Multimodality Imaging in Sarcomeric Hypertrophic Cardiomyopathy: Get It Right…on Time
Abstract
:1. Introduction
2. An Overview of the Main Imaging Techniques in Hypertrophic Cardiomyopathy
2.1. Echocardiography
2.1.1. Definition and Patterns of Hypertrophy
2.1.2. Systolic Function
2.1.3. Diastolic Function
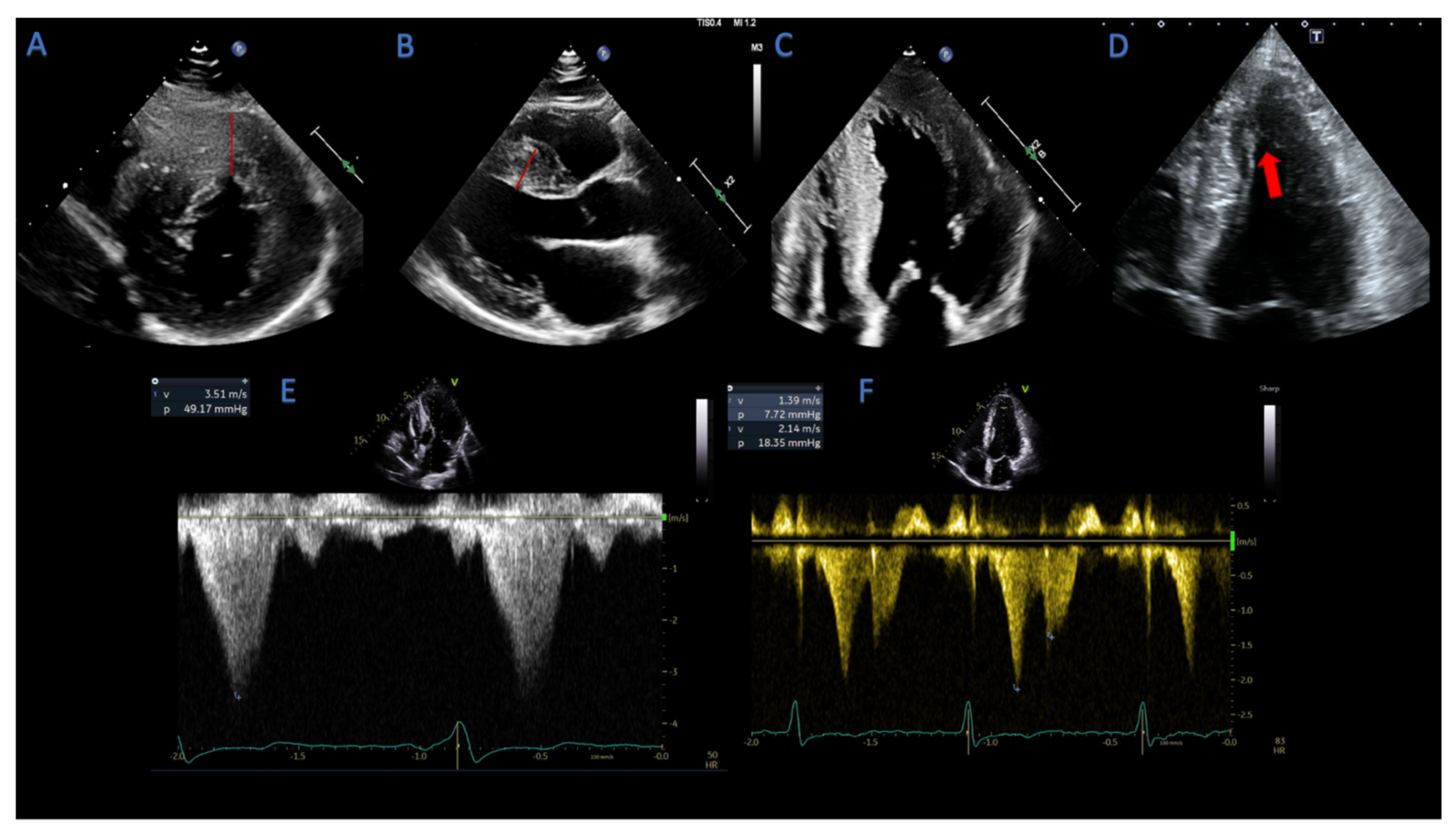
2.1.4. Assessment of LVOT- and Mid-Cavity Obstruction
2.1.5. Mitral Valve Abnormalities in HCM Patients
2.2. Stress Echocardiography
2.3. Cardiovascular Magnetic Resonance
3. A Practical Approach to Multimodality Imaging in HCM
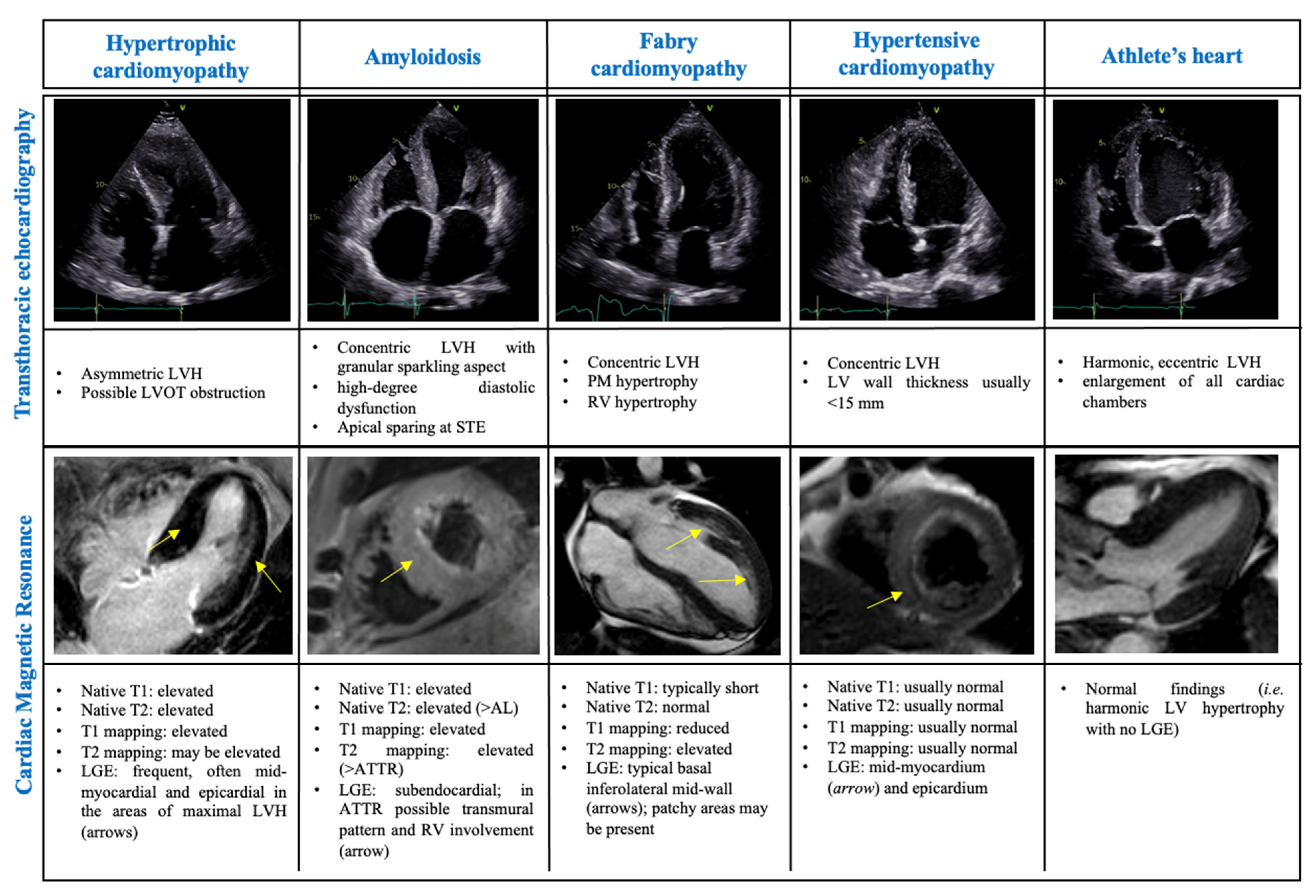
3.1. Differential Diagnosis of Phenocopies
3.1.1. The Athlete’s Heart
3.1.2. Amyloidosis
3.1.3. Hypertensive Cardiomyopathy
3.1.4. Fabry Cardiomyopathy
3.2. The Appropriate Exam at the Right Time: Indications for Imaging Referral
3.2.1. Imaging in “Non-Hypertrophic” Phase and “Classic Phenotype” HCM
3.2.2. Imaging in HCM “Adverse Remodeling and Overt Dysfunction”
3.3. Follow-Up in the Advanced Stages: A Role for Instrumental Examinations?
4. Future Perspectives: The Role of Artificial Intelligence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ciabatti, M.; Fumagalli, C.; Beltrami, M.; Vignini, E.; Martinese, L.; Tomberli, A.; Zampieri, M.; Bertini, A.; Carrassa, G.; Marchi, A.; et al. Prevalence, causes and predictors of cardiovascular hospitalization in patients with hypertrophic cardiomyopathy. Int. J. Cardiol. 2020, 318, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [CrossRef]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary. J. Am. Coll. Cardiol. 2020, 76, 3022–3055. [Google Scholar] [CrossRef] [PubMed]
- Cardim, N.; Galderisi, M.; Edvardsen, T.; Plein, S.; Popescu, B.A.; D’Andrea, A.; Bruder, O.; Cosyns, B.; Davin, L.; Donal, E.; et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: An expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur. Hear. J. Cardiovasc. Imaging 2015, 16, 280. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Phelan, D.; Abraham, T.; Armour, A.; Desai, M.Y.; Dragulescu, A.; Gilliland, Y.; Lester, S.J.; Maldonado, Y.; Mohiddin, S.; et al. Recommendations for Multimodality Cardiovascular Imaging of Patients with Hypertrophic Cardiomyopathy: An Update from the American Society of Echocardiography, in Collaboration with the American Society of Nuclear Cardiology, the Society for Cardiovascular Magnetic Resonance, and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2022, 35, 533–569. [Google Scholar] [CrossRef]
- Peyrou, J.; Réant, P.; Reynaud, A.; Cornolle, C.; Dijos, M.; Rooryck-Thambo, C.; Landelle, M.; Montaudon, M.; Laurent, F.; Roudaut, R.; et al. Morphological and functional abnormalities pattern in hypertrophy-free HCM mutation carriers detected with echocardiography. Int. J. Cardiovasc. Imaging 2016, 32, 1379–1389. [Google Scholar] [CrossRef]
- Stokke, T.M.; Hasselberg, N.E.; Smedsrud, M.K.; Sarvari, S.I.; Haugaa, K.H.; Smiseth, O.A.; Edvardsen, T.; Remme, E.W. Geometry as a Confounder When Assessing Ventricular Systolic Function. J. Am. Coll. Cardiol. 2017, 70, 942–954. [Google Scholar] [CrossRef]
- Thaman, R. Prevalence and clinical significance of systolic impairment in hypertrophic cardiomyopathy. Heart 2005, 91, 920–925. [Google Scholar] [CrossRef] [Green Version]
- Serri, K.; Reant, P.; Lafitte, M.; Berhouet, M.; Le Bouffos, V.; Roudaut, R.; Lafitte, S. Global and regional myocardial function quantification by two-dimensional strain: Application in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2006, 47, 1175–1181. [Google Scholar] [CrossRef] [Green Version]
- Wabich, E.; Dorniak, K.; Zienciuk-Krajka, A.; Nowak, R.; Raczak, G.; Daniłowicz-Szymanowicz, L. Segmental longitudinal strain as the most accurate predictor of the patchy pattern late gadolinium enhancement in hypertrophic cardiomyopathy. J. Cardiol. 2021, 77, 475–481. [Google Scholar] [CrossRef]
- Carasso, S.; Yang, H.; Woo, A.; Vannan, M.A.; Jamorski, M.; Wigle, E.D.; Rakowski, H. Systolic Myocardial Mechanics in Hypertrophic Cardiomyopathy: Novel Concepts and Implications for Clinical Status. J. Am. Soc. Echocardiogr. 2008, 21, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Voilliot, D.; Huttin, O.; Hammache, N.; Filippetti, L.; Vaugrenard, T.; Aliot, E.; Sadoul, N.; Juillière, Y.; Selton-Suty, C. Impact of Global and Segmental Hypertrophy on Two-Dimensional Strain Derived from Three-Dimensional Echocardiography in Hypertrophic Cardiomyopathy: Comparison with Healthy Subjects. J. Am. Soc. Echocardiogr. 2015, 28, 1093–1102. [Google Scholar] [CrossRef]
- Sun, J.P.; Xu, T.Y.; Ni, X.D.; Yang, X.S.; Hu, J.L.; Wang, S.C.; Li, Y.; Bahler, R.C.; Wang, J.G. Echocardiographic strain in hypertrophic cardiomyopathy and hypertensive left ventricular hypertrophy. Echocardiography 2019, 36, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Urbano Moral, J.A.; Arias Godinez, J.A.; Maron, M.S.; Malik, R.; Eagan, J.E.; Patel, A.R.; Pandian, N.G. Left ventricular twist mechanics in hypertrophic cardiomyopathy assessed by three-dimensional speckle tracking echocardiography. Am. J. Cardiol. 2011, 108, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Debonnaire, P.; Thijssen, J.; Leong, D.P.; Joyce, E.; Katsanos, S.; Hoogslag, G.E.; Schalij, M.J.; Atsma, D.E.; Bax, J.J.; Delgado, V.; et al. Global longitudinal strain and left atrial volume index improve prediction of appropriate implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy patients. Int. J. Cardiovasc. Imaging 2014, 30, 549–558. [Google Scholar] [CrossRef]
- Hartlage, G.R.; Kim, J.H.; Strickland, P.T.; Cheng, A.C.; Ghasemzadeh, N.; Pernetz, M.A.; Clements, S.D.; Williams, B.R. The prognostic value of standardized reference values for speckle-tracking global longitudinal strain in hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2015, 31, 557–565. [Google Scholar] [CrossRef]
- Wabich, E.; Zienciuk-Krajka, A.; Nowak, R.; Raczak, A.; Daniłowicz-Szymanowicz, L. Comprehensive echocardiography of left atrium and left ventricle using modern techniques helps in better revealing atrial fibrillation in patients with hypertrophic cardiomyopathy. Diagnostics 2021, 11, 1288. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Bayrak, F.; Kahveci, G.; Mutlu, B.; Sonmez, K.; Degertekin, M. Tissue Doppler imaging to predict clinical course of patients with hypertrophic cardiomyopathy. Eur. J. Echocardiogr. 2008, 9, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Yan, Z.N.; Rui, Y.F.; Fan, L.; Liu, C.; Li, J. Left ventricular short-axis systolic function changes in patients with hypertrophic cardiomyopathy detected by two-dimensional speckle tracking imaging. BMC Cardiovasc. Disord. 2018, 18, 13. [Google Scholar] [CrossRef]
- Garceau, P.; Carasso, S.; Woo, A.; Overgaard, C.; Schwartz, L.; Rakowski, H. Evaluation of left ventricular relaxation and filling pressures in obstructive hypertrophic cardiomyopathy: Comparison between invasive hemodynamics and two-dimensional speckle tracking. Echocardiography 2012, 29, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Al Wazzan, A.; Galli, E.; Lacout, M.; Paven, E.; L’official, G.; Schnell, F.; Oger, E.; Donal, E. Could echocardiographic left atrial characterization have additive value for detecting risks of atrial arrhythmias and stroke in patients with hypertrophic cardiomyopathy? Eur. Heart J. Cardiovasc. Imaging 2022, 43, ehac544.027. [Google Scholar] [CrossRef] [PubMed]
- Domsik, P.; Kalapos, A.; Chadaide, S.; Sepp, R.; Hausinger, P.; Forster, T.; Nemes, A. Three-dimensional speckle tracking echocardiography allows detailed evaluation of left atrial function in hypertrophic cardiomyopathy-insights from the MAGYAR-Path Study. Echocardiography 2014, 31, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Iio, C.; Inoue, K.; Nishimura, K.; Fujii, A.; Nagai, T.; Suzuki, J.; Okura, T.; Higaki, J.; Ogimoto, A. Characteristics of Left Atrial Deformation Parameters and Their Prognostic Impact in Patients with Pathological Left Ventricular Hypertrophy: Analysis by Speckle Tracking Echocardiography. Echocardiography 2015, 32, 1821–1830. [Google Scholar] [CrossRef]
- Guttmann, O.P.; Pavlou, M.; O’Mahony, C.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; Garcia-Pavia, P.; et al. Prediction of thrombo-embolic risk in patients with hypertrophic cardiomyopathy (HCM Risk-CVA). Eur. J. Heart Fail. 2015, 17, 837–845. [Google Scholar] [CrossRef] [Green Version]
- Aly, M.F.A.; Brouwer, W.P.; Kleijn, S.A.; Van Rossum, A.C.; Kamp, O. Three-dimensional speckle tracking echocardiography for the preclinical diagnosis of hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2014, 30, 523–533. [Google Scholar] [CrossRef]
- de la Rosa, A.; Shah, M.; Shiota, T.; Siegel, R.; Rader, F. Comparing echocardiographic characteristics in genotype positive-phenotype positive hypertrophic cardiomyopathy and hypertensive left ventricular hypertrophy. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 340–348. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Betocchi, S.; Casey, S.A.; Lesser, J.R.; Losi, M.A.; Cecchi, F.; Maron, B.J. Effect of Left Ventricular Outflow Tract Obstruction on Clinical Outcome in Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2003, 348, 295–303. [Google Scholar] [CrossRef]
- Boughner, D.R.; Schuld, R.L.; Persaud, J.A. Hypertrophic obstructive cardiomyopathy. Assessment by echocardiographic and Doppler ultrasound techniques. Heart 1975, 37, 917–923. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, B.J.; Haas, T.S.; Garberich, R.F.; Wang, W.; Link, M.S.; Maron, M.S. Hypertrophic Cardiomyopathy with Left Ventricular Apical Aneurysm. J. Am. Coll. Cardiol. 2017, 69, 761–773. [Google Scholar] [CrossRef]
- Jiang, L.; Levine, R.A.; King, M.E.; Weyman, A.E. An integrated mechanism for systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy based on echocardiographic observations. Am. Heart J. 1987, 113, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.L.; Barboza, J.S.; Lever, H.M. Diversity of Mitral Valve Abnormalities in Obstructive Hypertrophic Cardiomyopathy. Prog. Cardiovasc. Dis. 2012, 54, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.K.; Havndrup, O.; Pecini, R.; Dalsgaard, M.; Hassager, C.; Helqvist, S.; Kelbaek, H.; Jorgensen, E.; Kober, L.; Bundgaard, H. Comparison of Valsalva manoeuvre and exercise in echocardiographic evaluation of left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Eur. J. Echocardiogr. 2010, 11, 763–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maron, M.S.; Rowin, E.J.; Olivotto, I.; Casey, S.A.; Arretini, A.; Tomberli, B.; Garberich, R.F.; Link, M.S.; Chan, R.H.M.; Lesser, J.R.; et al. Contemporary Natural History and Management of Nonobstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, Q.; Olivotto, I.; Gardini, C.; Mori, F.; Peteiro, J.; Monserrat, L.; Fernandez, X.; Cortigiani, L.; Rigo, F.; Lopes, L.R.; et al. Prognostic role of stress echocardiography in hypertrophic cardiomyopathy: The International Stress Echo Registry. Int. J. Cardiol. 2016, 219, 331–338. [Google Scholar] [CrossRef]
- Nistri, S.; Olivotto, I.; Maron, M.S.; Grifoni, C.; Baldini, K.; Baldi, M.; Sgalambro, A.; Cecchi, F.; Maron, B.J. Timing and Significance of Exercise-Induced Left Ventricular Outflow Tract Pressure Gradients in Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2010, 106, 1301–1306. [Google Scholar] [CrossRef]
- Ha, J.-W.; Ahn, J.-A.; Kim, J.-M.; Choi, E.-Y.; Kang, S.-M.; Rim, S.-J.; Jang, Y.; Shim, W.-H.; Cho, S.-Y.; Oh, J.K.; et al. Abnormal Longitudinal Myocardial Functional Reserve Assessed by Exercise Tissue Doppler Echocardiography in Patients with Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2006, 19, 1314–1319. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Ha, J.-W.; Rim, S.-J.; Kim, S.-A.; Yoon, S.-J.; Shim, C.-Y.; Kim, J.-M.; Jang, Y.; Chung, N.; Cho, S.-Y. Incremental Value of Left Ventricular Diastolic Function Reserve Index for Predicting Exercise Capacity in Patients with Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2008, 21, 487–492. [Google Scholar] [CrossRef]
- Coppini, R.; Beltrami, M.; Doste, R.; Bueno-Orovio, A.; Ferrantini, C.; Vitale, G.; Pioner, J.M.; Santini, L.; Argirò, A.; Berteotti, M.; et al. Paradoxical prolongation of QT interval during exercise in patients with hypertrophic cardiomyopathy: Cellular mechanisms and implications for diastolic function. Eur. Heart J. Open 2022, 2, oeac034. [Google Scholar] [CrossRef]
- Pozios, I.; Pinheiro, A.; Corona-Villalobos, C.; Sorensen, L.L.; Dardari, Z.; Liu, H.; Cresswell, K.; Phillip, S.; Bluemke, D.A.; Zimmerman, S.L.; et al. Rest and Stress Longitudinal Systolic Left Ventricular Mechanics in Hypertrophic Cardiomyopathy: Implications for Prognostication. J. Am. Soc. Echocardiogr. 2018, 31, 578–586. [Google Scholar] [CrossRef]
- Badran, H.M.; Faheem, N.; Ibrahim, W.A.; Elnoamany, M.F.; Elsedi, M.; Yacoub, M. Systolic Function Reserve Using Two-Dimensional Strain Imaging in Hypertrophic Cardiomyopathy: Comparison with Essential Hypertension. J. Am. Soc. Echocardiogr. 2013, 26, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Soullier, C.; Obert, P.; Doucende, G.; Nottin, S.; Cade, S.; Perez-Martin, A.; Messner-Pellenc, P.; Schuster, I. Exercise Response in Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2012, 5, 324–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivotto, I.; Maron, B.J.; Montereggi, A.; Mazzuoli, F.; Dolara, A.; Cecchi, F. Prognostic value of systemic blood pressure response during exercise in a community-based patient population with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 1999, 33, 2044–2051. [Google Scholar] [CrossRef] [Green Version]
- Okeie, K.; Shimizu, M.; Yoshio, H.; Ino, H.; Yamaguchi, M.; Matsuyama, T.; Yasuda, T.; Taki, J.; Mabuchi, H. Left ventricular systolic dysfunction during exercise and dobutamine stress in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2000, 36, 856–863. [Google Scholar] [CrossRef] [Green Version]
- Sorajja, P.; Ommen, S.R.; Nishimura, R.A.; Gersh, B.J.; Berger, P.B.; Tajik, A.J. Adverse Prognosis of Patients with Hypertrophic Cardiomyopathy Who Have Epicardial Coronary Artery Disease. Circulation 2003, 108, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Peteiro, J.; Bouzas-Mosquera, A.; Fernandez, X.; Monserrat, L.; Pazos, P.; Estevez-Loureiro, R.; Castro-Beiras, A. Prognostic Value of Exercise Echocardiography in Patients with Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2012, 25, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Hindieh, W.; Weissler-Snir, A.; Hammer, H.; Adler, A.; Rakowski, H.; Chan, R.H. Discrepant Measurements of Maximal Left Ventricular Wall Thickness Between Cardiac Magnetic Resonance Imaging and Echocardiography in Patients with Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 10, e006309. [Google Scholar] [CrossRef] [Green Version]
- Rickers, C.; Wilke, N.M.; Jerosch-Herold, M.; Casey, S.A.; Panse, P.; Panse, N.; Weil, J.; Zenovich, A.G.; Maron, B.J. Utility of Cardiac Magnetic Resonance Imaging in the Diagnosis of Hypertrophic Cardiomyopathy. Circulation 2005, 112, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Maron, M.S.; Rowin, E.J.; Lin, D.; Appelbaum, E.; Chan, R.H.; Gibson, C.M.; Lesser, J.R.; Lindberg, J.; Haas, T.S.; Udelson, J.E.; et al. Prevalence and Clinical Profile of Myocardial Crypts in Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2012, 5, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Maron, M.S.; Olivotto, I.; Harrigan, C.; Appelbaum, E.; Gibson, C.M.; Lesser, J.R.; Haas, T.S.; Udelson, J.E.; Manning, W.J.; Maron, B.J. Mitral Valve Abnormalities Identified by Cardiovascular Magnetic Resonance Represent a Primary Phenotypic Expression of Hypertrophic Cardiomyopathy. Circulation 2011, 124, 40–47. [Google Scholar] [CrossRef]
- Gruner, C.; Chan, R.H.; Crean, A.; Rakowski, H.; Rowin, E.J.; Care, M.; Deva, D.; Williams, L.; Appelbaum, E.; Gibson, C.M.; et al. Significance of left ventricular apical–basal muscle bundle identified by cardiovascular magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Eur. Heart J. 2014, 35, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Appelbaum, E.; Harrigan, C.J.; Buros, J.; Gibson, C.M.; Hanna, C.; Lesser, J.R.; Udelson, J.E.; Manning, W.J.; Maron, B.J. Clinical Profile and Significance of Delayed Enhancement in Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2008, 1, 184–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fluechter, S.; Kuschyk, J.; Wolpert, C.; Doesch, C.; Veltmann, C.; Haghi, D.; Schoenberg, S.O.; Sueselbeck, T.; Germans, T.; Streitner, F.; et al. Extent of late gadolinium enhancement detected by cardiovascular magnetic resonance correlates with the inducibility of ventricular tachyarrhythmia in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2010, 12, 30. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.C.; McKenna, W.J.; McCrohon, J.A.; Elliott, P.M.; Smith, G.C.; Pennell, D.J. Toward clinical risk assessment inhypertrophic cardiomyopathy withgadolinium cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2003, 41, 1561–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hanlon, R.; Grasso, A.; Roughton, M.; Moon, J.C.; Clark, S.; Wage, R.; Webb, J.; Kulkarni, M.; Dawson, D.; Sulaibeekh, L.; et al. Prognostic Significance of Myocardial Fibrosis in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 867–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.Y.; López, B.; Coelho-Filho, O.R.; Lakdawala, N.K.; Cirino, A.L.; Jarolim, P.; Kwong, R.; González, A.; Colan, S.D.; Seidman, J.G.; et al. Myocardial Fibrosis as an Early Manifestation of Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2010, 363, 552–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florian, A.; Masci, P.G.; De Buck, S.; Aquaro, G.D.; Claus, P.; Todiere, G.; Van Cleemput, J.; Lombardi, M.; Bogaert, J. Geometric Assessment of Asymmetric Septal Hypertrophic Cardiomyopathy by CMR. JACC Cardiovasc. Imaging 2012, 5, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Vöhringer, M.; Mahrholdt, H.; Yilmaz, A.; Sechtem, U. Significance of Late Gadolinium Enhancement in Cardiovascular Magnetic Resonance Imaging (CMR). Herz Kardiovaskuläre Erkrank. 2007, 32, 129–137. [Google Scholar] [CrossRef]
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 Mapping. JACC Cardiovasc. Imaging 2016, 9, 67–81. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Ran, L.; Zhao, P.; Tang, D.; Han, R.; Ai, T.; Xia, L.; Tao, Q. MRI native T1 and T2 mapping of myocardial segments in hypertrophic cardiomyopathy: Tissue remodeling manifested prior to structure changes. Br. J. Radiol. 2019, 92, 20190634. [Google Scholar] [CrossRef]
- Kato, S.; Nakamori, S.; Bellm, S.; Jang, J.; Basha, T.; Maron, M.; Manning, W.J.; Nezafat, R. Myocardial Native T1 Time in Patients With Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2016, 118, 1057–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.A.; Naish, J.H.; Bishop, P.; Coutts, G.; Clark, D.; Zhao, S.; Ray, S.G.; Yonan, N.; Williams, S.G.; Flett, A.S.; et al. Comprehensive Validation of Cardiovascular Magnetic Resonance Techniques for the Assessment of Myocardial Extracellular Volume. Circ. Cardiovasc. Imaging 2013, 6, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2017, 18, 89. [Google Scholar] [CrossRef] [Green Version]
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’Andrea, A.; D’Ascenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: Recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s heart. Eur. Heart J. 2018, 39, 1949–1969. [Google Scholar] [CrossRef]
- Urhausen, A.; Kindermann, W. Echocardiographic Findings in Strength- and Endurance-Trained Athletes. Sport. Med. Int. J. Appl. Med. Sci. Sport Exerc. 1992, 13, 270–284. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, M.S.; Maron, B.J. Assessment of Left Ventricular Hypertrophy in a Trained Athlete: Differential Diagnosis of Physiologic Athlete’s Heart From Pathologic Hypertrophy. Prog. Cardiovasc. Dis. 2012, 54, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Niederseer, D.; Rossi, V.A.; Kissel, C.; Scherr, J.; Caselli, S.; Tanner, F.C.; Bohm, P.; Schmied, C. Role of echocardiography in screening and evaluation of athletes. Heart 2020, 107, 270–276. [Google Scholar] [CrossRef]
- Galderisi, M.; Cardim, N.; D’Andrea, A.; Bruder, O.; Cosyns, B.; Davin, L.; Donal, E.; Edvardsen, T.; Freitas, A.; Habib, G.; et al. Themulti-modality cardiac imaging approach to the Athletés heart: An expert consensus of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 353. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Pelliccia, A.; Alvino, F.; Solari, M.; Loffreno, A.; Cameli, M.; Focardi, M.; Bonifazi, M.; Mondillo, S. Effects of training on LV strain in competitive athletes. Heart 2015, 101, 1834–1839. [Google Scholar] [CrossRef]
- Bohm, P.; Schneider, G.; Linneweber, L.; Rentzsch, A.; Krämer, N.; Abdul-Khaliq, H.; Kindermann, W.; Meyer, T.; Scharhag, J. Right and left ventricular function and mass in male elite master athletes: A controlled contrast-enhanced cardiovascular magnetic resonance study. Circulation 2016, 133, 1927–1935. [Google Scholar] [CrossRef]
- Merlini, G.; Bellotti, V. Molecular Mechanisms of Amyloidosis. N. Engl. J. Med. 2003, 349, 583–596. [Google Scholar] [CrossRef] [Green Version]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk, R.H.; Quarta, C.C.; Dorbala, S. How to image cardiac amyloidosis. Circ. Cardiovasc. Imaging 2014, 7, 552–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, I.S.; Glockner, J.F.; Feng, D.L.; Araoz, P.A.; Martinez, M.W.; Edwards, W.D.; Gertz, M.A.; Dispenzieri, A.; Oh, J.K.; Bellavia, D.; et al. Role of Cardiac Magnetic Resonance Imaging in the Detection of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2010, 3, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Dungu, J.N.; Valencia, O.; Pinney, J.H.; Gibbs, S.D.J.; Rowczenio, D.; Gilbertson, J.A.; Lachmann, H.J.; Wechalekar, A.; Gillmore, J.D.; Whelan, C.J.; et al. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc. Imaging 2014, 7, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2021, 23, 512–526. [Google Scholar] [CrossRef]
- Devereux, R.B.; Pickering, T.G.; Alderman, M.H.; Chien, S.; Borer, J.S.; Laragh, J.H. Left ventricular hypertrophy in hypertension: Prevalence and relationship to pathophysiologic variables. Hypertension 1987, 9, II53–II60. [Google Scholar] [CrossRef] [Green Version]
- Olsen, M.H.; Wachtell, K.; Hermann, K.L.; Frandsen, E.; Dige-Petersen, H.; Rokkedal, J.; Devereux, R.B.; Ibsen, H. Is cardiovascular remodeling in patients with essential hypertension related to more than high blood pressure? A LIFE substudy. Am. Heart J. 2002, 144, 530–537. [Google Scholar] [CrossRef]
- Maron, M.S. Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2012, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.S.; Noda, A.; Izawa, H.; Yamada, A.; Obata, K.; Nagata, K.; Iwase, M.; Murohara, T.; Yokota, M. Discrimination of nonobstructive hypertrophic cardiomyopathy from hypertensive left ventricular hypertrophy on the basis of strain rate imaging by tissue Doppler ultrasonography. Circulation 2004, 110, 3808–3814. [Google Scholar] [CrossRef]
- Nagakura, T.; Takeuchi, M.; Yoshitani, H.; Nakai, H.; Nishikage, T.; Kokumai, M.; Otani, S.; Yoshiyama, M.; Yoshikawa, J. Hypertrophic cardiomyopathy is associated with more severe left ventricular dyssynchrony than is hypertensive left ventricular hypertrophy. Echocardiography 2007, 24, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Faber, L.; Heemann, A.; Sürig, M.; Michalowski, Z.; Gleichmann, U.; Klempt, H.W. Outflow acceleration assessed by continuous-wave Doppler echocardiography in left ventricular hypertrophy: An analysis of 103 consecutive cases. Cardiology 1998, 90, 220–226. [Google Scholar] [CrossRef]
- Rubinshtein, R.; Glockner, J.F.; Ommen, S.R.; Araoz, P.A.; Ackerman, M.J.; Sorajja, P.; Bos, J.M.; Tajik, A.J.; Valeti, U.S.; Nishimura, R.A.; et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ. Heart Fail. 2010, 3, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Hinojar, R.; Varma, N.; Child, N.; Goodman, B.; Jabbour, A.; Yu, C.Y.; Gebker, R.; Doltra, A.; Kelle, S.; Khan, S.; et al. T1 Mapping in Discrimination of Hypertrophic Phenotypes: Hypertensive Heart Disease and Hypertrophic Cardiomyopathy: Findings from the International T1 Multicenter Cardiovascular Magnetic Resonance Study. Circ. Cardiovasc. Imaging 2015, 8, e003285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieroni, M.; Moon, J.C.; Arbustini, E.; Barriales-Villa, R.; Camporeale, A.; Vujkovac, A.C.; Elliott, P.M.; Hagege, A.; Kuusisto, J.; Linhart, A.; et al. Cardiac Involvement in Fabry Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 922–936. [Google Scholar] [CrossRef]
- Perry, R.; Shah, R.; Saiedi, M.; Patil, S.; Ganesan, A.; Linhart, A.; Selvanayagam, J.B. The Role of Cardiac Imaging in the Diagnosis and Management of Anderson-Fabry Disease. JACC Cardiovasc. Imaging 2019, 12, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.C.; Sachdev, B.; Elkington, A.G.; McKenna, W.J.; Mehta, A.; Pennell, D.J.; Leed, P.J.; Elliott, P.M. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease: Evidence for a disease specific abnormality of the myocardial interstitium. Eur. Heart J. 2003, 24, 2151–2155. [Google Scholar] [CrossRef] [Green Version]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2 and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.B.; Chow, K.; Khan, A.; Chan, A.; Shanks, M.; Paterson, I.; Oudit, G.Y. T1 mapping with cardiovascular MRI is highly sensitive for fabry disease independent of hypertrophy and sex. Circ. Cardiovasc. Imaging 2013, 6, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Meucci, M.C.; Lillo, R.; Lombardo, A.; Lanza, G.A.; Bootsma, M.; Butcher, S.C.; Massetti, M.; Manna, R.; Bax, J.J.; Crea, F.; et al. Comparative analysis of right ventricular strain in Fabry cardiomyopathy and sarcomeric hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2022, jeac151. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, M.S.; Adler, A.; Albano, A.J.; Varnava, A.M.; Spears, D.; Marsy, D.; Heitner, S.B.; Cohen, E.; Leong, K.M.W.; et al. Importance of newer cardiac magnetic resonance–based risk markers for sudden death prevention in hypertrophic cardiomyopathy: An international multicenter study. Heart Rhythm 2022, 19, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Thiene, G.; Corrado, D.; Buja, G.; Melacini, P.; Nava, A. Hypertrophic cardiomyopathy and sudden death in the young: Pathologic evidence of myocardial ischemia. Hum. Pathol. 2000, 31, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Bravo, P.E. Is there a role for cardiac positron emission tomography in hypertrophic cardiomyopathy? J. Nucl. Cardiol. 2019, 26, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Cecchi, F.; Poggesi, C.; Yacoub, M.H. Patterns of Disease Progression in Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2012, 5, 535–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Mahony, C.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; McKenna, W.J.; et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD). Eur. Heart J. 2014, 35, 2010–2020. [Google Scholar] [CrossRef]
- Quarta, G.; Aquaro, G.D.; Pedrotti, P.; Pontone, G.; Dellegrottaglie, S.; Iacovoni, A.; Brambilla, P.; Pradella, S.; Todiere, G.; Rigo, F.; et al. Cardiovascular magnetic resonance imaging in hypertrophic cardiomyopathy: The importance of clinical context. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Beltrami, M.; Bartolini, S.; Milli, M.; Palazzuoli, A. The relevance of specific heart failure outpatient programs in the COVID era: An appropriate model for every disease. Rev. Cardiovasc. Med. 2021, 22, 677–690. [Google Scholar] [CrossRef]
- Melacini, P.; Basso, C.; Angelini, A.; Calore, C.; Bobbo, F.; Tokajuk, B.; Bellini, N.; Smaniotto, G.; Zucchetto, M.; Iliceto, S.; et al. Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur. Heart J. 2010, 31, 2111–2123. [Google Scholar] [CrossRef] [Green Version]
- Olivotto, I.; Girolami, F.; Ackerman, M.J.; Nistri, S.; Bos, J.M.; Zachara, E.; Ommen, S.R.; Theis, J.L.; Vaubel, R.A.; Re, F.; et al. Myofilament protein gene mutation screening and outcome of patients with hypertrophic cardiomyopathy. Mayo Clin. Proc. 2008, 83, 630–638. [Google Scholar] [CrossRef]
- Harris, K.M.; Spirito, P.; Maron, M.S.; Zenovich, A.G.; Formisano, F.; Lesser, J.R.; Mackey-Bojack, S.; Manning, W.J.; Udelson, J.E.; Maron, B.J. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation 2006, 114, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Pasqualucci, D.; Fornaro, A.; Castelli, G.; Rossi, A.; Arretini, A.; Chiriatti, C.; Targetti, M.; Girolami, F.; Corda, M.; Orrù, P.; et al. Clinical Spectrum, Therapeutic Options, and Outcome of Advanced Heart Failure in Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2015, 8, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Dearani, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Management of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 390–414. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.; Canter, C.; Hannan, M.; Semigran, M.; Uber, P.; Baran, D.; Danziger-Isakov, L.; Kirklin, J.; Kirk, R.; Kushwaha, S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Sorajja, P.; Allison, T.; Hayes, C.; Nishimura, R.A.; Lam, C.S.P.; Ommen, S.R. Prognostic utility of metabolic exercise testing in minimally symptomatic patients with obstructive hypertrophic cardiomyopathy. Am. J. Cardiol. 2012, 109, 1494–1498. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Haddad, F.; Knowles, J.W.; Caleshu, C.; Pavlovic, A.; Homburger, J.; Shmargad, Y.; Sinagra, G.; Magavern, E.; Wong, M.; et al. Cardiopulmonary responses and prognosis in hypertrophic cardiomyopathy. A potential role for comprehensive noninvasive hemodynamic assessment. JACC Heart Fail. 2015, 3, 408–418. [Google Scholar] [CrossRef]
- Masri, A.; Pierson, L.M.; Smedira, N.G.; Agarwal, S.; Lytle, B.W.; Naji, P.; Thamilarasan, M.; Lever, H.M.; Cho, L.S.; Desai, M.Y. Predictors of long-term outcomes in patients with hypertrophic cardiomyopathy undergoing cardiopulmonary stress testing and echocardiography. Am. Heart J. 2015, 169, 684–692.e1. [Google Scholar] [CrossRef]
- Coats, C.J.; Rantell, K.; Bartnik, A.; Patel, A.; Mist, B.; McKenna, W.J.; Elliott, P.M. Cardiopulmonary Exercise Testing and Prognosis in Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2015, 8, 1022–1031. [Google Scholar] [CrossRef]
- Magri, D.; Agostoni, P.; Sinagra, G.; Re, F.; Correale, M.; Limongelli, G.; Zachara, E.; Mastromarino, V.; Santolamazza, C.; Casenghi, M.; et al. Clinical and prognostic impact of chronotropic incompetence in patients with hypertrophic cardiomyopathy. Int. J. Cardiol. 2018, 271, 125–131. [Google Scholar] [CrossRef]
- Magrì, D.; Re, F.; Limongelli, G.; Agostoni, P.; Zachara, E.; Correale, M.; Mastromarino, V.; Santolamazza, C.; Casenghi, M.; Pacileo, G.; et al. Heart failure progression in hypertrophic cardiomyopathy—Possible insights from cardiopulmonary exercise testing. Circ. J. 2016, 80, 2204–2211. [Google Scholar] [CrossRef]
- Magrì, D.; Limongelli, G.; Re, F.; Agostoni, P.; Zachara, E.; Correale, M.; Mastromarino, V.; Santolamazza, C.; Casenghi, M.; Pacileo, G.; et al. Cardiopulmonary exercise test and sudden cardiac death risk in hypertrophic cardiomyopathy. Heart 2016, 102, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Topilsky, Y.; Pereira, N.L.; Shah, D.K.; Boilson, B.; Schirger, J.A.; Kushwaha, S.S.; Joyce, L.D.; Park, S.J. Left ventricular assist device therapy in patients with restrictive and hypertrophic cardiomyopathy. Circ. Heart Fail. 2011, 4, 266–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.R.; Saeed, O.; Naftel, D.; Myers, S.; Kirklin, J.; Jorde, U.P.; Goldstein, D.J. Outcomes of Restrictive and Hypertrophic Cardiomyopathies After LVAD: An INTERMACS Analysis. J. Card. Fail. 2017, 23, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Kalsmith, B.M.; Udelson, J.E.; Li, W.; DeNofrio, D. Survival after cardiac transplantation in patients with hypertrophic cardiomyopathy. Circ. Heart Fail. 2010, 3, 574–579. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef]
- Maron, B.J.; Haas, T.S.; Murphy, C.J.; Ahluwalia, A.; Rutten-Ramos, S. Incidence and Causes of Sudden Death in U.S. College Athletes. J. Am. Coll. Cardiol. 2014, 63, 1636–1643. [Google Scholar] [CrossRef] [Green Version]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; Culasso, F.; Di Paolo, F.M.; Spataro, A.; Biffi, A.; Caselli, G.; Piovano, P. Clinical Significance of Abnormal Electrocardiographic Patterns in Trained Athletes. Circulation 2000, 102, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Monzo, L.; Martino, A.; Lanzillo, C.; Bencivenga, S.; Acitelli, A.; Fedele, E.; Salustri, E.; Bona, R.D.; Maresca, L.; Silvetti, E.; et al. Electrocardiographic voltage criteria in patients with hypertrophic cardiomyopathy. J. Cardiovasc. Med. 2020, 21, 696–703. [Google Scholar] [CrossRef]
- Ko, W.-Y.; Siontis, K.C.; Attia, Z.I.; Carter, R.E.; Kapa, S.; Ommen, S.R.; Demuth, S.J.; Ackerman, M.J.; Gersh, B.J.; Arruda-Olson, A.M.; et al. Detection of Hypertrophic Cardiomyopathy Using a Convolutional Neural Network-Enabled Electrocardiogram. J. Am. Coll. Cardiol. 2020, 75, 722–733. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Lu, D.-Y.; Kudchadkar, S.M.; Greenland, G.V.; Lingamaneni, P.; Corona-Villalobos, C.P.; Guan, Y.; Marine, J.E.; Olgin, J.E.; Zimmerman, S.; et al. Identifying Ventricular Arrhythmias and Their Predictors by Applying Machine Learning Methods to Electronic Health Records in Patients With Hypertrophic Cardiomyopathy (HCM-VAr-Risk Model). Am. J. Cardiol. 2019, 123, 1681–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmy, A.S.; Rausch, J.; Neisius, U.; Chan, R.H.; Maron, M.S.; Appelbaum, E.; Menze, B.; Nezafat, R. Automated Cardiac MR Scar Quantification in Hypertrophic Cardiomyopathy Using Deep Convolutional Neural Networks. JACC Cardiovasc. Imaging 2018, 11, 1917–1918. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic Value of Quantitative Contrast-Enhanced Cardiovascular Magnetic Resonance for the Evaluation of Sudden Death Risk in Patients With Hypertrophic Cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayes-Genis, A.; Liu, P.P.; Lanfear, D.E.; de Boer, R.A.; González, A.; Thum, T.; Emdin, M.; Januzzi, J.L. Omics phenotyping in heart failure: The next frontier. Eur. Heart J. 2020, 41, 3477–3484. [Google Scholar] [CrossRef] [PubMed]
| Non-Hypertrophic Phase |
|---|
| ETT: every 2 years in carriers for a mutation associated with the development of HCM. First-degree relatives: every 1–2 years between 10 and 20 years old, and every 2–5 years in adults. CMR: in case of suboptimal/borderline echo images, high-risk families, ECG-positive/ETT-negative patients. |
| Classic phenotype |
| ETT: every year. Stress echocardiography: every 6 months in obstructive HCM, every 2 years in remaining patients. CMR: every 3 to 5 years. |
| Adverse remodeling and overt dysfunction |
| ETT: every 6 months or sooner (based on clinical status). CPET-ETT combination: every 6 months to 1 year (based on clinical status and before referral to a heart transplantation center). CMR: every 2 years. |
| Non-hypertrophic | Imaging tests may be normal. Abnormal findings may include a reduction in e’ or abnormalities in myocardial strain + other non-specific patterns * |
| Classic phenotype | EF ≥ 65%, frequently abnormal LV strain frequent LVOTO normal diastole or delayed relaxation with reduced e’ mild-moderate LA dilation LGE: absent or <5% + other non-specific patterns * |
| Adverse remodeling | EF 50–65% less frequent LVOTO (a previously significant LVOTO may disappear) pseudonormal-restrictive diastole with reduced e’ moderate to severe LA dilation LGE 10–15% + other non-specific patterns * |
| Overt dysfunction | EF < 50% no LVOTO pseudonormal-restrictive diastole with severely reduced e’ severe biatrial dilation LGE 25–50% + other non-specific patterns * |
| First Author (Ref) | Year | Patients, N | End Point | CPET Prognostic Value |
|---|---|---|---|---|
| Sorajja et al. [105] | 2012 | 182 | HF progression, death | Peak VO2 < 60% predicted |
| Finocchiaro et al. [106] | 2015 | 156 | All-cause mortality, heart transplant, deterioration to septal reduction | VE/VCO2 > 34 |
| Masri et al. [107] | 2015 | 1005 | All-cause mortality, sudden cardiac death | Peak VO2 < 50% predicted |
| Coats et al. [108] | 2015 | 1898 | All-cause mortality, heart transplant | Reduction of 21% of mortality/HT risk for each 1 mL/kg/min increase in peak VO2 and by 29% for each 1 mL/kg/min increase in AT. Risk of mortality/HT is decreased by 18% for each unit increase in VE/VCO2 slope |
| Magri et al. [111] | 2016 | 623 | Sudden cardiac death | VE/VCO2 slope > 31 |
| Magri et al. [110] | 2016 | 620 | HF progression | Circulatory power (predicted peak VO2% peak SBP), VE/VCO2 slope (Mean peak VO2 = 21 mL/kg/min, mean VE/VCO2 slope = 29) |
| Magri et al. [109] | 2018 | 681 | HF events, sudden cardiac death | Peak HR < 70% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galluzzo, A.; Fiorelli, F.; Rossi, V.A.; Monzo, L.; Montrasio, G.; Camilli, M.; Halasz, G.; Uccello, G.; Mollace, R.; Beltrami, M. Multimodality Imaging in Sarcomeric Hypertrophic Cardiomyopathy: Get It Right…on Time. Life 2023, 13, 171. https://doi.org/10.3390/life13010171
Galluzzo A, Fiorelli F, Rossi VA, Monzo L, Montrasio G, Camilli M, Halasz G, Uccello G, Mollace R, Beltrami M. Multimodality Imaging in Sarcomeric Hypertrophic Cardiomyopathy: Get It Right…on Time. Life. 2023; 13(1):171. https://doi.org/10.3390/life13010171
Chicago/Turabian StyleGalluzzo, Alessandro, Francesca Fiorelli, Valentina A. Rossi, Luca Monzo, Giulia Montrasio, Massimiliano Camilli, Geza Halasz, Giuseppe Uccello, Rocco Mollace, and Matteo Beltrami. 2023. "Multimodality Imaging in Sarcomeric Hypertrophic Cardiomyopathy: Get It Right…on Time" Life 13, no. 1: 171. https://doi.org/10.3390/life13010171
APA StyleGalluzzo, A., Fiorelli, F., Rossi, V. A., Monzo, L., Montrasio, G., Camilli, M., Halasz, G., Uccello, G., Mollace, R., & Beltrami, M. (2023). Multimodality Imaging in Sarcomeric Hypertrophic Cardiomyopathy: Get It Right…on Time. Life, 13(1), 171. https://doi.org/10.3390/life13010171






