.0578 | 9.96 × 10−11 | 2.45 × 10−9 | Calcium-dependent protein kinase 1 OS = Arabidopsis thaliana OX = 3702 GN = CPK1 PE = 1 SV = 1 |
| PB.12258.1 | 1.0192 | 0.00019 | 0.002038 | Calcium-dependent protein kinase 30 OS = Arabidopsis thaliana OX = 3702 GN = CPK30 PE = 1 SV = 1 |
| PB.14842.1 | 3.9140 | 1.78 × 10−241 | 7.04 × 10−239 | Calcium-dependent protein kinase 8 OS = Arabidopsis thaliana OX = 3702 GN = CPK8 PE = 1 SV = 1 |
| PB.13498.1 | 1.0004 | 7.03 × 10−11 | 1.75 × 10−9 | Calcium-dependent protein kinase 8 OS = Arabidopsis thaliana OX = 3702 GN = CPK8 PE = 1 SV = 1 |
| Gene ID | Log2 Ratio | p-Value | q-Value | Gene Description |
|---|---|---|---|---|
| PB.6276.1 | 3.4312 | 1.57 × 10−80 | 2.10 × 10−78 | bZIP transcription factor 17 OS = Arabidopsis thaliana OX = 3702 GN = BZIP17 PE = 1 SV = 2 |
| PB.22986.1 | 3.3479 | 1.30 × 10−8 | 2.66 × 10−7 | ABSCISIC ACID-INSENSITIVE 5-like protein 5 OS = Arabidopsis thaliana OX = 3702 GN = ABF2 PE = 1 SV = 1 |
| PB.21759.1 | 3.2678 | 2.75 × 10−87 | 3.94 × 10−8 | bZIP transcription factor 17 OS = Arabidopsis thaliana OX = 3702 GN = BZIP17 PE = 1 SV = 2 |
| PB.19784.1 | 2.9457 | 4.47 × 10−15 | 1.40 × 10−13 | bZIP transcription factor 17 OS = Arabidopsis thaliana OX = 3702 GN = BZIP17 PE = 1 SV = 2 |
| PB.19784.1 | 2.9457 | 4.47 × 10−15 | 1.40 × 10−13 | bZIP transcription factor 17 OS = Arabidopsis thaliana OX = 3702 GN = BZIP17 PE = 1 SV = 2 |
| PB.19784.1 | 2.9457 | 4.47 × 10−15 | 1.40 × 10−13 | bZIP transcription factor 17 OS = Arabidopsis thaliana OX = 3702 GN = BZIP17 PE = 1 SV = 2 |
| PB.30413.1 | 2.2121 | 1.54 × 10−50 | 1.27 × 10−48 | bZIP transcription factor 53 OS = Arabidopsis thaliana OX = 3702 GN = BZIP53 PE = 1 SV = 1 |
| PB.20861.1 | 1.1184 | 6.48 × 10−8 | 1.22 × 10−6 | Probable transcription factor PosF21 OS = Arabidopsis thaliana OX = 3702 GN = POSF21 PE = 2 SV = 1 |
| Gene ID | Log2 Ratio | p-Value | q-Value | Gene Description |
|---|---|---|---|---|
| PB.15241.1 | 8.3623 | 1.41 × 10−9 | 3.15 × 10−8 | NAC domain-containing protein 91 OS = Arabidopsis thaliana OX = 3702 GN = NAC091 PE = 1 SV = 1 |
| PB.12957.1 | 8.1420 | 6.04 × 10−10 | 1.40 × 10−8 | NAC domain-containing protein 78 OS = Arabidopsis thaliana OX = 3702 GN = NAC078 PE = 2 SV = 2 |
| PB.31443.1 | 4.7942 | 1.32 × 10−91 | 2.01 × 10−89 | NAC domain-containing protein 2 OS = Arabidopsis thaliana OX = 3702 GN = NAC002 PE = 1 SV = 2 |
| PB.25504.1 | 4.3026 | 1.63 × 10−230 | 5.91 × 10−228 | NAC domain-containing protein 2 OS = Solanum lycopersicum OX = 4081 GN = NAP2 PE = 2 SV = 1 |
| PB.27680.1 | 3.6323 | 2.95 × 10−73 | 3.49 × 10−71 | NAC domain-containing protein JA2L OS = Solanum lycopersicum OX = 4081 GN = JA2L PE = 2 SV = 1 |
| PB.29002.1 | 3.3875 | 7.42 × 10−156 | 1.85 × 10−153 | NAC domain-containing protein 2 OS = Arabidopsis thaliana OX = 3702 GN = NAC002 PE = 1 SV = 2 |
| PB.29160.1 | 3.1996 | 9.66 × 10−225 | 3.41 × 10−222 | NAC domain-containing protein 2 OS = Arabidopsis thaliana OX = 3702 GN = NAC002 PE = 1 SV = 2 |
| PB.12308.1 | 2.6077 | 2.87 × 10−82 | 3.91 × 10−80 | NAC domain-containing protein 78 OS = Arabidopsis thaliana OX = 3702 GN = NAC078 PE = 2 SV = 2 |
| PB.17150.1 | 1.7393 | 0.007178 | 0.045244 | NAC domain-containing protein 17 OS = Arabidopsis thaliana OX = 3702 GN = NAC017 PE = 2 SV = 1 |
| PB.12444.1 | 1.4019 | 3.30 × 10−14 | 9.93 × 10−13 | NAC domain-containing protein 82 OS = Arabidopsis thaliana OX = 3702 GN = NAC082 PE = 1 SV = 1 |
| PB.23584.1 | 1.2042 | 5.20 × 10−13 | 1.48 × 10−11 | NAC domain-containing protein 53 OS = Arabidopsis thaliana OX = 3702 GN = NAC053 PE = 2 SV = 1 |
| Gene ID | Log2 Ratio | p-Value | q-Value | Gene Description |
|---|---|---|---|---|
| PB.28576.1 | 2.1307 | 4.14 × 10−5 | 0.000509 | Probable WRKY transcription factor 41 OS = Arabidopsis thaliana OX = 3702 GN = WRKY41 PE = 2 SV = 2 |
| PB.9306.1 | 2.7500 | 7.58 × 10−15 | 2.35 × 10−13 | Probable WRKY transcription factor 46 OS = Arabidopsis thaliana OX = 3702 GN = WRKY46 PE = 1 SV = 1 |
| PB.26632.1 | 1.2038 | 8.50 × 10−12 | 2.24 × 10−10 | Probable WRKY transcription factor 40 OS = Arabidopsis thaliana OX = 3702 GN = WRKY40 PE = 1 SV = 1 |
| PB.17400.1 | 1.8484 | 1.52 × 10−14 | 4.65 × 10−13 | Probable WRKY transcription factor 53 OS = Arabidopsis thaliana OX = 3702 GN = WRKY53 PE = 1 SV = 1 |
| PB.27665.1 | 1.1763 | 0.000231 | 0.002425 | Probable WRKY transcription factor 40 OS = Arabidopsis thaliana OX = 3702 GN = WRKY40 PE = 1 SV = 1 |
| PB.31800.1 | 1.4388 | 0.000696 | 0.006323 | Probable WRKY transcription factor 75 OS = Arabidopsis thaliana OX = 3702 GN = WRKY75 PE = 2 SV = 1 |
| PB.9501.1 | 1.2791 | 2.97 × 10−5 | 0.000375 | F-box/LRR-repeat protein 3 OS = Arabidopsis thaliana OX = 3702 GN = FBL3 PE = 2 SV = 1 |
| PB.30350.1 | 2.1258 | 1.62 × 10−51 | 1.36 × 10−49 | Probable WRKY transcription factor 70 OS = Arabidopsis thaliana OX = 3702 GN = WRKY70 PE = 1 SV = 1 |
| PB.28576.1 | 2.1307 | 4.14 × 10−5 | 0.000509 | Probable WRKY transcription factor 41 OS = Arabidopsis thaliana OX = 3702 GN = WRKY41 PE = 2 SV = 2 |
| Gene ID | Log2 Ratio | p-Value | q-Value | Gene Description |
|---|---|---|---|---|
| PB.28723.1 | −1.3956 | 2.71 × 10−7 | 4.75 × 10−6 | Transcription factor bHLH30 OS = Arabidopsis thaliana OX = 3702 GN = BHLH30 PE = 1 SV = 1 |
| PB.8767.1 | −1.6598 | 3.01 × 10−17 | 1.04 × 10−15 | Transcription factor bHLH74 OS = Arabidopsis thaliana OX = 3702 GN = BHLH74 PE = 1 SV = 1 |
| PB.30938.1 | −1.4028 | 1.41 × 10−11 | 3.69 × 10−10 | Transcription factor bHLH147 OS = Arabidopsis thaliana OX = 3702 GN = BHLH147 PE = 1 SV = 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Wassie, M.; Zhou, W.; Wang, H.; You, J.; Ma, G.; Zhang, M. Transcriptomic Analysis Provides Novel Insights into the Heat Stress-Induced Response in Codonopsis tangshen. Life 2023, 13, 168. https://doi.org/10.3390/life13010168
Jiang X, Wassie M, Zhou W, Wang H, You J, Ma G, Zhang M. Transcriptomic Analysis Provides Novel Insights into the Heat Stress-Induced Response in Codonopsis tangshen. Life. 2023; 13(1):168. https://doi.org/10.3390/life13010168
Chicago/Turabian StyleJiang, Xiaogang, Misganaw Wassie, Wuxian Zhou, Hua Wang, Jinwen You, Guangjing Ma, and Meide Zhang. 2023. "Transcriptomic Analysis Provides Novel Insights into the Heat Stress-Induced Response in Codonopsis tangshen" Life 13, no. 1: 168. https://doi.org/10.3390/life13010168






