Lipid Changes in the Peri-Implantation Period with Mass Spectrometry Imaging: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Literature Search
2.3. Study Selection, Risk of Bias Assessment
2.4. Data Extraction and Synthesis
3. Results
3.1. Lipids in Embryos Early after Fertilization
3.2. Lipids in the Receptive Uterus
3.3. Lipids in Implanting Embryos and Uterus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caprioli, R.M.; Farmer, T.B.; Gile, J. Molecular imaging of biological samples: Localization of peptides and proteins using MALDI-TOF MS. Anal. Chem. 1997, 69, 4751–4760. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.; Islam, A.; Eto, F.; Sato, T.; Kahyo, T.; Setou, M. Mass spectrometry-based phospholipid imaging: Methods and findings. Expert Rev. Proteom. 2020, 17, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Cornett, D.S.; Reyzer, M.L.; Chaurand, P.; Caprioli, R.M. MALDI imaging mass spectrometry: Molecular snapshots of biochemical systems. Nat. Methods 2007, 4, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Tuck, M.; Grelard, F.; Blanc, L.; Desbenoit, N. MALDI-MSI Towards Multimodal Imaging: Challenges and Perspectives. Front. Chem. 2022, 10, 904688. [Google Scholar] [CrossRef] [PubMed]
- Cazares, L.H.; Troyer, D.A.; Wang, B.; Drake, R.R.; Semmes, O.J. MALDI tissue imaging: From biomarker discovery to clinical applications. Anal. Bioanal. Chem. 2011, 401, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lee, M.; Fairn, G.D. Phospholipid subcellular localization and dynamics. J. Biol. Chem. 2018, 293, 6230–6240. [Google Scholar] [CrossRef]
- Raphael, W.; Sordillo, L.M. Dietary polyunsaturated fatty acids and inflammation: The role of phospholipid biosynthesis. Int. J. Mol. Sci. 2013, 14, 21167–21188. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Dowhan, W. Molecular basis for membrane phospholipid diversity: Why are there so many lipids? Annu. Rev. Biochem. 1997, 66, 199–232. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Introduction to fatty acids and lipids. World Rev. Nutr. Diet. 2015, 112, 1–16. [Google Scholar] [CrossRef]
- Ryan, D.J.; Spraggins, J.M.; Caprioli, R.M. Protein identification strategies in MALDI imaging mass spectrometry: A brief review. Curr. Opin. Chem. Biol. 2019, 48, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Hale, O.J.; Cooper, H.J. Native Mass Spectrometry Imaging of Proteins and Protein Complexes by Nano-DESI. Anal. Chem. 2021, 93, 4619–4627. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Buijs, R.; Eijkel, G.B.; Giskes, F.; Dyachenko, A.; van der Horst, J.; Byelov, D.; Spaanderman, D.J.; Heck, A.J.R.; Porta Siegel, T.; et al. Ion Imaging of Native Protein Complexes Using Orthogonal Time-of-Flight Mass Spectrometry and a Timepix Detector. J. Am. Soc. Mass Spectrom. 2021, 32, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Seeley, E.H.; Caprioli, R.M. Molecular imaging of proteins in tissues by mass spectrometry. Proc. Natl. Acad. Sci. USA 2008, 105, 18126–18131. [Google Scholar] [CrossRef] [PubMed]
- Stoeckli, M.; Chaurand, P.; Hallahan, D.E.; Caprioli, R.M. Imaging mass spectrometry: A new technology for the analysis of protein expression in mammalian tissues. Nat. Med. 2001, 7, 493–496. [Google Scholar] [CrossRef]
- Eberlin, L.S.; Ferreira, C.R.; Dill, A.L.; Ifa, D.R.; Cooks, R.G. Desorption electrospray ionization mass spectrometry for lipid characterization and biological tissue imaging. Biochim. Biophys. Acta 2011, 1811, 946–960. [Google Scholar] [CrossRef]
- Greco, F.; Quercioli, L.; Pucci, A.; Rocchiccioli, S.; Ferrari, M.; Recchia, F.A.; McDonnell, L.A. Mass Spectrometry Imaging as a Tool to Investigate Region Specific Lipid Alterations in Symptomatic Human Carotid Atherosclerotic Plaques. Metabolites 2021, 11, 250. [Google Scholar] [CrossRef]
- Lim, J.; Aguilan, J.T.; Sellers, R.S.; Nagajyothi, F.; Weiss, L.M.; Angeletti, R.H.; Bortnick, A.E. Lipid mass spectrometry imaging and proteomic analysis of severe aortic stenosis. J. Mol. Histol. 2020, 51, 559–571. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Lambertsen, K.L.; Clausen, B.H.; Meyer, M.; Bhandari, D.R.; Larsen, S.T.; Poulsen, S.S.; Spengler, B.; Janfelt, C.; Hansen, H.S. Mass spectrometry imaging of biomarker lipids for phagocytosis and signalling during focal cerebral ischaemia. Sci. Rep. 2016, 6, 39571. [Google Scholar] [CrossRef]
- Holzlechner, M.; Eugenin, E.; Prideaux, B. Mass spectrometry imaging to detect lipid biomarkers and disease signatures in cancer. Cancer Rep. 2019, 2, e1229. [Google Scholar] [CrossRef]
- Mouchlis, V.D.; Dennis, E.A. Membrane Association Allosterically Regulates Phospholipase A(2) Enzymes and Their Specificity. Acc. Chem. Res. 2022, 55, 3303–3311. [Google Scholar] [CrossRef] [PubMed]
- Beermann, C.; Möbius, M.; Winterling, N.; Schmitt, J.J.; Boehm, G. sn-position determination of phospholipid-linked fatty acids derived from erythrocytes by liquid chromatography electrospray ionization ion-trap mass spectrometry. Lipids 2005, 40, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Bowman, A.P.; Bogie, J.F.J.; Hendriks, J.J.A.; Haidar, M.; Belov, M.; Heeren, R.M.A.; Ellis, S.R. Evaluation of lipid coverage and high spatial resolution MALDI-imaging capabilities of oversampling combined with laser post-ionisation. Anal. Bioanal. Chem. 2020, 412, 2277–2289. [Google Scholar] [CrossRef] [PubMed]
- McMillen, J.C.; Fincher, J.A.; Klein, D.R.; Spraggins, J.M.; Caprioli, R.M. Effect of MALDI matrices on lipid analyses of biological tissues using MALDI-2 postionization mass spectrometry. J. Mass Spectrom. 2020, 55, e4663. [Google Scholar] [CrossRef] [PubMed]
- Claes, B.S.R.; Bowman, A.P.; Poad, B.L.J.; Young, R.S.E.; Heeren, R.M.A.; Blanksby, S.J.; Ellis, S.R. Mass Spectrometry Imaging of Lipids with Isomer Resolution Using High-Pressure Ozone-Induced Dissociation. Anal. Chem. 2021, 93, 9826–9834. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- de Mouzon, J.; Chambers, G.M.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Banker, M.; Dyer, S.; Kupka, M.; Adamson, G.D. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted reproductive technology 2012dagger. Hum. Reprod. 2020, 35, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, V.A.; Barad, D.H.; Albertini, D.F.; Darmon, S.K.; Gleicher, N. Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reprod. Biol. Endocrinol. 2017, 15, 6. [Google Scholar] [CrossRef]
- Ye, Q.; Zeng, X.; Cai, S.; Qiao, S.; Zeng, X. Mechanisms of lipid metabolism in uterine receptivity and embryo development. Trends Endocrinol. Metab. 2021, 32, 1015–1030. [Google Scholar] [CrossRef]
- Paria, B.C.; Wang, H.; Dey, S.K. Endocannabinoid signaling in synchronizing embryo development and uterine receptivity for implantation. Chem. Phys. Lipids 2002, 121, 201–210. [Google Scholar] [CrossRef]
- Liu, W.M.; Duan, E.K.; Cao, Y.J. Effects of anandamide on embryo implantation in the mouse. Life Sci. 2002, 71, 1623–1632. [Google Scholar] [CrossRef]
- Schmid, P.C.; Paria, B.C.; Krebsbach, R.J.; Schmid, H.H.; Dey, S.K. Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proc. Natl. Acad. Sci. USA 1997, 94, 4188–4192. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Bisogno, T.; Valensise, H.; Lazzarin, N.; Fezza, F.; Manna, C.; Di Marzo, V.; Finazzi-Agro, A. Low fatty acid amide hydrolase and high anandamide levels are associated with failure to achieve an ongoing pregnancy after IVF and embryo transfer. Mol. Hum. Reprod. 2002, 8, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kaczynski, P.; Kowalewski, M.P.; Waclawik, A. Prostaglandin F2α promotes angiogenesis and embryo–maternal interactions during implantation. Reproduction 2016, 151, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Pakrasi, P.L.; Jain, A.K. Cyclooxygenase-2 derived PGE2 and PGI2 play an important role via EP2 and PPARdelta receptors in early steps of oil induced decidualization in mice. Placenta 2008, 29, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Freeman, D.A.; Vanderwall, D.K.; Woods, G.L. Prostaglandin E2 secretion by oviductal transport-stage equine embryos. Biol. Reprod. 1991, 45, 540–543. [Google Scholar] [CrossRef]
- Niringiyumukiza, J.D.; Cai, H.; Xiang, W. Prostaglandin E2 involvement in mammalian female fertility: Ovulation, fertilization, embryo development and early implantation. Reprod. Biol. Endocrinol. 2018, 16, 43. [Google Scholar] [CrossRef]
- Vilella, F.; Ramirez, L.; Berlanga, O.; Martinez, S.; Alama, P.; Meseguer, M.; Pellicer, A.; Simon, C. PGE2 and PGF2alpha concentrations in human endometrial fluid as biomarkers for embryonic implantation. J. Clin. Endocrinol. Metab. 2013, 98, 4123–4132. [Google Scholar] [CrossRef]
- Demiral Keles, I.; Ulgen, E.; Erkan, M.B.; Celik, S.E.; Aydin, Y.; Onem, A.N.; Kandemir, H.; Arslanoglu, T.; Apak, M.R.; Sezerman, U.; et al. Comparison of endometrial prostanoid profiles in three infertile subgroups: The missing part of receptivity? Fertil. Steril. 2020, 113, 670–678.e1. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); Cochrane: London, UK, 2021; Available online: www.training.cochrane.org/handbook (accessed on 15 December 2021).
- Byron, C.W.; Small, K.; Brodley, C.E.; Lau, J.; Trikalinos, T.A. Deploying an interactive machine learning system in an evidence-based practice center: Abstrackr. In Proceedings of the IHI ’12: ACM International Health Informatics Symposium, Miami, FL, USA, 28–30 January 2012; pp. 819–824. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Sato, B.; Katagiri, Y.U.; Miyado, K.; Okino, N.; Ito, M.; Akutsu, H.; Okita, H.; Umezawa, A.; Fujimoto, J.; Toshimori, K.; et al. Lipid rafts enriched in monosialylGb5Cer carrying the stage-specific embryonic antigen-4 epitope are involved in development of mouse preimplantation embryos at cleavage stage. BMC Dev. Biol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Montani, D.A.; Cordeiro, F.B.; Regiani, T.; Victorino, A.B.; Pilau, E.J.; Gozzo, F.C.; Ferreira, C.R.; Fraietta, R.; Lo Turco, E.G. The follicular microenviroment as a predictor of pregnancy: MALDI-TOF MS lipid profile in cumulus cells. J. Assist. Reprod. Genet. 2012, 29, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Eberlin, L.S.; Ferreira, C.R.; Cooks, R.G. Three-dimensional chemical imaging of a whole pig fetus by desorption electrospray ionization mass spectrometry. Reprod. Fertil. Dev. 2012, 24, 144–145. [Google Scholar] [CrossRef]
- Hayasaka, T.; Goto-Inoue, N.; Zaima, N.; Kimura, Y.; Setou, M. Organ-specific distributions of lysophosphatidylcholine and triacylglycerol in mouse embryo. Lipids 2009, 44, 837–848. [Google Scholar] [CrossRef]
- Banliat, C.; Dubuisson, F.; Corbin, E.; Beurois, J.; Tomas, D.; Le Bourhis, D.; Salvetti, P.; Labas, V.; Mermillod, P.; Saint-Dizier, M. Intraoviductal concentrations of steroid hormones during in vitro culture changed phospholipid profiles and cryotolerance of bovine embryos. Mol. Reprod. Dev. 2019, 86, 661–672. [Google Scholar] [CrossRef]
- Annes, K.; Sudano, M.J.; Belaz, K.R.A.; Tata, A.; Santos, V.G.; da Fonseca Junior, A.M.; Dos Santos, É.C.; Eberlin, M.N.; Milazzotto, M.P. Lipid characterization of in vitro -produced bovine embryos with distinct kinetics of development. Zygote 2019, 27, 413–422. [Google Scholar] [CrossRef]
- Sudano, M.J.; Rascado, T.D.; Tata, A.; Belaz, K.R.; Santos, V.G.; Valente, R.S.; Mesquita, F.S.; Ferreira, C.R.; Araujo, J.P.; Eberlin, M.N.; et al. Lipidome signatures in early bovine embryo development. Theriogenology 2016, 86, 472–484.e1. [Google Scholar] [CrossRef]
- Leão, B.C.; Rocha-Frigoni, N.A.; Cabral, E.C.; Franco, M.F.; Ferreira, C.R.; Eberlin, M.N.; Filgueiras, P.R.; Mingoti, G.Z. Membrane lipid profile monitored by mass spectrometry detected differences between fresh and vitrified in vitro-produced bovine embryos. Zygote 2015, 23, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Tata, A.; Sudano, M.J.; Santos, V.G.; Landim-Alvarenga, F.D.C.; Ferreira, C.R.; Eberlin, M.N. Optimal single-embryo mass spectrometry fingerprinting. J. Mass Spectrom. 2013, 48, 844–849. [Google Scholar] [CrossRef]
- Banliat, C.; Le Bourhis, D.; Bernardi, O.; Tomas, D.; Labas, V.; Salvetti, P.; Guyonnet, B.; Mermillod, P.; Saint-Dizier, M. Oviduct fluid extracellular vesicles change the phospholipid composition of bovine embryos developed in vitro. Int. J. Mol. Sci. 2020, 21, 5326. [Google Scholar] [CrossRef] [PubMed]
- Sudano, M.J.; Santos, V.G.; Tata, A.; Ferreira, C.R.; Paschoal, D.M.; Machado, R.; Buratini, J.; Eberlin, M.N.; Landim-Alvarenga, F.D.C. Phosphatidylcholine and sphingomyelin profiles vary in bos taurus indicus and bos taurus taurus in vitro- and in vivo-produced blastocysts. Biol. Reprod. 2012, 87, 130. [Google Scholar] [CrossRef] [PubMed]
- Sprícigo, J.F.W.; Leme, L.O.; Guimarães, A.L.; Oliveira Neto, J.C.; Silva, P.C.P.; Moreira, N.H.; Pivato, I.; Silva, B.D.M.; Ramos, A.F.; Dode, M.A.N. Phospholipid composition and resistance to vitrification of in vivo blastocyst of a Brazilian naturalized porcine breed. Arq. Bras. Med. Vet. Zootec. 2019, 71, 837–847. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; Ferreira, C.R.; Orlandi, C.M.B.; Sartori, V.C.; Ferreira, H.N.; Gozzo, F.C.; Saraiva, S.A.; Pilau, E.J.; Eberlin, M.N. Single equine embryo lipid fingerprinting by mass spectrometry. Reprod. Fertil. Dev. 2010, 23, 160–161. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; Ferreira, M.S.; De Oliveira, D.N.; Canevarolo, R.; Achilles, M.A.; D’Ercole, D.L.; Bols, P.E.; Visintin, J.A.; Killian, G.J.; Catharino, R.R. Analysis and characterisation of bovine oocyte and embryo biomarkers by matrix-assisted desorption ionisation mass spectrometry imaging. Reprod. Fertil. Dev. 2016, 28, 293–301. [Google Scholar] [CrossRef]
- De Melo, A.A.; Camillo, J.; Oleinki, T.D.; Cordeiro, F.B.; Lo Turco, E.G.; Santos, T.G. Lipid profile analysis from mice embryos vitrified. Fertil. Steril. 2015, 104, e194. [Google Scholar] [CrossRef]
- Burnum, K.E.; Cornett, D.S.; Puolitaival, S.M.; Milne, S.B.; Myers, D.S.; Tranguch, S.; Brown, H.A.; Dey, S.K.; Caprioli, R.M. Spatial and temporal alterations of phospholipids determined by mass spectrometry during mouse embryo implantation. J. Lipid Res. 2009, 50, 2290–2298. [Google Scholar] [CrossRef]
- Lanekoff, I.; Burnum-Johnson, K.; Thomas, M.; Cha, J.; Dey, S.K.; Yang, P.; Prieto Conaway, M.C.; Laskin, J. Three-dimensional imaging of lipids and metabolites in tissues by nanospray desorption electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2063–2071. [Google Scholar] [CrossRef]
- Lanekoff, I.; Cha, J.; Kyle, J.E.; Dey, S.K.; Laskin, J.; Burnum-Johnson, K.E. Trp53 deficient mice predisposed to preterm birth display region-specific lipid alterations at the embryo implantation site. Sci. Rep. 2016, 6, 33023. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.S.; de Oliveira, D.N.; Gonçalves, R.F.; Catharino, R.R. Lipid characterization of embryo zones by silica plate laser desorption ionization mass spectrometry imaging (SP-LDI-MSI). Anal. Chim. Acta 2014, 807, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Belaz, K.A.; Tata, A.; França, M.R.; Santos da Silva, M.I.; Vendramini, P.H.; Fernandes, A.M.A.P.; D’Alexandri, F.L.; Eberlin, M.N.; Binelli, M. Phospholipid profile and distribution in the receptive oviduct and uterus during early diestrus in cattle. Biol. Reprod. 2016, 95, 127. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Saraiva, S.A.; Catharino, R.R.; Garcia, J.S.; Gozzo, F.C.; Sanvido, G.B.; Santos, L.F.A.; Lo Turco, E.G.; Pontes, J.H.F.; Basso, A.C.; et al. Single embryo and oocyte lipid fingerprinting by mass spectrometry. J. Lipid Res. 2010, 51, 1218–1227. [Google Scholar] [CrossRef]
- Bertevello, P.S.; Teixeira-Gomes, A.P.; Seyer, A.; Carvalho, A.V.; Labas, V.; Blache, M.C.; Banliat, C.; Cordeiro, L.A.V.; Duranthon, V.; Papillier, P.; et al. Lipid identification and transcriptional analysis of controlling enzymes in bovine ovarian follicle. Int. J. Mol. Sci. 2018, 19, 3261. [Google Scholar] [CrossRef] [PubMed]
- Fraher, D.; Sanigorski, A.; Mellett, N.A.; Meikle, P.J.; Sinclair, A.J.; Gibert, Y. Zebrafish Embryonic Lipidomic Analysis Reveals that the Yolk Cell Is Metabolically Active in Processing Lipid. Cell Rep. 2016, 14, 1317–1329. [Google Scholar] [CrossRef]
- Haggarty, P.; Wood, M.; Ferguson, E.; Hoad, G.; Srikantharajah, A.; Milne, E.; Hamilton, M.; Bhattacharya, S. Fatty acid metabolism in human preimplantation embryos. Hum. Reprod. 2006, 21, 766–773. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Eberlin, L.S.; Hallett, J.E.; Cooks, R.G. Lipid fingerprinting of individual bovine blastocysts by desorption ionization electrospray mass spectrometry. Reprod. Fertil. Dev. 2012, 24, 132. [Google Scholar] [CrossRef]
- Sun, T.; Lee, B.; Kinchen, J.; Wang, E.T.; Gonzalez, T.L.; Chan, J.L.; Rotter, J.I.; Chen, Y.I.; Taylor, K.; Goodarzi, M.O.; et al. Differences in First-Trimester Maternal Metabolomic Profiles in Pregnancies Conceived From Fertility Treatments. J. Clin. Endocrinol. Metab. 2019, 104, 1005–1019. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hayasaka, T.; Setou, M.; Itoh, H.; Kanayama, N. Comparison of phospholipid molecular species between terminal and stem villi of human term placenta by imaging mass spectrometry. Placenta 2010, 31, 245–248. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Wang, M.; Lu, J.; Cai, Y.; Li, B. In vitro fertilization alters phospholipid profiles in mouse placenta. J. Assist. Reprod. Genet. 2019, 36, 557–567. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Guan, L.; Zhang, H.; Chen, P.; Gong, X.; Li, D.; Liang, X.; Huang, M.; Bi, H. Lipid Profiling of Peri-implantation Endometrium in Patients With Premature Progesterone Rise in the Late Follicular Phase. J. Clin. Endocrinol. Metab. 2019, 104, 5555–5565. [Google Scholar] [CrossRef] [PubMed]
- Matorras, R.; Martinez-Arranz, I.; Arretxe, E.; Iruarrizaga-Lejarreta, M.; Corral, B.; Ibanez-Perez, J.; Exposito, A.; Prieto, B.; Elortza, F.; Alonso, C. The lipidome of endometrial fluid differs between implantative and non-implantative IVF cycles. J. Assist. Reprod. Genet. 2020, 37, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Nabi, M.M.; Mamun, M.A.; Islam, A.; Hasan, M.M.; Waliullah, A.S.M.; Tamannaa, Z.; Sato, T.; Kahyo, T.; Setou, M. Mass spectrometry in the lipid study of cancer. Expert Rev. Proteom. 2021, 18, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Luberto, C.; Haley, J.D.; Del Poeta, M. Imaging with mass spectrometry, the next frontier in sphingolipid research? A discussion on where we stand and the possibilities ahead. Chem. Phys. Lipids 2019, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S.; Yamamoto, S.; Hayasaka, T.; Konishi, Y.; Yamaguchi-Okada, M.; Goto-Inoue, N.; Sugiura, Y.; Setou, M.; Namba, H. Imaging mass spectrometry revealed the production of lyso-phosphatidylcholine in the injured ischemic rat brain. Neuroscience 2010, 168, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Janfelt, C.; Wellner, N.; Leger, P.L.; Kokesch-Himmelreich, J.; Hansen, S.H.; Charriaut-Marlangue, C.; Hansen, H.S. Visualization by mass spectrometry of 2-dimensional changes in rat brain lipids, including N-acylphosphatidylethanolamines, during neonatal brain ischemia. FASEB J. 2012, 26, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Feng, Q.; Wang, Z. Mass Spectrometry Imaging as a New Method: To Reveal the Pathogenesis and the Mechanism of Traditional Medicine in Cerebral Ischemia. Front. Pharm. 2022, 13, 887050. [Google Scholar] [CrossRef]
- Luan, H.; Wang, X.; Cai, Z. Mass spectrometry-based metabolomics: Targeting the crosstalk between gut microbiota and brain in neurodegenerative disorders. Mass Spectrom. Rev. 2019, 38, 22–33. [Google Scholar] [CrossRef]
- Strnad, S.; PraZienkova, V.; Holubova, M.; Sykora, D.; Cvacka, J.; Maletinska, L.; Zelezna, B.; Kunes, J.; Vrkoslav, V. Mass spectrometry imaging of free-floating brain sections detects pathological lipid distribution in a mouse model of Alzheimer’s-like pathology. Analyst 2020, 145, 4595–4605. [Google Scholar] [CrossRef]
- Angelini, R.; Yutuc, E.; Wyatt, M.F.; Newton, J.; Yusuf, F.A.; Griffiths, L.; Cooze, B.J.; El Assad, D.; Frache, G.; Rao, W.; et al. Visualizing Cholesterol in the Brain by On-Tissue Derivatization and Quantitative Mass Spectrometry Imaging. Anal. Chem. 2021, 93, 4932–4943. [Google Scholar] [CrossRef] [PubMed]
- Mezger, S.T.P.; Mingels, A.M.A.; Bekers, O.; Cillero-Pastor, B.; Heeren, R.M.A. Trends in mass spectrometry imaging for cardiovascular diseases. Anal. Bioanal. Chem. 2019, 411, 3709–3720. [Google Scholar] [CrossRef] [PubMed]
- Lagarrigue, M.; Lavigne, R.; Guevel, B.; Com, E.; Chaurand, P.; Pineau, C. Matrix-assisted laser desorption/ionization imaging mass spectrometry: A promising technique for reproductive research. Biol. Reprod. 2012, 86, 74. [Google Scholar] [CrossRef] [PubMed]
- Chansela, P.; Goto-Inoue, N.; Zaima, N.; Hayasaka, T.; Sroyraya, M.; Kornthong, N.; Engsusophon, A.; Tamtin, M.; Chaisri, C.; Sobhon, P.; et al. Composition and localization of lipids in Penaeus merguiensis ovaries during the ovarian maturation cycle as revealed by imaging mass spectrometry. PLoS ONE 2012, 7, e33154. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, A.; Ramirez, C.E.; Michalkova, V.; Nouzova, M.; Noriega, F.G.; Francisco, F.L. Three Dimensional Secondary Ion Mass Spectrometry Imaging (3D-SIMS) of Aedes aegypti ovarian follicles. J. Anal. Spectrom. 2019, 34, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Uzbekova, S.; Elis, S.; Teixeira-Gomes, A.P.; Desmarchais, A.; Maillard, V.; Labas, V. MALDI Mass Spectrometry Imaging of Lipids and Gene Expression Reveals Differences in Fatty Acid Metabolism between Follicular Compartments in Porcine Ovaries. Biology 2015, 4, 216–236. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.S.; Lockyer, N.P.; Vaidyanathan, S.; Vickerman, J.C. TOF-SIMS 3D biomolecular imaging of Xenopus laevis oocytes using buckminsterfullerene (C60) primary ions. Anal. Chem. 2007, 79, 2199–2206. [Google Scholar] [CrossRef]
- Fu, T.; Knittelfelder, O.; Geffard, O.; Clement, Y.; Testet, E.; Elie, N.; Touboul, D.; Abbaci, K.; Shevchenko, A.; Lemoine, J.; et al. Shotgun lipidomics and mass spectrometry imaging unveil diversity and dynamics in Gammarus fossarum lipid composition. iScience 2021, 24, 102115. [Google Scholar] [CrossRef]
- Bodzon-Kulakowska, A.; Arena, R.; Mielczarek, P.; Hartman, K.; Kozoł, P.; Gibuła-Tarlowska, E.; Wrobel, T.P.; Gąsior, Ł.; Polański, Z.; Ptak, G.E.; et al. Mouse single oocyte imaging by MALDI-TOF MS for lipidomics. Cytotechnology 2020, 72, 455–468. [Google Scholar] [CrossRef]
- Pirro, V.; Guffey, S.C.; Sepúlveda, M.S.; Mahapatra, C.T.; Ferreira, C.R.; Jarmusch, A.K.; Cooks, R.G. Lipid dynamics in zebrafish embryonic development observed by DESI-MS imaging and nanoelectrospray-MS. Mol. Biosyst. 2016, 12, 2069–2079. [Google Scholar] [CrossRef]
- Dueñas, M.E.; Essner, J.J.; Lee, Y.J. 3D MALDI Mass Spectrometry Imaging of a Single Cell: Spatial Mapping of Lipids in the Embryonic Development of Zebrafish. Sci. Rep. 2017, 7, 14946. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Fletcher, J.S.; Thuret, R.; Henderson, A.; Papalopulu, N.; Vickerman, J.C.; Lockyer, N.P. Spatiotemporal lipid profiling during early embryo development of Xenopus laevis using dynamic ToF-SIMS imaging. J. Lipid Res. 2014, 55, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.; Ferreira, C.R.; Eberlin, L.S.; Jarmusch, A.K.; Pirro, V.; Rodrigues, A.C.B.; Favaron, P.O.; Miglino, M.A.; Cooks, R.G. Metabolites and Lipids Associated with Fetal Swine Anatomy via Desorption Electrospray Ionization-Mass Spectrometry Imaging. Sci. Rep. 2019, 9, 7247. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Patterson, N.H.; Laveaux Charbonneau, J.; Chaurand, P. Orthogonal organic and aqueous-based washes of tissue sections to enhance protein sensitivity by MALDI imaging mass spectrometry. J. Mass Spectrom. 2013, 48, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Garikapati, V.; Karnati, S.; Bhandari, D.R.; Baumgart-Vogt, E.; Spengler, B. High-resolution atmospheric-pressure MALDI mass spectrometry imaging workflow for lipidomic analysis of late fetal mouse lungs. Sci. Rep. 2019, 9, 3192. [Google Scholar] [CrossRef]
- Yamazaki, K.; Masaki, N.; Kohmura-Kobayashi, Y.; Yaguchi, C.; Hayasaka, T.; Itoh, H.; Setou, M.; Kanayama, N. Decrease in Sphingomyelin (d18:1/16:0) in Stem Villi and Phosphatidylcholine (16:0/20:4) in Terminal Villi of Human Term Placentas with Pathohistological Maternal Malperfusion. PLoS ONE 2015, 10, e0142609. [Google Scholar] [CrossRef]
- Bidne, K.L.; Rister, A.L.; McCain, A.R.; Hitt, B.D.; Dodds, E.D.; Wood, J.R. Maternal obesity alters placental lysophosphatidylcholines, lipid storage, and the expression of genes associated with lipid metabolismdouble dagger. Biol. Reprod. 2021, 104, 197–210. [Google Scholar] [CrossRef]
- Apparicio, M.; Ferreira, C.R.; Tata, A.; Santos, V.G.; Alves, A.E.; Mostachio, G.Q.; Pires-Butler, E.A.; Motheo, T.F.; Padilha, L.C.; Pilau, E.J.; et al. Chemical composition of lipids present in cat and dog oocyte by matrix-assisted desorption ionization mass spectrometry (MALDI- MS). Reprod. Domest. Anim. 2012, 47 (Suppl. 6), 113–117. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Pirro, V.; Eberlin, L.S.; Hallett, J.E.; Cooks, R.G. Developmental phases of individual mouse preimplantation embryos characterized by lipid signatures using desorption electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 2012, 404, 2915–2926. [Google Scholar] [CrossRef]
- Gonzalez-Serrano, A.F.; Pirro, V.; Ferreira, C.R.; Oliveri, P.; Eberlin, L.S.; Heinzmann, J.; Lucas-Hahn, A.; Niemann, H.; Cooks, R.G. Desorption electrospray ionization mass spectrometry reveals lipid metabolism of individual oocytes and embryos. PLoS ONE 2013, 8, e74981. [Google Scholar] [CrossRef]
- de Lima, C.B.; Ferreira, C.R.; Milazzotto, M.P.; Sobreira, T.J.P.; Vireque, A.A.; Cooks, R.G. Comprehensive lipid profiling of early stage oocytes and embryos by MRM profiling. J. Mass Spectrom. 2018, 53, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Zander-Fox, D.; Villarosa, L.; McPherson, N.O. Albumin used in human IVF contain different levels of lipids and modify embryo and fetal growth in a mouse model. J. Assist. Reprod. Genet. 2021, 38, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
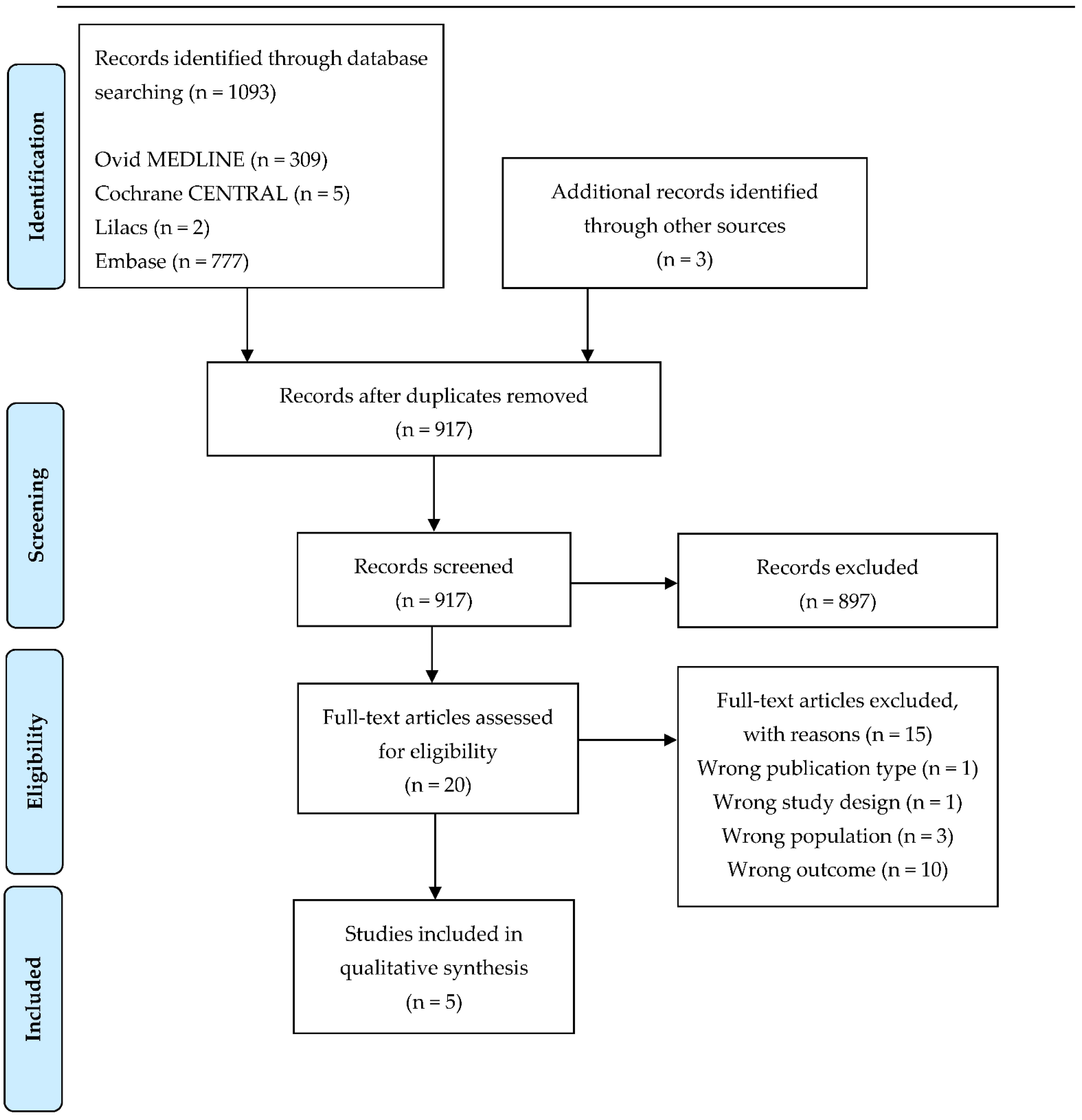
| Author, Year of Publication | Investigated Animals | Type of Fertilization | Age of Embryos | Type of Samples | Imaging Method |
|---|---|---|---|---|---|
| Burnum KE, 2009 [61] | CD-1 mouse | Normal | Day 4, 5, 6, 7, 8 | Implanting embryos with uterus | MALDI-TOF, MALDI-FTICR |
| Lanekoff I, 2015 [62] | CD-1 mouse | Normal | Day 6 | Implanting embryos with uterus | Nano-DESI MSI |
| Lanekoff I, 2016 [63] | p53f/f vs. p53d/d mouse | Normal | Day 8 | Implanting embryos with uterus | Nano-DESI MSI |
| Ferreira MS, 2014 [64] | Cattle | IVF | Day 2–3 | 2-cell, 8-cell embryo | MALDI-LTQ-XL |
| Belaz KRA, 2016 [65] | Cattle | IVF | Day 4, 7 | Uterus after induced ovulation | MALDI-MS |
| Time | Place | Lipid | Result |
|---|---|---|---|
| 2-cell stage (bovine) [64] | Blastomere | PE(16:0/18:1); Cer(18:1/22:0) | ↑ |
| Zona pellucida | PA(20:0/20:3); PE(24:0/20:0) | ↑ | |
| 8-cell stage (bovine) [64] | Blastomere | PE(16:0/18:1); Cer(18:1/22:0) | ↑ |
| Zona pellucida | PA(20:0/20:3); PE(24:0/20:0) | ↑ | |
| Receptive uterus (bovine), day 7 [65] | Myometrium | SM34:1 | ↑ |
| Luminal epithel | PC35:2 | ↑ | |
| Uterus region | PC38:7; PC38:5; PC38:4 | ↑ | |
| Whole section | SM34:2, PC31:0, PC32:0, PC47:0, PC40:6 | ↑ | |
| Receptive uterus (mouse), day 4 [61] | Luminal epithelial cells | SM16:0 (-/16:0), PC34:0 (16:0/18:0), PC34:1 (16:0/18:1), PC36:1 (18:0/18:1), PC34:2 (16:0/18:2), PC36:2 (18:0/18:2), PC36:4 (16:0/20:4), PC38:4 (18:0/20:4), PI34:0 (16:0/18:0), PI40:6 (18:0/22:6) | ↑ |
| PC32:0 (16:0/16:0), PC40:6 (18:0/22:6), PE34:1 (16:0/18:1) | ↓ | ||
| Uterus (mouse), day 5 [61] | Uterine stroma cells | SM16:0 (-/16:0), PC34:0 (16:0/18:0), PC34:1 (16:0/18:1), PC36:1 (18:0/18:1), PC34:2 (16:0/18:2), PC36:2 (18:0/18:2), PC36:4 (16:0/20:4), PC38:4 (18:0/20:4), PC40:6 (18:0/22:6), PI34:2 (16:0/18:2), PI36:2 (18:0/18:2), PI40:6 (18:0/22:6) | ↑ |
| PC32:0 (16:0/16:0) | ↓ | ||
| Uterus (mouse), day 6 [61] | PDZ | SM16:0 (-/16:0), PC34:1 (16:0/18:1), PC36:1 (18:0/18:1), PC34:2 (16:0/18:2), PC36:2 (18:0/18:2), PC36:4 (16:0/20:4), PC38:4 (18:0/20:4), PC40:6 (18:0/22:6), | ↑ |
| PC32:0 (16:0/16:0) | ↓ | ||
| M pole | PC34:1 (16:0/18:1), PE34:1 (16:0/18:1), PI38:5 (18:1/20:4) | ↑ | |
| AM pole | PC36:2 (18:0/18:2), PI34:2 (16:0/18:2) | ↑ | |
| Uterus (mouse), day 6 [62] | PDZ | LPC18:0 | ↓ |
| PDZ | FA18:2 | ↑ | |
| AM pole | PC36:2 | ↑ | |
| Uterus (mouse), day 7 [61] | PDZ | PC32:0(16:0/16:0) | ↓ |
| M pole | PC34:0 (16:0/18:0), PC34:1 (16:0/18:1), PE34:1 (16:0/18:1), PI38:5 (18:1/20:4) | ↑ | |
| AM pole | PC34:2 (16:0/18:2), PC36:2 (18:0/18:2), PC36:4 (16:0/20:4), PC38:4 (18:0/20:4), PC40:6 (18:0/22:6), PI34:2 (16:0/18:2), PI36:2 (18:0/18:2), PS36:2 (18:0/18:2), PI40:6 (18:0/22:6) | ↑ | |
| Uterus (mouse), day 8 [61] | M pole | PC34:1(16:0/18:1), PE34:1 (16:0/18:1), PG 34:1 (16:0/18:1), PS36:1 (18:0/18:1) | ↑ |
| AM pole | PC34:2 (16:0/18:2), PC36:2 (18:0/18:2), PC36:4 (16:0/20:4), PC38:4 (18:0/20:4), PC40:6 (18:0/22:6), PI34:2 (16:0/18:2), PI36:2 (18:0/18:2), PS36:2 (18:0/18:2), PI40:6 (18:0/22:6) | ↑ | |
| Uterus (mouse), day 8 [63] | M pole | PC34:1, PC36:1 (18:0/18:1), PC 36:3 (18:1/18:2) | ↑ |
| AM pole | PC36:2 (18:0/18:2), PC38:4, PC40:6, DG34:2, DG36:4, DG36:3, DG36:2, DG38:4 | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gitta, S.; Márk, L.; Szentpéteri, J.L.; Szabó, É. Lipid Changes in the Peri-Implantation Period with Mass Spectrometry Imaging: A Systematic Review. Life 2023, 13, 169. https://doi.org/10.3390/life13010169
Gitta S, Márk L, Szentpéteri JL, Szabó É. Lipid Changes in the Peri-Implantation Period with Mass Spectrometry Imaging: A Systematic Review. Life. 2023; 13(1):169. https://doi.org/10.3390/life13010169
Chicago/Turabian StyleGitta, Stefánia, László Márk, József L. Szentpéteri, and Éva Szabó. 2023. "Lipid Changes in the Peri-Implantation Period with Mass Spectrometry Imaging: A Systematic Review" Life 13, no. 1: 169. https://doi.org/10.3390/life13010169
APA StyleGitta, S., Márk, L., Szentpéteri, J. L., & Szabó, É. (2023). Lipid Changes in the Peri-Implantation Period with Mass Spectrometry Imaging: A Systematic Review. Life, 13(1), 169. https://doi.org/10.3390/life13010169









