The Role of Brain-Derived Neurotrophic Factor (BDNF) in Depression and Cardiovascular Disease: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Study Design
2.3. BDNF Level Measurement
2.4. Search Strategy
2.5. Data Extraction
2.6. Quality Assessment
2.7. Data Synthesis
3. Results
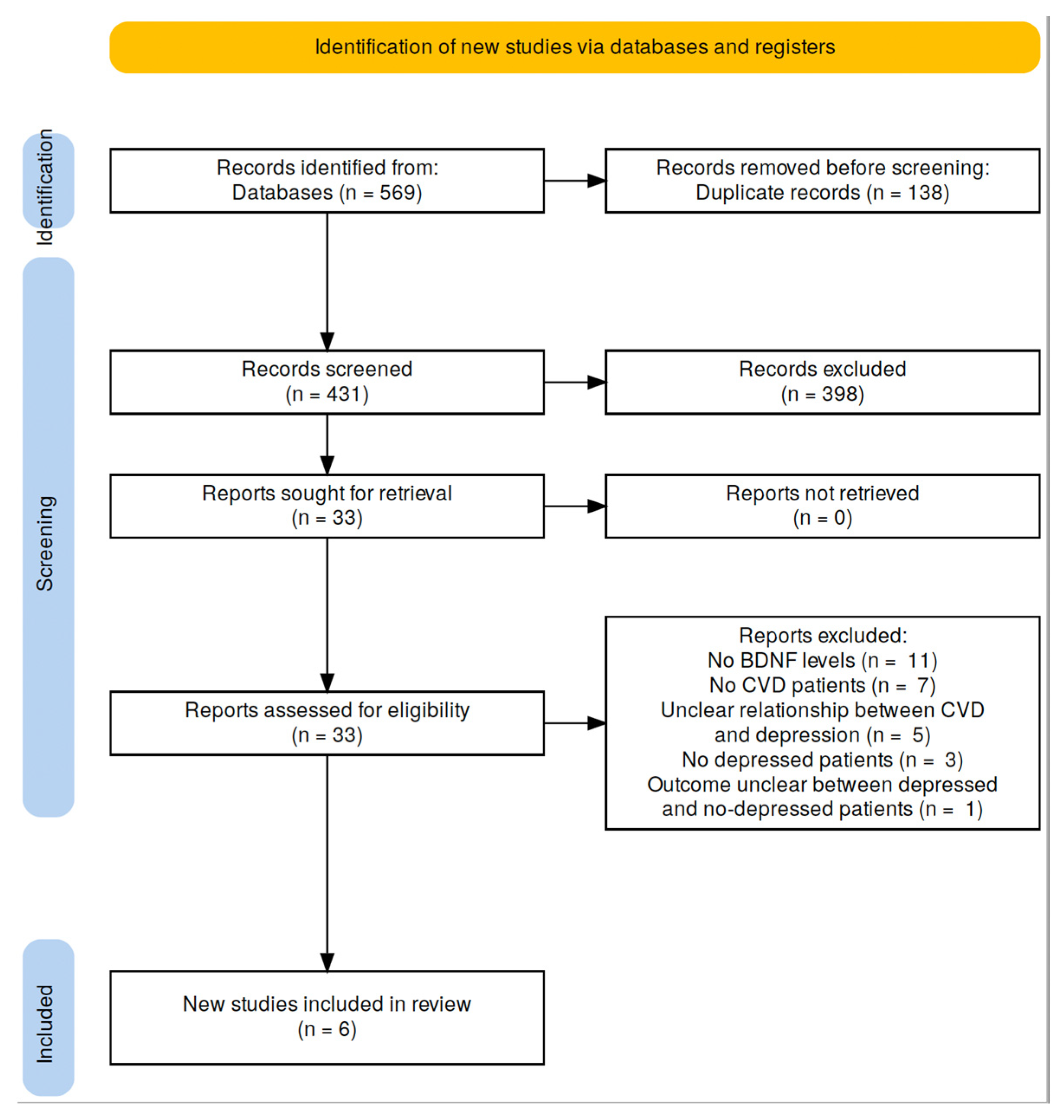
3.1. Search Results
3.2. Study Characteristics
3.3. Risk of Bias
3.4. Role of BDNF in Patients with Stroke
3.5. Coronary Heart Disease and Altered BDNF Levels
4. Discussion
4.1. Relationship between Stroke and Depression
4.2. Relationship between Coronary Artery Disease and Depression
4.3. Relationship between Depression and CVD
4.4. Limitations of the Included Studies
4.5. Limitations of this Systematic Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronado, F.; Melvin, S.C.; Bell, R.A.; Zhao, G. Global Responses to Prevent, Manage, and Control Cardiovascular Diseases. Prev. Chronic Dis. 2022, 19, E84. [Google Scholar] [CrossRef]
- World Health Organization Depression. Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 10 July 2023).
- Cho, Y.; Lee, J.K.; Kim, D.H.; Park, J.H.; Choi, M.; Kim, H.J.; Nam, M.J.; Lee, K.U.; Han, K.; Park, Y.G. Factors associated with quality of life in patients with depression: A nationwide population-based study. PLoS ONE 2019, 14, e0219455. [Google Scholar] [CrossRef]
- Bahall, M.; Legall, G.; Khan, K. Quality of life among patients with cardiac disease: The impact of comorbid depression. Health Qual. Life Outcomes 2020, 18, 189. [Google Scholar] [CrossRef]
- Komalasari, R.; Nurjanah; Yoche, M.M. Quality of Life of People with Cardiovascular Disease: A Descriptive Study. Asian Pac. Isl. Nurs. J. 2019, 4, 92–96. [Google Scholar] [CrossRef]
- Basu, S.; Bendavid, E.; Sood, N. Health and Economic Implications of National Treatment Coverage for Cardiovascular Disease in India: Cost-Effectiveness Analysis. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 541–551. [Google Scholar] [CrossRef]
- Suhrcke, M.; Urban, D. Are cardiovascular diseases bad for economic growth? Health Econ. 2010, 19, 1478–1496. [Google Scholar] [CrossRef]
- Andlin-Sobocki, P.; Wittchen, H.U. Cost of affective disorders in Europe. Eur. J. Neurol. 2005, 12 (Suppl. S1), 34–38. [Google Scholar] [CrossRef]
- Bock, J.O.; Luppa, M.; Brettschneider, C.; Riedel-Heller, S.; Bickel, H.; Fuchs, A.; Gensichen, J.; Maier, W.; Mergenthal, K.; Schafer, I.; et al. Impact of depression on health care utilization and costs among multimorbid patients--from the MultiCare Cohort Study. PLoS ONE 2014, 9, e91973. [Google Scholar] [CrossRef]
- Ziwei, Z.; Hua, Y.; Liu, A. Bidirectional association between depressive symptoms and cardiovascular disease in the middle-aged and elderly Chinese: A 5-year longitudinal study. BMJ Open 2023, 13, e071175. [Google Scholar] [CrossRef]
- Jia, Z.; Li, S. Risk of Cardiovascular Disease Mortality in Relation to Depression and 14 Common Risk Factors. Int. J. Gen. Med. 2021, 14, 441–449. [Google Scholar] [CrossRef]
- Baune, B.T.; Stuart, M.; Gilmour, A.; Wersching, H.; Heindel, W.; Arolt, V.; Berger, K. The relationship between subtypes of depression and cardiovascular disease: A systematic review of biological models. Transl. Psychiatry 2012, 2, e92. [Google Scholar] [CrossRef] [PubMed]
- Henao Perez, M.; Lopez Medina, D.C.; Lemos Hoyos, M.; Rios Zapata, P. Depression and the risk of adverse outcomes at 5 years in patients with coronary heart disease. Heliyon 2020, 6, e05425. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.C.; Celano, C.M.; Beach, S.R.; Motiwala, S.R.; Januzzi, J.L. Depression and cardiac disease: Epidemiology, mechanisms, and diagnosis. Cardiovasc. Psychiatry Neurol. 2013, 2013, 695925. [Google Scholar] [CrossRef] [PubMed]
- Rawashdeh, S.I.; Ibdah, R.; Kheirallah, K.A.; Al-Kasasbeh, A.; Raffee, L.A.; Alrabadi, N.; Albustami, I.S.; Haddad, R.; Ibdah, R.M.; Al-Mistarehi, A.H. Prevalence Estimates, Severity, and Risk Factors of Depressive Symptoms among Coronary Artery Disease Patients after Ten Days of Percutaneous Coronary Intervention. Clin. Pract. Epidemiol. Ment. Health 2021, 17, 103–113. [Google Scholar] [CrossRef]
- Ayerbe, L.; Ayis, S.; Wolfe, C.D.; Rudd, A.G. Natural history, predictors and outcomes of depression after stroke: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 14–21. [Google Scholar] [CrossRef]
- de Bekker, A.; Geerlings, M.I.; Uitewaal-Poslawsky, I.E.; de Man-van Ginkel, J.M. Depression in Stroke Survivors: Ten-Year Follow-Up. Determinants of the Natural Course of Depressive Symptoms in Stroke Survivors in the Netherlands: The SMART-Medea Study. J. Stroke Cerebrovasc. Dis. 2022, 31, 106272. [Google Scholar] [CrossRef]
- Hackett, M.L.; Pickles, K. Part I: Frequency of depression after stroke: An updated systematic review and meta-analysis of observational studies. Int. J. Stroke 2014, 9, 1017–1025. [Google Scholar] [CrossRef]
- Hashimoto, K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: Emerging links between cardiovascular disease and depression. Prog. Neurobiol. 2013, 100, 15–29. [Google Scholar] [CrossRef]
- Dhar, A.K.; Barton, D.A. Depression and the Link with Cardiovascular Disease. Front. Psychiatry 2016, 7, 33. [Google Scholar] [CrossRef]
- Ogunmoroti, O.; Osibogun, O.; Spatz, E.S.; Okunrintemi, V.; Mathews, L.; Ndumele, C.E.; Michos, E.D. A systematic review of the bidirectional relationship between depressive symptoms and cardiovascular health. Prev. Med. 2022, 154, 106891. [Google Scholar] [CrossRef]
- Fujitani, M.; Otani, Y.; Miyajima, H. Do Neurotrophins Connect Neurological Disorders and Heart Diseases? Biomolecules 2021, 11, 1730. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Camilli, M.; Del Buono, M.G.; Russo, M.; Rinaldi, R.; Canonico, F.; Pedicino, D.; Severino, A.; D’Amario, D.; Trani, C.; et al. Brain-derived neurotrophic factor in patients with acute coronary syndrome. Transl. Res. 2021, 231, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Mojtabavi, H.; Shaka, Z.; Momtazmanesh, S.; Ajdari, A.; Rezaei, N. Circulating brain-derived neurotrophic factor as a potential biomarker in stroke: A systematic review and meta-analysis. J. Transl. Med. 2022, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Carniel, B.P.; da Rocha, N.S. Brain-derived neurotrophic factor (BDNF) and inflammatory markers: Perspectives for the management of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 108, 110151. [Google Scholar] [CrossRef] [PubMed]
- Arosio, B.; Guerini, F.R.; Voshaar, R.C.O.; Aprahamian, I. Blood Brain-Derived Neurotrophic Factor (BDNF) and Major Depression: Do We Have a Translational Perspective? Front. Behav. Neurosci. 2021, 15, 626906. [Google Scholar] [CrossRef]
- Bahls, M.; Konemann, S.; Markus, M.R.P.; Wenzel, K.; Friedrich, N.; Nauck, M.; Volzke, H.; Steveling, A.; Janowitz, D.; Grabe, H.J.; et al. Brain-derived neurotrophic factor is related with adverse cardiac remodeling and high NTproBNP. Sci. Rep. 2019, 9, 15421. [Google Scholar] [CrossRef]
- Kaess, B.M.; Preis, S.R.; Lieb, W.; Beiser, A.S.; Yang, Q.; Chen, T.C.; Hengstenberg, C.; Erdmann, J.; Schunkert, H.; Seshadri, S.; et al. Circulating brain-derived neurotrophic factor concentrations and the risk of cardiovascular disease in the community. J. Am. Heart Assoc. 2015, 4, e001544. [Google Scholar] [CrossRef]
- Duman, R.S.; Li, N. A neurotrophic hypothesis of depression: Role of synaptogenesis in the actions of NMDA receptor antagonists. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2475–2484. [Google Scholar] [CrossRef]
- Kuhlmann, S.L.; Tschorn, M.; Arolt, V.; Beer, K.; Brandt, J.; Grosse, L.; Haverkamp, W.; Muller-Nordhorn, J.; Rieckmann, N.; Waltenberger, J.; et al. Serum brain-derived neurotrophic factor and stability of depressive symptoms in coronary heart disease patients: A prospective study. Psychoneuroendocrinology 2017, 77, 196–202. [Google Scholar] [CrossRef]
- Sen, S.; Duman, R.; Sanacora, G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: Meta-analyses and implications. Biol. Psychiatry 2008, 64, 527–532. [Google Scholar] [CrossRef]
- Tschorn, M.; Kuhlmann, S.L.; Rieckmann, N.; Beer, K.; Grosse, L.; Arolt, V.; Waltenberger, J.; Haverkamp, W.; Muller-Nordhorn, J.; Hellweg, R.; et al. Brain-derived neurotrophic factor, depressive symptoms and somatic comorbidity in patients with coronary heart disease. Acta. Neuropsychiatr. 2021, 33, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Ejiri, J.; Inoue, N.; Kobayashi, S.; Shiraki, R.; Otsui, K.; Honjo, T.; Takahashi, M.; Ohashi, Y.; Ichikawa, S.; Terashima, M.; et al. Possible role of brain-derived neurotrophic factor in the pathogenesis of coronary artery disease. Circulation 2005, 112, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhong, J.; Zou, B.; Fang, L.; Chen, J.; Deng, X.; Zhang, L.; Zhao, X.; Qu, Z.; Lei, Y.; et al. Meta-analyses of comparative efficacy of antidepressant medications on peripheral BDNF concentration in patients with depression. PLoS ONE 2017, 12, e0172270. [Google Scholar] [CrossRef] [PubMed]
- Fioranelli, M.; Spadafora, L.; Bernardi, M.; Roccia, M.G.; Del Buono, M.G.; Cacioli, G.; Biondi-Zoccai, G. Impact of low-dose Brain-Derived Neurotrophic Factor (BDNF) on atrial fibrillation recurrence. Minerva. Cardiol. Angiol. 2023. [Google Scholar] [CrossRef]
- Jehn, C.F.; Becker, B.; Flath, B.; Nogai, H.; Vuong, L.; Schmid, P.; Luftner, D. Neurocognitive function, brain-derived neurotrophic factor (BDNF) and IL-6 levels in cancer patients with depression. J. Neuroimmunol. 2015, 287, 88–92. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Serra-Millas, M. Are the changes in the peripheral brain-derived neurotrophic factor levels due to platelet activation? World J. Psychiatry 2016, 6, 84–101. [Google Scholar] [CrossRef]
- Baccaro, A.; Wang, Y.P.; Candido, M.; Conforto, A.B.; Brunoni, A.R.; Leite, C.D.C.; Busatto Filho, G.; Lotufo, P.A.; Bensenor, I.M.; Goulart, A.C. Post-stroke depression and cognitive impairment: Study design and preliminary findings in a Brazilian prospective stroke cohort (EMMA study). J. Affect. Disord. 2019, 245, 72–81. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.D.; Zeng, J.W.; Chen, X.Y.; Wang, R.D.; Cheng, S.Y. Serum Brain-derived neurotrophic factor levels in post-stroke depression. J. Affect. Disord. 2014, 168, 373–379. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Lan, Y.; Miao, J.; Pan, C.; Sun, W.; Li, G.; Wang, Y.; Zhao, X.; Zhu, Z.; et al. Blood biomarkers of post-stroke depression after minor stroke at three months in males and females. BMC Psychiatry 2022, 22, 162. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Z.; Sun, D.; Xu, Z.; Yuan, Y.; Zhang, X.; Li, L. Low serum BDNF may indicate the development of PSD in patients with acute ischemic stroke. Int. J. Geriatr. Psychiatry 2011, 26, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lu, T.; Xu, G.; Yue, X.; Zhu, W.; Ma, M.; Liu, W.; Zhu, S.; Liu, X. Decreased serum brain-derived neurotrophic factor (BDNF) is associated with post-stroke depression but not with BDNF gene Val66Met polymorphism. Clin. Chem. Lab. Med. 2011, 49, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Yin, H.; Guo, L.; Ma, H.; Wang, H.; Liu, F.; Liang, Y.; Liu, A.; Geng, Q. Comorbidity of depression and anxiety leads to a poor prognosis following angina pectoris patients: A prospective study. BMC Psychiatry 2021, 21, 202. [Google Scholar] [CrossRef]
- Hare, D.L.; Toukhsati, S.R.; Johansson, P.; Jaarsma, T. Depression and cardiovascular disease: A clinical review. Eur. Heart J. 2014, 35, 1365–1372. [Google Scholar] [CrossRef]
- Duan, J.; Huang, K.; Zhang, X.; Wang, R.; Chen, Z.; Wu, Z.; Huang, C.; Yang, C.; Yang, L. Role of depressive symptoms in the prognosis of heart failure and its potential clinical predictors. ESC Heart Fail. 2022, 9, 2676–2685. [Google Scholar] [CrossRef]
- Freedland, K.E.; Carney, R.M. Depression as a risk factor for adverse outcomes in coronary heart disease. BMC Med. 2013, 11, 131. [Google Scholar] [CrossRef]
- Choi, W.; Kim, J.W.; Kang, H.J.; Kim, H.K.; Kang, H.C.; Lee, J.Y.; Kim, S.W.; Hong, Y.J.; Ahn, Y.; Jeong, M.H.; et al. Interaction effects of diabetes and brain-derived neurotrophic factor on suicidal ideation in patients with acute coronary syndrome. Sci. Rep. 2022, 12, 6602. [Google Scholar] [CrossRef]
- Angelucci, F.; Brene, S.; Mathe, A.A. BDNF in schizophrenia, depression and corresponding animal models. Mol. Psychiatry 2005, 10, 345–352. [Google Scholar] [CrossRef]
- Qiao, H.; An, S.C.; Xu, C.; Ma, X.M. Role of proBDNF and BDNF in dendritic spine plasticity and depressive-like behaviors induced by an animal model of depression. Brain Res. 2017, 1663, 29–37. [Google Scholar] [CrossRef]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front. Cell Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Belanger, J.C.; Bouchard, V.; Le Blanc, J.; Starnino, L.; Welman, M.; Chabot-Blanchet, M.; Busseuil, D.; Chertkow, H.; D’Antono, B.; Lordkipanidze, M. Brain-Derived Neurotrophic Factor Mitigates the Association Between Platelet Dysfunction and Cognitive Impairment. Front. Cardiovasc. Med. 2021, 8, 739045. [Google Scholar] [CrossRef] [PubMed]
- Gejl, A.K.; Enevold, C.; Bugge, A.; Andersen, M.S.; Nielsen, C.H.; Andersen, L.B. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci. Rep. 2019, 9, 9655. [Google Scholar] [CrossRef] [PubMed]
- Czira, M.E.; Wersching, H.; Baune, B.T.; Berger, K. Brain-derived neurotrophic factor gene polymorphisms, neurotransmitter levels, and depressive symptoms in an elderly population. Age 2012, 34, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, A.N.; Overman, J.J.; Zhong, S.; Mueller, R.; Lynch, G.; Carmichael, S.T. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J. Neurosci. 2011, 31, 3766–3775. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.H.; Cui, Y.; Huang, S.M.; Zhang, B. The Role of Brain-Derived Neurotrophic Factor Signaling in Central Nervous System Disease Pathogenesis. Front. Hum. Neurosci. 2022, 16, 924155. [Google Scholar] [CrossRef]
- Bus, B.A.; Molendijk, M.L.; Penninx, B.J.; Buitelaar, J.K.; Kenis, G.; Prickaerts, J.; Elzinga, B.M.; Voshaar, R.C. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology 2011, 36, 228–239. [Google Scholar] [CrossRef]
- Jin, H.; Chen, Y.; Wang, B.; Zhu, Y.; Chen, L.; Han, X.; Ma, G.; Liu, N. Association between brain-derived neurotrophic factor and von Willebrand factor levels in patients with stable coronary artery disease. BMC Cardiovasc. Disord. 2018, 18, 23. [Google Scholar] [CrossRef]
- Feng, N.; Huke, S.; Zhu, G.; Tocchetti, C.G.; Shi, S.; Aiba, T.; Kaludercic, N.; Hoover, D.B.; Beck, S.E.; Mankowski, J.L.; et al. Constitutive BDNF/TrkB signaling is required for normal cardiac contraction and relaxation. Proc. Natl. Acad. Sci. USA 2015, 112, 1880–1885. [Google Scholar] [CrossRef]
- Fulgenzi, G.; Tomassoni-Ardori, F.; Babini, L.; Becker, J.; Barrick, C.; Puverel, S.; Tessarollo, L. BDNF modulates heart contraction force and long-term homeostasis through truncated TrkB.T1 receptor activation. J. Cell Biol. 2015, 210, 1003–1012. [Google Scholar] [CrossRef]
- Hildreth, V.; Anderson, R.H.; Henderson, D.J. Autonomic innervation of the developing heart: Origins and function. Clin. Anat. 2009, 22, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Hang, P.Z.; Zhu, H.; Li, P.F.; Liu, J.; Ge, F.Q.; Zhao, J.; Du, Z.M. The Emerging Role of BDNF/TrkB Signaling in Cardiovascular Diseases. Life 2021, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Yokoyama, M.; Toko, H.; Tateno, K.; Moriya, J.; Shimizu, I.; Nojima, A.; Ito, T.; Yoshida, Y.; Kobayashi, Y.; et al. Brain-derived neurotrophic factor protects against cardiac dysfunction after myocardial infarction via a central nervous system-mediated pathway. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Hang, P.; Zhao, J.; Cai, B.; Tian, S.; Huang, W.; Guo, J.; Sun, C.; Li, Y.; Du, Z. Brain-derived neurotrophic factor regulates TRPC3/6 channels and protects against myocardial infarction in rodents. Int. J. Biol. Sci. 2015, 11, 536–545. [Google Scholar] [CrossRef]
- Monisha, K.G.; Prabu, P.; Chokkalingam, M.; Murugesan, R.; Milenkovic, D.; Ahmed, S. Clinical utility of brain-derived neurotrophic factor as a biomarker with left ventricular echocardiographic indices for potential diagnosis of coronary artery disease. Sci. Rep. 2020, 10, 16359. [Google Scholar] [CrossRef]
- Kermani, P.; Hempstead, B. BDNF Actions in the Cardiovascular System: Roles in Development, Adulthood and Response to Injury. Front. Physiol. 2019, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Zhong, J.H.; Zhou, X.F. Development of mature BDNF-specific sandwich ELISA. J. Neurochem. 2015, 134, 75–85. [Google Scholar] [CrossRef]
- Polacchini, A.; Metelli, G.; Francavilla, R.; Baj, G.; Florean, M.; Mascaretti, L.G.; Tongiorgi, E. A method for reproducible measurements of serum BDNF: Comparison of the performance of six commercial assays. Sci. Rep. 2015, 5, 17989. [Google Scholar] [CrossRef]
- Khodadi, E. Platelet Function in Cardiovascular Disease: Activation of Molecules and Activation by Molecules. Cardiovasc. Toxicol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Gelle, T.; Samey, R.A.; Plansont, B.; Bessette, B.; Jauberteau-Marchan, M.O.; Lalloue, F.; Girard, M. BDNF and pro-BDNF in serum and exosomes in major depression: Evolution after antidepressant treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110229. [Google Scholar] [CrossRef]
- Zara, M.; Amadio, P.; Campodonico, J.; Sandrini, L.; Barbieri, S.S. Exosomes in Cardiovascular Diseases. Diagnostics 2020, 10, 943. [Google Scholar] [CrossRef]
- Lin, C.C.; Huang, T.L. Brain-derived neurotrophic factor and mental disorders. Biomed. J. 2020, 43, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Falaschi, V.; Palego, L.; Marazziti, D.; Betti, L.; Musetti, L.; Maglio, A.; Dell’Oste, V.; Sagona, S.; Felicioli, A.; Carpita, B.; et al. Variation of Circulating Brain-Derived Neurotrophic Factor (BDNF) in Depression: Relationships with Inflammatory Indices, Metabolic Status and Patients’ Clinical Features. Life 2023, 13, 1555. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Stewart, R.; Kim, S.Y.; Kim, J.W.; Kang, H.J.; Lee, J.Y.; Kim, S.W.; Shin, I.S.; Kim, M.C.; Hong, Y.J.; et al. Interaction between BDNF val66met polymorphism and personality on long-term cardiac outcomes in patients with acute coronary syndrome. PLoS ONE 2019, 14, e0226802. [Google Scholar] [CrossRef]
- Zheleznyakova, G.Y.; Cao, H.; Schioth, H.B. BDNF DNA methylation changes as a biomarker of psychiatric disorders: Literature review and open access database analysis. Behav. Brain Funct. 2016, 12, 17. [Google Scholar] [CrossRef] [PubMed]
| Author | Country | Study Design | Observation Period | Relationship Direction | CVD at Recruitment | CVD Diagnosis | Outcome | Outcome Evaluation | Endpoints |
|---|---|---|---|---|---|---|---|---|---|
| Baccaro et al. (2019) [39] | Brazil | Prospective | April 2006–September 2014 | CVD → depression | Stroke | STEPS-STROKE criteria confirmed by CT + TOAST for ischemic stroke | PSD | PHQ-9 ≥ 10 | Within 3 months from stroke onset (subclinical phase) |
| Kuhlmann et al. (2017) [30] | Germany | Prospective | December 2012–November 2014 | CVD → depression | CHD | Medical charts | Depressive symptoms | PHQ-9 ≥ 7 | PHQ-9 at baseline and at 6 months |
| Li et al. (2014) [40] | China | Prospective | NR | CVD → depression | First episode of acute ischemic stroke | TOAST | Depression | HAM-D | Baseline and after 3 months from stroke |
| Qiu et al. (2022) [41] | China | Prospective | May 2018–August 2019 | CVD → depression | Minor stroke (NIHSS score < 3) | Confirmed by CT or MRI | PSD | HAM-D > 7 | Baseline and after 3 months from stroke |
| Yang et al. (2011) [42] | China | Prospective | June 2007–June 2008 | CVD → depression | Stroke with acute cerebral infarction | TOAST | PSD | HAM-D and MADRS | 3–7–14 days after admission |
| Zhou et al. (2011) [43] | China | Retrospective | April 2008–November 2008 | CVD → depression | Ischemic stroke | WHO criteria | PSD | HAM-D | 7 days and at 1, 3, and 6 months after onset of stroke |
| Author | Analytical Methods | BDNF Evaluation | Differentiation mBDNF from proBDNF | Endpoints | Sample Size |
|---|---|---|---|---|---|
| Baccaro et al. (2019) [39] | (1) Sample collection (2) Aliquots frozen at −80 °C | ELISA (HMYOMAG-56k-02, Millipore®, St. Charles, MO, USA) | Unclear | Within 3 months from stroke onset (subclinical phase) | 103 |
| Kuhlmann et al. (2017) [30] | (1) Sample collection (2) Clotting time 30–60 min at room temperature (3) Centrifugation at 3.500 rpm for 15 min at 4 °C (4) Serum refrigeration at 20 °C | ELISA (Promega Inc., Mannheim, Germany) | No | PHQ-9 at baseline and at 6 months | 190 |
| Li et al. (2014) [40] | (1) Sample collection (2) Samples stored before analysis at −80 °C | ELISA (DuoSet ELISA Development, R&S Systems, USA) | Unclear | Baseline and after 3 months from stroke | 216 |
| Qiu et al. (2022) [41] | NR | NR | NR | Baseline and after 3 months from stroke | 530 |
| Yang et al. (2011) [42] | (1) Sample collection (2) Kept at room temperature for 1 h (3) Kept for 1 h at 48 °C (4) Centrifugation at 2000× g for 10 min at 48 °C (5) Kept frozen at −80 °C | ELISA (Promega Inc., Madison, WI, USA) | Yes | 3–7–14 days after admission | 100 |
| Zhou et al. (2011) [43] | (1) Sample collection (2) Kept at room temperature for 30 min (3) Centrifugation for 15 min at 1000× g (4) Stored at −80 °C | BDNF Emax Immunoassay System kit (R&D Systems, Minneapolis, MN, USA) | Yes | 7 days and at 1, 3, and 6 months after onset of stroke | 112 |
| Author | Sample Size | Follow-Up | Age | Sex (Male/Female Ratio) | NIHSS | Number of Patients with Depression | Findings |
|---|---|---|---|---|---|---|---|
| Baccaro et al. (2019) [39] | 103 | 71 days | Median: 63 years | 60/43 | NR | 14 | No statistically significant difference in serum BDNF levels between patients with and without PSD (p = 0.35) |
| Li et al. (2014) [40] | 216 | Depressed patients: 72.8 ± 11.2; not depressed patients: 63.9 ± 9.1 | 88/128 | Depressed: median 8 (IQR 4–14); not depressed: median 5 (IQR 2–8) | 59 | BDNF serum levels in depressed patients: 8.1 ng/mL (5.6–9.4); BDNF serum level in not depressed patients: 13.7 ng/mL (10.4–16.5), p < 0.0001. Inverse correlation between lower serum BDNF levels at admission and higher HAM-D score at 3 months (r = 0.361, p < 0.0001). | |
| Qiu et al. (2022) [41] | 530 | Female: 58.8 ± 12.3; male: 58.0 ± 11.5 | 415/115 | NIHSS < 3 | 168 | Serum BDNF levels statistically different in women with and without PSD: p = 0.029, OR = 0.916, 95% CI: 0.846–0.991 | |
| Yang et al. (2011) [42] | 100 | PSD: 68.95 ± 9.28; no PSD: 68.43 ± 11.18; HC: 65.12 ± 10.27 | 77/73 | PSD: median 7 (IQR 4~9.5); no PSD: median 3 (IQR 2~4) | 37 | Serum BDNF levels lower in PSD patients than in non-PSD patients 1 day after stroke. No significant differences on day 7 (F = 2.796, p = 0.064). | |
| Zhou et al. (2011) [43] | 112 | 6 mo. | PSD: 61.7 ± 8.5; no PSD: 63.5 ± 12.5 | 53/59 | PSD: median 7 (IQR 1–24); no PSD: median 5 (IQR 1–13) | 35 | Diagnosis, serum BDNF level lower in PSD patients compared to non-PSD patients (p = 0.027). Acute stage: no significant differences. No significant differences in patients without PSD comparing BDNF levels at 7 days and 6 months |
| Author | Sample Size | Follow-Up | Age | Sex (Male/ Female Ratio) | Number of Patients with Depression | Findings |
|---|---|---|---|---|---|---|
| Kuhlmann et al. (2017) [30] | 190 | 65 ± 11 | 145/45 | Incident depressed: 23; persistently depressed: 48; remitted depressed: 25, persistently non-depressed: 94 | Depressed patients: BDNF significantly lower in persistently depressed patients (p < 0.05). BDNF not predictive for depression in incident depressed patients (OR: 1.50, 95% CI: 0.95–2.39, p = 0.081). Persistent depressive symptoms more common in patients with lowed BDNF concentration at admission (OR: 0.37, 95% CI: 0.19–0.74, p = 0.005). Acute coronary syndrome predictive factor for depressive symptoms (OR: 4.60, 95% CI: 1.12–18.97, p = 0.035). Charlson Comorbidity Index significantly predicts depressive symptoms in initially non-depressed patients (OR: 1.61, 95% CI: 1.06–2.46, p = 0.026). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fioranelli, M.; Roccia, M.G.; Przybylek, B.; Garo, M.L. The Role of Brain-Derived Neurotrophic Factor (BDNF) in Depression and Cardiovascular Disease: A Systematic Review. Life 2023, 13, 1967. https://doi.org/10.3390/life13101967
Fioranelli M, Roccia MG, Przybylek B, Garo ML. The Role of Brain-Derived Neurotrophic Factor (BDNF) in Depression and Cardiovascular Disease: A Systematic Review. Life. 2023; 13(10):1967. https://doi.org/10.3390/life13101967
Chicago/Turabian StyleFioranelli, Massimo, Maria Grazia Roccia, Bianca Przybylek, and Maria Luisa Garo. 2023. "The Role of Brain-Derived Neurotrophic Factor (BDNF) in Depression and Cardiovascular Disease: A Systematic Review" Life 13, no. 10: 1967. https://doi.org/10.3390/life13101967
APA StyleFioranelli, M., Roccia, M. G., Przybylek, B., & Garo, M. L. (2023). The Role of Brain-Derived Neurotrophic Factor (BDNF) in Depression and Cardiovascular Disease: A Systematic Review. Life, 13(10), 1967. https://doi.org/10.3390/life13101967





