6-Nitrodopamine Is the Most Potent Endogenous Positive Inotropic Agent in the Isolated Rat Heart
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Treatment with NO Synthesis Inhibitor Nω-Nitro-L-arginine Methyl Ester Hydrochloride (L-NAME)
2.3. Rat Isolated Ventricles
2.4. Basal Release of Catecholamines from Rat Ventricles
2.5. Determination of Catecholamines by Liquid Chromatography Coupled to Tandem Mass Spectrometry (LC-MS/MS)
2.6. Isolated Langendorff’s Perfused Heart
2.7. Langendorff’s Perfused Heart Analysis and Experimental Design
2.8. Effect of Atenolol Infusion on the Positive Inotropic Effect Induced by Catecholamines
2.9. Statistical Analysis
2.10. Chemical and Reagents
3. Results
3.1. Basal Release of Catecholamines from Isolated Ventricles
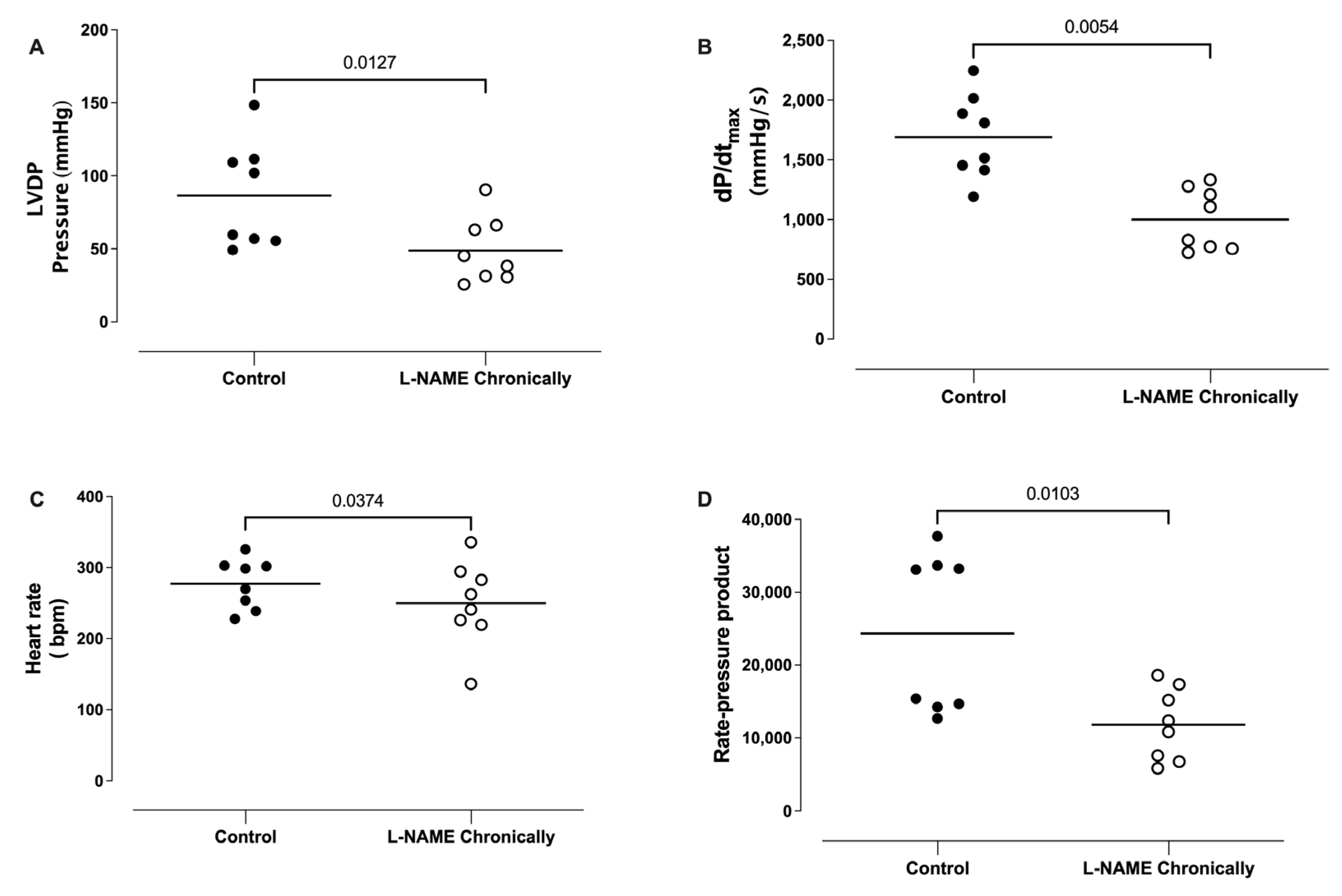
3.2. Effect of the Chronic Administration of L-NAME on the Left Ventricular Developed Pressure (LVDP), dP/dtmax, Heart Rate (HR), and Rate Pressure Product (RPP)
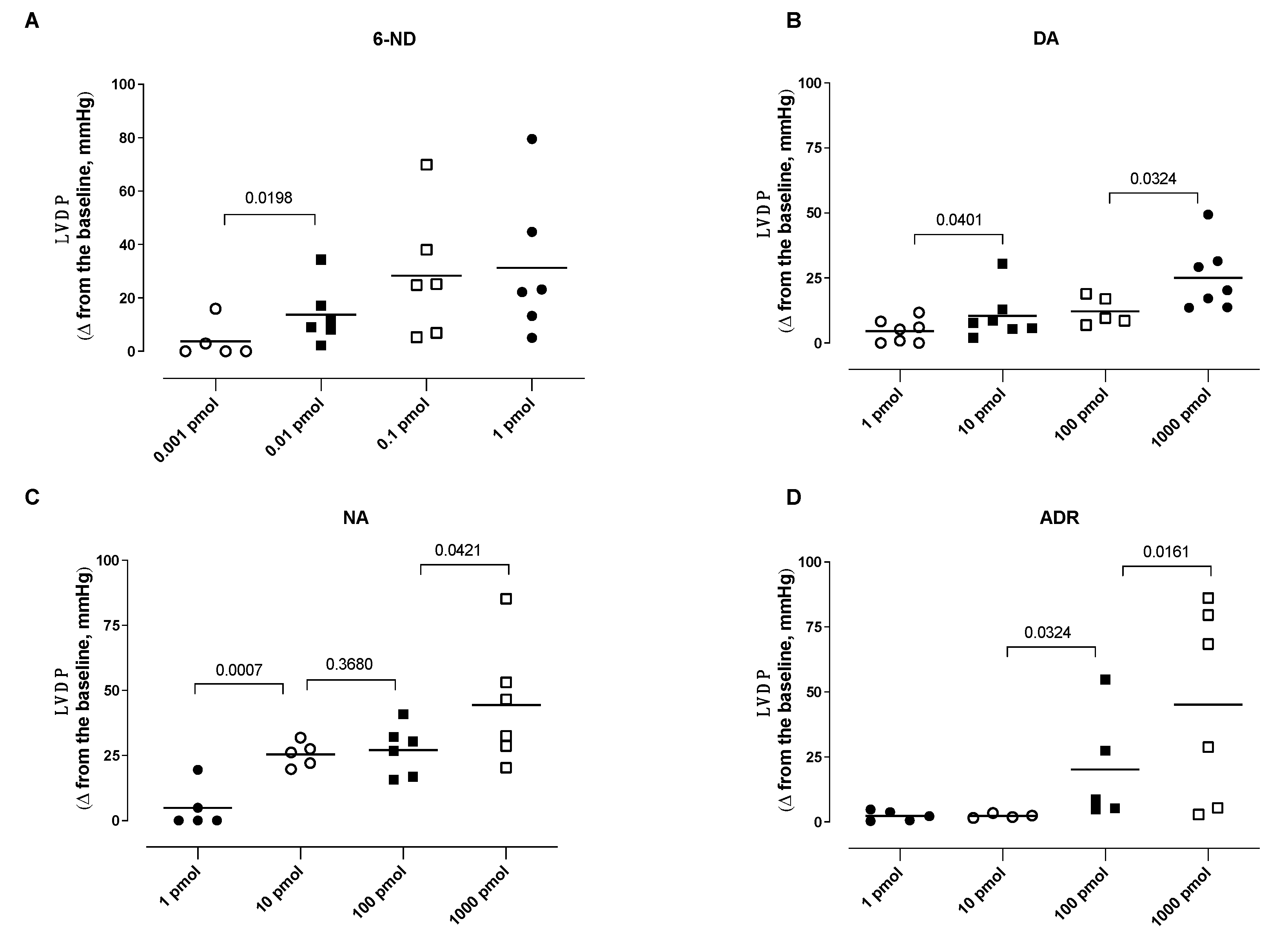
3.3. Effect of Bolus Injections of Catecholamines on the Left Ventricle Developed Pressure (LVDP)
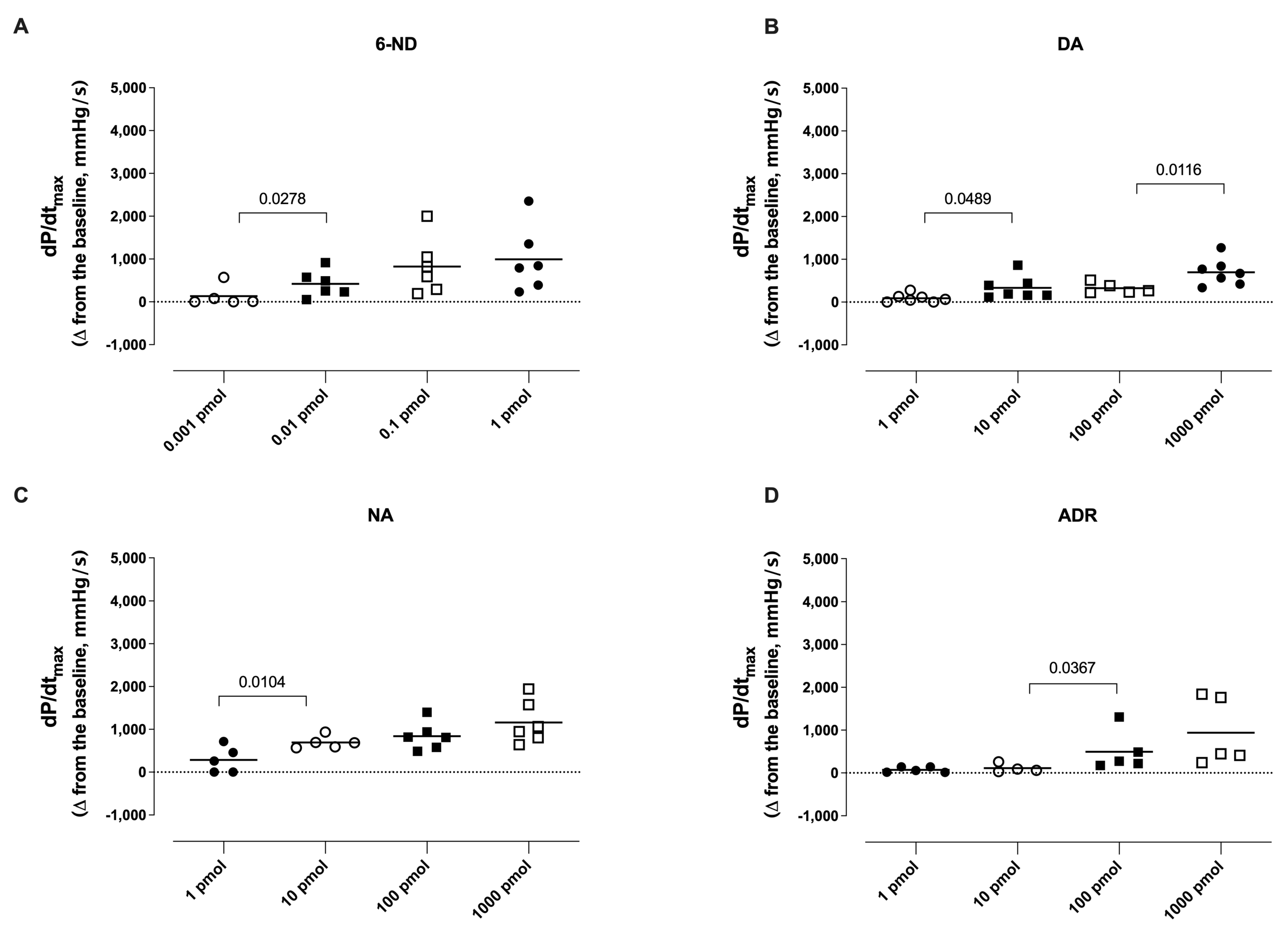
3.4. Effect of Bolus Injections of Catecholamines on dP/dtmax
3.5. Effect of Bolus Injections of Catecholamines on the Heart Rate
3.6. Effect of Atenolol Infusion on the Increases in the Left Ventricle Developed Pressure (LVDP) Induced by Catecholamines
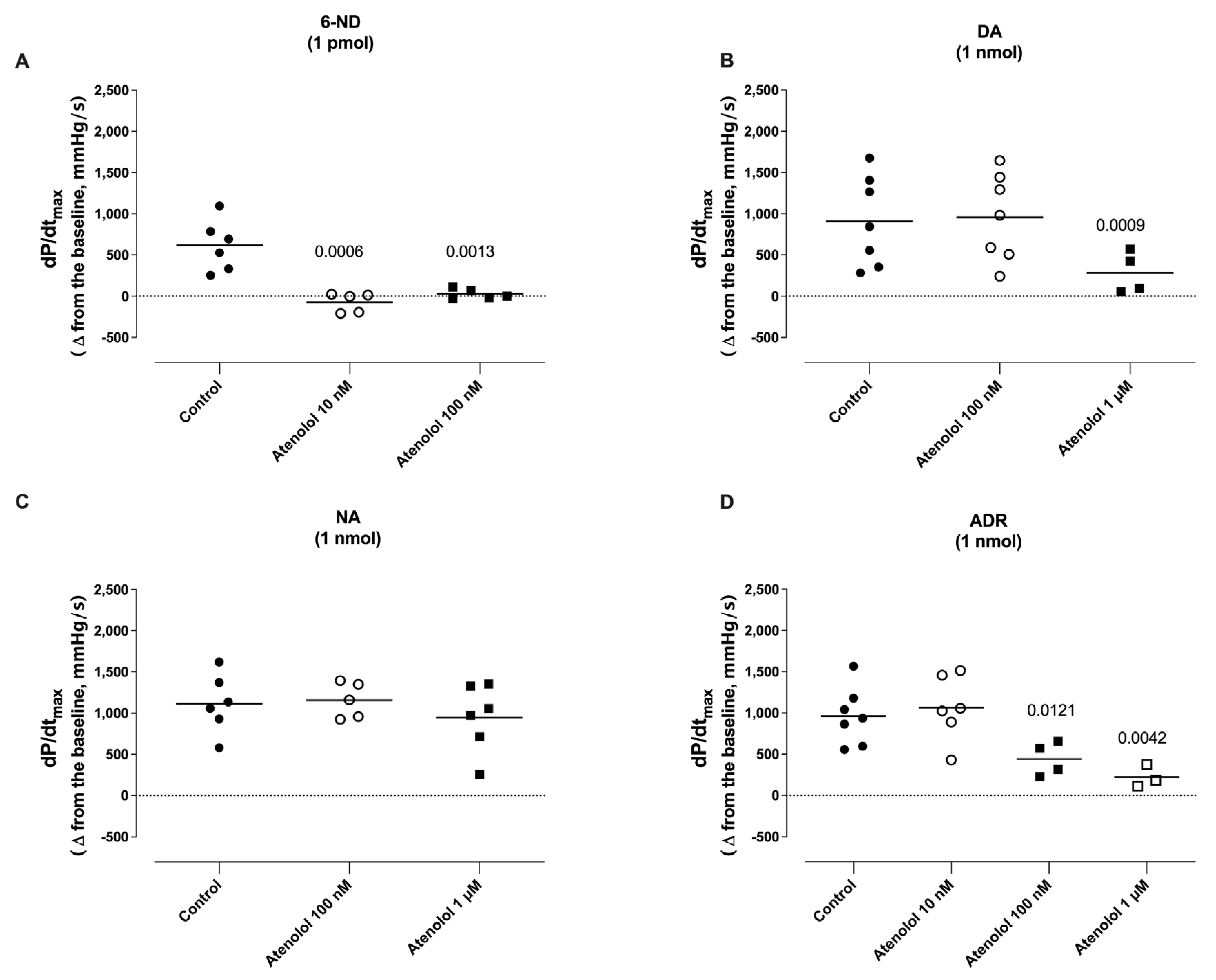
3.7. Effect of Atenolol Infusion on the Increases in dP/dtmax Induced by Catecholamines
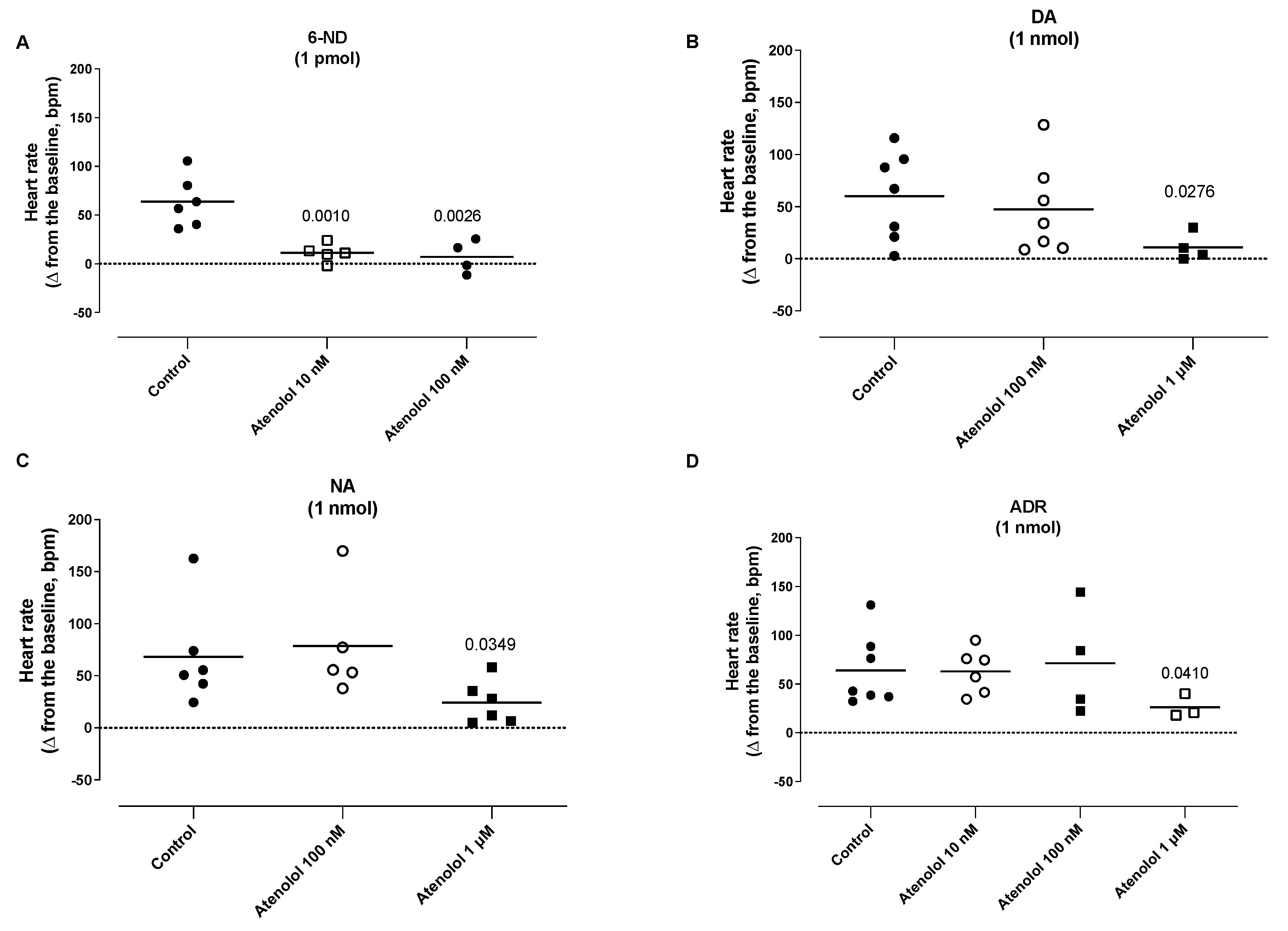
3.8. Effect of Atenolol Infusion on the Increases in the Heart Rate (HR) Induced by Catecholamines
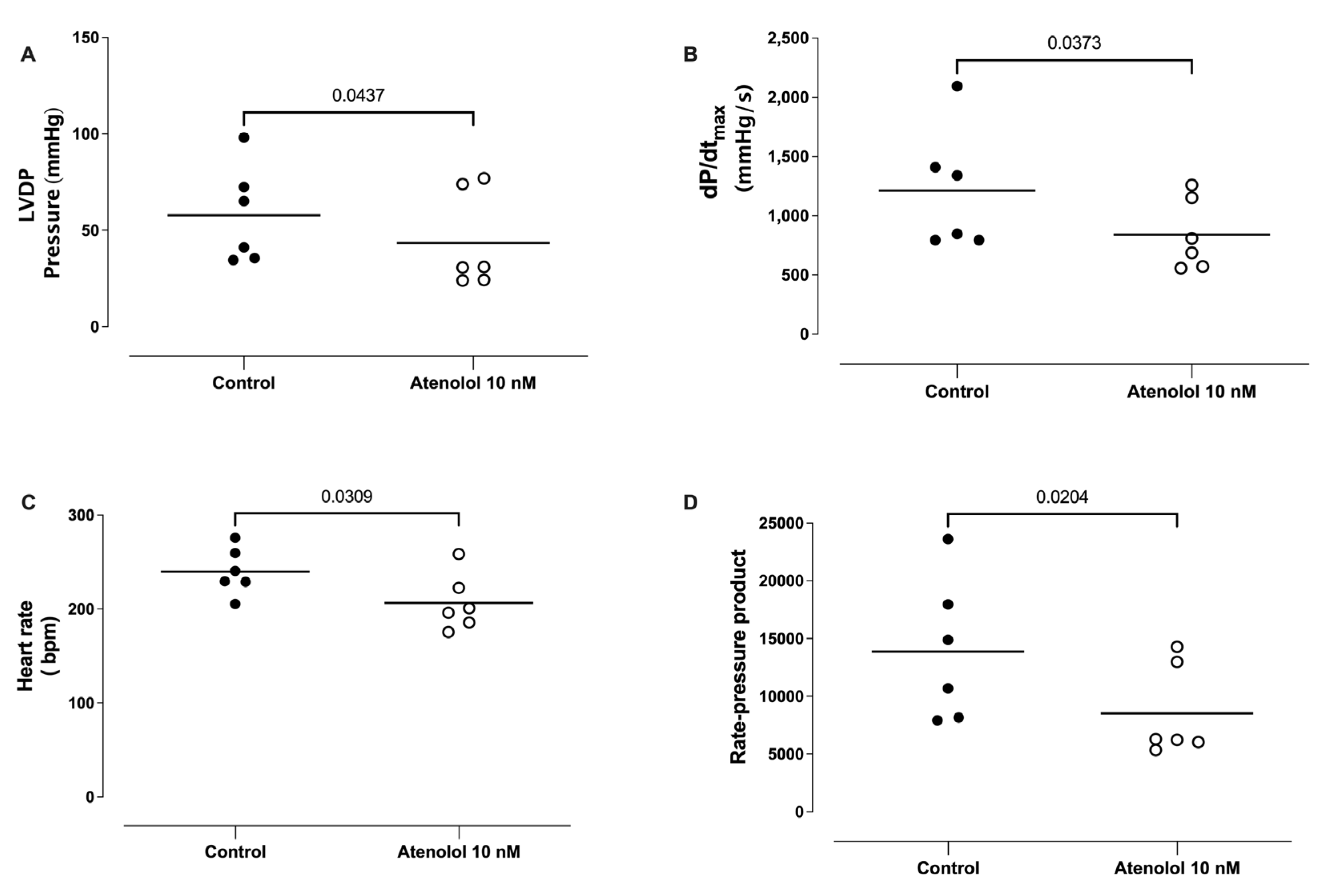
3.9. Effect of Atenolol Infusion on the Basal LVDP, dP/dtmax, HR, and Rate Pressure Product
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heitz, A.; Schwartz, J.; Velly, J. β-Adrenoceptors of the human myocardium: Determination of β1 and β2 subtypes by radioligand binding. Br. J. Pharmacol. 1983, 80, 711–717. [Google Scholar] [CrossRef]
- Juberg, E.N.; Minneman, K.P.; Abel, P.W. β1- and β2-adrenoceptor binding and functional response in right and left atria of rat heart. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1985, 330, 193–202. [Google Scholar] [CrossRef]
- Vago, T.; Bevilacqua, M.; Dagani, R.; Meroni, R.; Frigeni, G.; Santoli, C.; Norbiato, G. Comparison of rat and human left ventricle beta-adrenergic receptors: Subtype heterogeneity delineated by direct radioligand binding. Biochem. Biophys. Res. Commun. 1984, 121, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Y.; Fan, X.; Li, Z.; Cheng, Y. A pathway and network review on beta-adrenoceptor signaling and beta blockers in cardiac remodeling. Heart Fail. Rev. 2013, 19, 799–814. [Google Scholar] [CrossRef]
- Gauthier, C.; Leblais, V.; Kobzik, L.; Trochu, J.N.; Khandoudi, N.; Bril, A.; Balligand, J.L.; Le Marec, H. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J. Clin. Investig. 1998, 102, 1377–1384. [Google Scholar] [CrossRef]
- Vandecasteele, G.; Bedioune, I. Investigating cardiac β-adrenergic nuclear signaling with FRET-based biosensors. Ann. d’Endocrinologie 2021, 82, 198–200. [Google Scholar] [CrossRef]
- Fu, Q.; Chen, X.; Xiang, Y.K. Compartmentalization of β-adrenergic signals in cardiomyocytes. Trends Cardiovasc. Med. 2013, 23, 250–256. [Google Scholar] [CrossRef]
- Marzo, K.P.; Frey, M.J.; Wilson, J.R.; Liang, B.T.; Manning, D.R.; Lanoce, V.; Molinoff, P.B. Beta-adrenergic receptor-G protein-adenylate cyclase complex in experimental canine congestive heart failure produced by rapid ventricular pacing. Circ. Res. 1991, 69, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Zaccolo, M. Phosphodiesterases and compartmentalized cAMP signalling in the heart. Eur. J. Cell Biol. 2006, 85, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.K. Compartmentalization of β-Adrenergic Signals in Cardiomyocytes. Circ. Res. 2011, 109, 231–244. [Google Scholar] [CrossRef]
- Alousi, A.A.; Stankus, G.P.; Stuart, J.C.; Walton, L.H. Characterization of the Cardiotonic Effects of Milrinone, a New and Potent Cardiac Bipyridine, on Isolated Tissues from Several Animal Species. J. Cardiovasc. Pharmacol. 1983, 5, 804–811. [Google Scholar] [CrossRef]
- Dage, R.C.; Roebel, E.L.; Hsieh, C.P.; Weiner, D.L.; Woodward, J.K. Cardiovascular properties of a new cardiotonic agent: MDL 17,043 (1.3-dihydro-4-methyl-5-[4-(methylthio)-benzoyl]-2H-imidazol-2-one). J. Cardiovasc. Pharmacol. 1982, 4, 500–508. [Google Scholar] [CrossRef]
- Christ, T.; Engel, A.; Ravens, U.; Kaumann, A.J. Cilostamide potentiates more the positive inotropic effects of (−)-adrenaline through β2-adrenoceptors than the effects of (−)-noradrenaline through β1-adrenoceptors in human atrial myocardium. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2006, 374, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Britto-Júnior, J.; Coelho-Silva, W.C.; Murari, G.F.; Nash, C.E.S.; Mónica, F.Z.; Antunes, E.; De Nucci, G. 6-Nitrodopamine is released by human umbilical cord vessels and modulates vascular reactivity. Life Sci. 2021, 276, 119425. [Google Scholar] [CrossRef] [PubMed]
- Britto-Júnior, J.; Campos, R.; Peixoto, M.; Lima, A.T.; Jacintho, F.F.; Mónica, F.Z.; Moreno, R.A.; Antunes, E.; De Nucci, G. 6-Nitrodopamine is an endogenous selective dopamine receptor antagonist in Chelonoidis carbonaria aorta. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 260, 109403. [Google Scholar] [CrossRef] [PubMed]
- Britto-Júnior, J.; Lima, A.; Santos-Xavier, J.; Gonzalez, P.; Mónica, F.; Campos, R.; de Souza, V.; Schenka, A.; Antunes, E.; De Nucci, G. Relaxation of thoracic aorta and pulmonary artery rings of marmosets (Callithrix spp.) by endothelium-derived 6-nitrodopamine. Braz. J. Med. Biol. Res. 2023, 56, e12622. [Google Scholar] [CrossRef]
- Britto-Júnior, J.; da Silva-Filho, W.P.; Amorim, A.C.; Campos, R.; Moraes, M.O.; Moraes, M.E.A.; Fregonesi, A.; Monica, F.Z.; Antunes, E.; De Nucci, G. 6-nitrodopamine is a major endogenous modulator of human vas deferens contractility. Andrology 2022, 10, 1540–1547. [Google Scholar] [CrossRef]
- Britto-Júnior, J.; Ximenes, L.; Ribeiro, A.; Fregonesi, A.; Campos, R.; Kiguti, L.R.d.A.; Mónica, F.Z.; Antunes, E.; De Nucci, G. 6-Nitrodopamine is an endogenous mediator of rat isolated epididymal vas deferens contractions induced by electric-field stimulation. Eur. J. Pharmacol. 2021, 911, 174544. [Google Scholar] [CrossRef]
- Britto-Júnior, J.; de Oliveira, M.G.; Gati, C.d.R.; Campos, R.; Moraes, M.O.; Moraes, M.E.A.; Mónica, F.Z.; Antunes, E.; De Nucci, G. 6-NitroDopamine is an endogenous modulator of rat heart chronotropism. Life Sci. 2022, 307, 120879. [Google Scholar] [CrossRef]
- Britto-Júnior, J.; Prado, G.L.P.D.; Chiavegatto, S.; Cunha, F.; Moraes, M.O.; Moraes, M.E.A.; Monica, F.Z.; Antunes, E.; De Nucci, G. The importance of the endothelial nitric oxide synthase on the release of 6-nitrodopamine from mouse isolated atria and ventricles and their role on chronotropism. Nitric Oxide 2023, 138–139, 26–33. [Google Scholar] [CrossRef]
- Endoh, M.; Shimizu, T.; Yanagisawa, T. Characterization of adrenoceptors mediating positive inotropic responses in the ventricular myocardium of the dog. Br. J. Pharmacol. 1978, 64, 53–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Langendorff, O. Untersuchungen am überlebenden Säugethierherzen. Pflug. Arch. 1895, 61, 291–332. [Google Scholar] [CrossRef]
- Andersen, M.L. Guia Brasileiro de Produção, Manutenção ou Utilização de Animais em Atividade de Ensino ou Pesquisa Cientifica, Conselho Nacional de Controle de Experimentação Animal; Ministério da Ciência, Tecnologia e Inovação: Brasília, Brasil, 2016. [Google Scholar]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Ribeiro, O.M.; Antunes, E.; de Nucci, G.; Lovisolo, S.M.; Zatz, R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension 1992, 20, 298–303. [Google Scholar] [CrossRef] [PubMed]
- van Oene, J.C.; Sminia, P.; Mulder, A.H.; Horn, A.S. The purported dopamine agonist DPI inhibits [3H]noradrenaline release from rat cortical slices but not [3H]dopamine and [14C]acetylcholine release from rat striatal slices in-vitro. J. Pharm. Pharmacol. 1983, 35, 786–792. [Google Scholar] [CrossRef]
- Britto-Júnior, J.; Pinheiro, D.H.A.; Justo, A.F.O.; Murari, G.M.F.; Campos, R.; Mariano, F.V.; de Souza, V.B.; Schenka, A.A.; Mónica, F.Z.; Antunes, E.; et al. Endothelium-derived dopamine modulates EFS-induced contractions of human umbilical vessels. Pharmacol. Res. Perspect. 2020, 8, e00612. [Google Scholar] [CrossRef]
- Campos, R.; Pinheiro, D.H.A.; Britto-Júnior, J.; de Castro, H.A.; Mendes, G.D.; Moraes, M.O.; Moraes, M.E.A.; Lopes-Martins, R.B.; Antunes, N.J.; De Nucci, G. Quantification of 6-nitrodopamine in Krebs-Henseleit’s solution by LC-MS/MS for the assessment of its basal release from Chelonoidis carbonaria aortae in vitro. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1173, 122668. [Google Scholar] [CrossRef]
- Meesters, R.J.; Voswinkel, S. Bioanalytical Method Development and Validation: From the USFDA 2001 to the USFDA 2018 Guidance for Industry. J. Appl. Bioanal. 2018, 4, 67–73. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Cristofaro, M.; Breschi, M.C.; Calderone, V. Cardioprotective effects of different flavonoids against myocardial ischaemia/reperfusion injury in Langendorff-perfused rat hearts. J. Pharm. Pharmacol. 2013, 65, 750–756. [Google Scholar] [CrossRef]
- Arnhold, J.; Monzani, E.; Furtmüller, P.G.; Zederbauer, M.; Casella, L.; Obinger, C. Kinetics and Thermodynamics of Halide and Nitrite Oxidation by Mammalian Heme Peroxidases. Eur. J. Inorg. Chem. 2006, 2006, 3801–3811. [Google Scholar] [CrossRef]
- Burner, U.; Furtmüller, P.G.; Kettle, A.J.; Koppenol, W.H.; Obinger, C. Mechanism of Reaction of Myeloperoxidase with Nitrite. J. Biol. Chem. 2000, 275, 20597–20601. [Google Scholar] [CrossRef] [PubMed]
- Moreno, H.; Metze, K.; Bento, A.C.; Antunes, E.; Zatz, R.; Nucci, G. Chronic nitric oxide inhibition as a model of hypertensive heart muscle disease. Basic Res. Cardiol. 1996, 91, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Amrani, M.; O’Shea, J.; Allen, N.J.; Harding, S.E.; Jaäyakumar, J.; Pepper, J.R.; Moncada, S.; Yacoub, M.H. Role of basal release of nitric oxide on coronary flow and mechanical performance of the isolated rat heart. J Physiol. 1992, 456, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Preckel, B.; Kojda, G.; Schlack, W.; Ebel, D.; Kottenberg, K.; Noack, E.; Thämer, V. Inotropic Effects of Glyceryl Trinitrate and Spontaneous NO Donors in the Dog Heart. Circulation 1997, 96, 2675–2682. [Google Scholar] [CrossRef]
- Kojda, G.; Kottenberg, K.; Nix, P.; Schlüter, K.D.; Piper, H.M.; Noack, E. Low Increase in cGMP Induced by Organic Nitrates and Nitrovasodilators Improves Contractile Response of Rat Ventricular Myocytes. Circ. Res. 1996, 78, 91–101. [Google Scholar] [CrossRef]
- Brady, A.J.; Warren, J.B.; Poole-Wilson, P.A.; Williams, T.J.; Harding, S.E. Nitric oxide attenuates cardiac myocyte contraction. Am. J. Physiol. Circ. Physiol. 1993, 265, H176–H182. [Google Scholar] [CrossRef]
- Müller-Strahl, G.; Kottenberg, K.; Zimmer, H.; Noack, E.; Kojda, G. Inhibition of nitric oxide synthase augments the positive inotropic effect of nitric oxide donors in the rat heart. J. Physiol. 2000, 522, 311–320. [Google Scholar] [CrossRef]
- Klabunde, R.E.; Kimber, N.D.; Kuk, J.E.; Helgren, M.C.; Förstermann, U. NGMethyl-L-arginine decreases contractility, cGMP and cAMP in isoproterenol-stimulated rat hearts in vitro. Eur. J. Pharmacol. 1992, 223, 1–7. [Google Scholar] [CrossRef]
- Cotton, J.M.; Kearney, M.T.; MacCarthy, P.A.; Grocott-Mason, R.M.; McClean, D.R.; Heymes, C.; Richardson, P.J.; Shah, A.M. Effects of nitric oxide synthase inhibition on Basal function and the force-frequency relationship in the normal and failing human heart in vivo. Circulation 2001, 104, 2318–2323. [Google Scholar] [CrossRef]
- Stamler, J.S.; Loh, E.; Roddy, A.M.; Currie, E.K.; Creager, A.M. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation 1994, 89, 2035–2040. [Google Scholar] [CrossRef]
- Gardiner, S.; Compton, A.; Kemp, P.; Bennett, T. Regional and cardiac haemodynamic effects of NG-nitro-l-arginine methyl ester in conscious, Long Evans rats. Br. J. Pharmacol. 1990, 101, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Brett, S.E.; Cockcroft, J.R.; Mant, T.G.; Ritter, J.M.; Chowienczyk, P.J. Haemodynamic effects of inhibition of nitric oxide synthase and of L-arginine at rest and during exercise. J. Hypertens. 1998, 16, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Kiely, D.G.; Lee, A.F.C.; Struthers, A.D.; Lipworth, B.J. Nitric oxide: An important role in the maintenance of systemic and pulmonary vascular tone in man. Br. J. Clin. Pharmacol. 1998, 46, 263–266. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zivkovic, V.; Djuric, D.; Turjacanin-Pantelic, D.; Marinkovic, Z.; Stefanovic, D.; Srejovic, I.; Jakovljevic, V. The effects of cyclooxygenase and nitric oxide synthase inhibition on cardiodynamic parameters and coronary flow in isolated rat hearts. Exp. Clin. Cardiol. 2013, 18, e102–e110. [Google Scholar]
- Rassaf, T.; Poll, L.W.; Brouzos, P.; Lauer, T.; Totzeck, M.; Kleinbongard, P.; Gharini, P.; Andersen, K.; Schulz, R.; Heusch, G.; et al. Positive effects of nitric oxide on left ventricular function in humans. Eur. Heart J. 2006, 27, 1699–1705. [Google Scholar] [CrossRef]
- Brillante, D.G.; O’sullivan, A.J.; Johnstone, M.T.; Howes, L.G. Predictors of inotropic and chronotropic effects ofN G-monomethyl-l-arginine. Eur. J. Clin. Investig. 2009, 39, 273–279. [Google Scholar] [CrossRef]
- Marck, P.V.; Pierre, S.V. Na/K-ATPase Signaling and Cardiac Pre/Postconditioning with Cardiotonic Steroids. Int. J. Mol. Sci. 2018, 19, 2336. [Google Scholar] [CrossRef]
- Sukoyan, G.V.; Berberashvili, T.M.; Karsanov, N.V. Submolecular mechanisms underlying in vitro and in vivo effect of cardiac glycosides on contractile activity of myocardial myofibrils during heart failure. Bull. Exp. Biol. Med. 2006, 141, 424–426. [Google Scholar] [CrossRef]
- Khan, H.; Metra, M.; Blair, J.E.A.; Vogel, M.; Harinstein, M.E.; Filippatos, G.S.; Sabbah, H.N.; Porchet, H.; Valentini, G.; Gheorghiade, M. Istaroxime, a first in class new chemical entity exhibiting SERCA-2 activation and Na–K-ATPase inhibition: A new promising treatment for acute heart failure syndromes? Heart Fail. Rev. 2009, 14, 277–287. [Google Scholar] [CrossRef]
- Patocka, J.; Nepovimova, E.; Wu, W.; Kuca, K. Digoxin: Pharmacology and toxicology—A review. Environ. Toxicol. Pharmacol. 2020, 79, 103400. [Google Scholar] [CrossRef]
- Bossu, A.; Kostense, A.; Beekman, H.D.; Houtman, M.J.; van der Heyden, M.A.; Vos, M.A. Istaroxime, a positive inotropic agent devoid of proarrhythmic properties in sensitive chronic atrioventricular block dogs. Pharmacol. Res. 2018, 133, 132–140. [Google Scholar] [CrossRef]
- Pathak, A.; Lebrin, M.; Vaccaro, A.; Senard, J.M.; Despas, F. Pharmacology of levosimendan: Inotropic, vasodilatory and cardioprotective effects. J. Clin. Pharm. Ther. 2013, 38, 341–349. [Google Scholar] [CrossRef]
- Szilágyi, S.; Pollesello, P.; Levijoki, J.; Kaheinen, P.; Haikala, H.; Édes, I.; Papp, Z. The effects of levosimendan and OR-1896 on isolated hearts, myocyte-sized preparations and phosphodiesterase enzymes of the guinea pig. Eur. J. Pharmacol. 2004, 486, 67–74. [Google Scholar] [CrossRef]
- Despas, F.; Trouillet, C.; Franchitto, N.; Labrunee, M.; Galinier, M.; Senard, J.-M.; Pathak, A. Levosimedan improves hemodynamics functions without sympathetic activation in severe heart failure patients: Direct evidence from sympathetic neural recording. Acute Card. Care 2009, 12, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Honerjager, P. Pharmacology of positive inotropic phosphodiesterase III inhibitors. Eur. Heart J. 1989, 10, 25–31. [Google Scholar] [CrossRef]
- Overgaard, C.B.; Džavík, V. Inotropes and Vasopressors: Review of Physiology and Clinical Use in Cardiovascular Disease. Circulation 2008, 118, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Cone, J.; Wang, S.; Tandon, N.; Fong, M.; Sun, B.; Sakurai, K.; Yoshitake, M.; Kambayashi, J.-I.; Liu, Y. Comparison of the Effects of Cilostazol and Milrinone on Intracellular cAMP Levels and Cellular Function in Platelets and Cardiac Cells. J. Cardiovasc. Pharmacol. 1999, 34, 497–504. [Google Scholar] [CrossRef]
- Britto-Júnior, J.; Lima, A.T.; Fuguhara, V.; Monica, F.Z.; Antunes, E.; De Nucci, G. Investigation on the positive chronotropic action of 6-nitrodopamine in the rat isolated atria. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 1279–1290. [Google Scholar] [CrossRef]
- Nash, C.E.S.; Antunes, N.J.; Coelho-Silva, W.C.; Campos, R.; De Nucci, G. Quantification of cyclic AMP and cyclic GMP levels in Krebs-Henseleit solution by LC-MS/MS: Application in washed platelet aggregation samples. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2022, 1211, 123472. [Google Scholar] [CrossRef]
- Lefkowitz, R.J.; Rockman, H.A.; Koch, W.J. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation 2000, 101, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Motiejunaite, J.; Amar, L.; Vidal-Petiot, E. Adrenergic receptors and cardiovascular effects of catecholamines. Ann. d’Endocrinologie 2020, 82, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart. J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Sinnenberg, L.; Givertz, M.M. Acute heart failure. Trends Cardiovasc. Med. 2020, 30, 104–112. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, 1810–1852. [Google Scholar] [CrossRef] [PubMed]
- The Xamoterol in Severe Heart Failure Study Group. Xamoterol in severe heart failure. Lancet 1990, 336, 1–6, Erratum in Lancet 1990, 336, 698. [Google Scholar] [CrossRef]
- Njoroge, J.N.; Teerlink, J.R. Pathophysiology and Therapeutic Approaches to Acute Decompensated Heart Failure. Circ. Res. 2021, 128, 1468–1486. [Google Scholar] [CrossRef]



| Suppliers | Chemicals |
|---|---|
| Sigma-Aldrich Chemicals Co. (MO, USA) | Dopamine, adrenaline, and L-NAME (Nω-Nitro-L-arginine methyl ester hydrochloride) |
| Cayman Chemicals (MI, USA) | Noradrenaline and tetrodotoxin (TTX) |
| Toronto Research Chemicals (ON, Canada). | 6-Nitrodopamine-d4 and 6-Nitrodopamine |
| CDN Isotopes (QC, Canada) | Adrenaline-d6 hydrochloride, DL-noradrenaline-d6 hydrochloride, and dopamine-d3 hydrochloride, |
| Merck KGaA (Hesse, Germany) | Sodium chloride (NaCl), dextrose, calcium chloride (CaCl2), magnesium sulfate (MgSO4), sodium bicarbonate (NaHCO3), potassium chloride (KCl), potassium phosphate monobasic (KH2PO4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Britto-Júnior, J.; Medeiros-Teixeira, L.R.; Lima, A.T.; Dassow, L.C.; Lopes-Martins, R.Á.B.; Campos, R.; Moraes, M.O.; Moraes, M.E.A.; Antunes, E.; De Nucci, G. 6-Nitrodopamine Is the Most Potent Endogenous Positive Inotropic Agent in the Isolated Rat Heart. Life 2023, 13, 2012. https://doi.org/10.3390/life13102012
Britto-Júnior J, Medeiros-Teixeira LR, Lima AT, Dassow LC, Lopes-Martins RÁB, Campos R, Moraes MO, Moraes MEA, Antunes E, De Nucci G. 6-Nitrodopamine Is the Most Potent Endogenous Positive Inotropic Agent in the Isolated Rat Heart. Life. 2023; 13(10):2012. https://doi.org/10.3390/life13102012
Chicago/Turabian StyleBritto-Júnior, José, Lincoln Rangel Medeiros-Teixeira, Antonio Tiago Lima, Letícia Costa Dassow, Rodrigo Álvaro Brandão Lopes-Martins, Rafael Campos, Manoel Odorico Moraes, Maria Elisabete A. Moraes, Edson Antunes, and Gilberto De Nucci. 2023. "6-Nitrodopamine Is the Most Potent Endogenous Positive Inotropic Agent in the Isolated Rat Heart" Life 13, no. 10: 2012. https://doi.org/10.3390/life13102012
APA StyleBritto-Júnior, J., Medeiros-Teixeira, L. R., Lima, A. T., Dassow, L. C., Lopes-Martins, R. Á. B., Campos, R., Moraes, M. O., Moraes, M. E. A., Antunes, E., & De Nucci, G. (2023). 6-Nitrodopamine Is the Most Potent Endogenous Positive Inotropic Agent in the Isolated Rat Heart. Life, 13(10), 2012. https://doi.org/10.3390/life13102012





