Artificial Intelligence to Early Predict Liver Metastases in Patients with Colorectal Cancer: Current Status and Future Prospectives
Abstract
:1. Introduction
2. Material and Methods
3. Results
4. Discussion
4.1. Colorectal Cancer and Liver Metastases
4.2. Artificial Intelligence
4.3. Chemotherapy Regimen
4.4. Current Status and Future Perspectives
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rocca, A.; Cipriani, F.; Belli, G.; Berti, S.; Boggi, U.; Bottino, V.; Cillo, U.; Cescon, M.; Cimino, M.; Corcione, F.; et al. The Italian Consensus on minimally invasive simultaneous resections for synchronous liver metastasis and primary colorectal cancer: A Delphi methodology. Updates Surg. 2021, 73, 1247–1265. [Google Scholar] [CrossRef] [PubMed]
- Taberna, M.; Gil Moncayo, F.; Jané-Salas, E.; Antonio, M.; Arribas, L.; Vilajosana, E.; Peralvez Torres, E.; Mesía, R. The Multidisciplinary Team (MDT) Approach and Quality of Care. Front. Oncol. 2020, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Tebala, G.D.; Bond-Smith, G. Multidisciplinary treatment of cancer. Updates Surg. 2021, 73, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Surci, N.; Marchegiani, G.; Salvia, R. Response to: “Multidisciplinary treatment of cancer”. Updates Surg. 2021, 73, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Aldrighetti, L.; Guzzetti, E.; Pulitanò, C.; Cipriani, F.; Catena, M.; Paganelli, M.; Ferla, G. Case-matched analysis of totally laparoscopic versus open liver resection for HCC: Short and middle term results. J. Surg. Oncol. 2010, 102, 82–86. [Google Scholar] [CrossRef]
- Marte, G.; Scuderi, V.; Rocca, A.; Surfaro, G.; Migliaccio, C.; Ceriello, A. Laparoscopic splenectomy: A single center experience. Unusual cases and expanded inclusion criteria for laparoscopic approach. Updates Surg. 2013, 65, 115–119. [Google Scholar] [CrossRef]
- Casadei, R.; Ricci, C.; D’Ambra, M.; Marrano, N.; Alagna, V.; Rega, D.; Monari, F.; Minni, F. Laparoscopic versus open distal pancreatectomy in pancreatic tumours: A case-control study. Updates Surg. 2010, 62, 171–174. [Google Scholar] [CrossRef]
- Mege, D.; Frontali, A.; Pellino, G.; Adegbola, S.; Maggiori, L.; Warusavitarne, J.; Panis, Y. Laparoscopic subtotal colectomy with double-end ileosigmoidostomy in right iliac fossa facilitates second-stage surgery in patients with inflammatory bowel disease. Surg. Endosc. 2020, 34, 186–191. [Google Scholar] [CrossRef]
- Rocca, A.; Scacchi, A.; Cappuccio, M.; Avella, P.; Bugiantella, W.; De Rosa, M.; Costa, G.; Polistena, A.; Codacci-Pisanelli, M.; Amato, B.; et al. Robotic surgery for colorectal liver metastases resection: A systematic review. Int. J. Med. Robot. 2021, 17, e2330. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Costa, G.; De Rosa, M.; Codacci Pisanelli, M.; Frezza, B.; De Prizio, M.; Bravi, I.; Scacchi, A.; Gallo, G.; Amato, B.; et al. Minimally Invasive Approach to Gastric GISTs: Analysis of a Multicenter Robotic and Laparoscopic Experience with Literature Review. Cancers 2021, 13, 4351. [Google Scholar] [CrossRef]
- Giuliani, A.; Avella, P.; Segreto, A.L.; Izzo, M.L.; Buondonno, A.; Coluzzi, M.; Cappuccio, M.; Brunese, M.C.; Vaschetti, R.; Scacchi, A.; et al. Postoperative Outcomes Analysis After Pancreatic Duct Occlusion: A Safe Option to Treat the Pancreatic Stump After Pancreaticoduodenectomy in Low-Volume Centers. Front. Surg. 2021, 8, 804675. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Porfidia, C.; Russo, R.; Tamburrino, A.; Avella, P.; Vaschetti, R.; Bianco, P.; Calise, F. Neuraxial anesthesia in hepato-pancreatic-bilio surgery: A first western pilot study of 46 patients. Updates Surg. 2023, 75, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Buondonno, A.; Avella, P.; Cappuccio, M.; Scacchi, A.; Vaschetti, R.; Di Marzo, G.; Maida, P.; Luciani, C.; Amato, B.; Brunese, M.C.; et al. A Hub and Spoke Learning Program in Bariatric Surgery in a Small Region of Italy. Front. Surg. 2022, 9, 855527. [Google Scholar] [CrossRef] [PubMed]
- Busnatu, Ș.; Niculescu, A.G.; Bolocan, A.; Petrescu, G.E.D.; Păduraru, D.N.; Năstasă, I.; Lupușoru, M.; Geantă, M.; Andronic, O.; Grumezescu, A.M.; et al. Clinical Applications of Artificial Intelligence-An Updated Overview. J. Clin. Med. 2022, 11, 2265. [Google Scholar] [CrossRef] [PubMed]
- Santone, A.; Belfiore, M.P.; Mercaldo, F.; Varriano, G.; Brunese, L. On the Adoption of Radiomics and Formal Methods for COVID-19 Coronavirus Diagnosis. Diagnostics 2021, 11, 293. [Google Scholar] [CrossRef]
- Carrano, F.M.; Sileri, P.; Batt, S.; Di Lorenzo, N. Blockchain in surgery: Are we ready for the digital revolution? Updates Surg. 2022, 74, 3–6. [Google Scholar] [CrossRef]
- Viganò, L.; Ammirabile, A.; Zwanenburg, A. Radiomics in liver surgery: Defining the path toward clinical application. Updates Surg. 2023, 75, 1387–1390. [Google Scholar] [CrossRef]
- Taghavi, M.; Trebeschi, S.; Simões, R.; Meek, D.B.; Beckers, R.C.J.; Lambregts, D.M.J.; Verhoef, C.; Houwers, J.B.; van der Heide, U.A.; Beets-Tan, R.G.H.; et al. Machine learning-based analysis of CT radiomics model for prediction of colorectal metachronous liver metastases. Abdom. Radiol. 2021, 46, 249–256. [Google Scholar] [CrossRef]
- Santone, A.; Brunese, M.C.; Donnarumma, F.; Guerriero, P.; Mercaldo, F.; Reginelli, A.; Miele, V.; Giovagnoni, A.; Brunese, L. Radiomic features for prostate cancer grade detection through formal verification. Radiol. Med. 2021, 126, 688–697. [Google Scholar] [CrossRef]
- Brunese, L.; Mercaldo, F.; Reginelli, A.; Santone, A. Formal methods for prostate cancer Gleason score and treatment prediction using radiomic biomarkers. Magn. Reson. Imaging 2020, 66, 165–175. [Google Scholar] [CrossRef]
- Rocca, A.; Brunese, M.C.; Santone, A.; Avella, P.; Bianco, P.; Scacchi, A.; Scaglione, M.; Bellifemine, F.; Danzi, R.; Varriano, G.; et al. Early Diagnosis of Liver Metastases from Colorectal Cancer through CT Radiomics and Formal Methods: A Pilot Study. J. Clin. Med. 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Stollmayer, R.; Budai, B.K.; Tóth, A.; Kalina, I.; Hartmann, E.; Szoldán, P.; Bérczi, V.; Maurovich-Horvat, P.; Kaposi, P.N. Diagnosis of focal liver lesions with deep learning-based multi-channel analysis of hepatocyte-specific contrast-enhanced magnetic resonance imaging. World J. Gastroenterol. 2021, 27, 5978–5988. [Google Scholar] [CrossRef] [PubMed]
- Schlanger, D.; Graur, F.; Popa, C.; Moiș, E.; Al Hajjar, N. The role of artificial intelligence in pancreatic surgery: A systematic review. Updates Surg. 2022, 74, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Laino, M.E.; Fiz, F.; Morandini, P.; Costa, G.; Maffia, F.; Giuffrida, M.; Pecorella, I.; Gionso, M.; Wheeler, D.R.; Cambiaghi, M.; et al. A virtual biopsy of liver parenchyma to predict the outcome of liver resection. Updates Surg. 2023, 75, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Barretta, M.L.; Picone, C.; Avallone, A.; Belli, A.; Patrone, R.; Ferrante, M.; Cozzi, D.; Grassi, R.; et al. Radiomics in hepatic metastasis by colorectal cancer. Infect. Agent. Cancer 2021, 16, 39. [Google Scholar] [CrossRef]
- Adam, R.; de Gramont, A.; Figueras, J.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; Sobrero, A.; et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat. Rev. 2015, 41, 729–741. [Google Scholar] [CrossRef]
- Conticchio, M.; Maggialetti, N.; Rescigno, M.; Brunese, M.C.; Vaschetti, R.; Inchingolo, R.; Calbi, R.; Ferraro, V.; Tedeschi, M.; Fantozzi, M.R.; et al. Hepatocellular Carcinoma with Bile Duct Tumor Thrombus: A Case Report and Literature Review of 890 Patients Affected by Uncommon Primary Liver Tumor Presentation. J. Clin. Med. 2023, 12, 423. [Google Scholar] [CrossRef]
- Avella, P.; Vaschetti, R.; Cappuccio, M.; Gambale, F.; Meis, L.D.E.; Rafanelli, F.; Brunese, M.C.; Guerra, G.; Scacchi, A.; Rocca, A. The role of liver surgery in simultaneous synchronous colorectal liver metastases and colorectal cancer resections: A literature review of 1730 patients underwent open and minimally invasive surgery. Minerva Surg. 2022, 77, 582–590. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Mattace Raso, M.; Gabelloni, M.; Avallone, A.; Ottaiano, A.; Tatangelo, F.; Brunese, M.C.; et al. Radiomics and Machine Learning Analysis Based on Magnetic Resonance Imaging in the Assessment of Colorectal Liver Metastases Growth Pattern. Diagnostics 2022, 12, 1115. [Google Scholar] [CrossRef]
- Viganò, L.; Jayakody Arachchige, V.S.; Fiz, F. Is precision medicine for colorectal liver metastases still a utopia? New perspectives by modern biomarkers, radiomics, and artificial intelligence. World J. Gastroenterol. 2022, 28, 608–623. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Andolfi, E.; Fontani, A.; Calise, F.; Rocca, A.; Giuliani, A. Robot-assisted liver surgery in a general surgery unit with a “Referral Centre Hub&Spoke Learning Program”. Early outcomes after our first 70 consecutive patients. Minerva Chir. 2018, 73, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Rompianesi, G.; Pegoraro, F.; Ceresa, C.D.; Montalti, R.; Troisi, R.I. Artificial intelligence in the diagnosis and management of colorectal cancer liver metastases. World J. Gastroenterol. 2022, 28, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Capretti, G.; Bonifacio, C.; De Palma, C.; Nebbia, M.; Giannitto, C.; Cancian, P.; Laino, M.E.; Balzarini, L.; Papanikolaou, N.; Savevski, V.; et al. A machine learning risk model based on preoperative computed tomography scan to predict postoperative outcomes after pancreatoduodenectomy. Updates Surg. 2022, 74, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chai, F.; Wei, J.; Yue, Y.; Cheng, J.; Gu, D.; Zhang, Y.; Tong, T.; Sheng, W.; Hong, N.; et al. Identification of Predominant Histopathological Growth Patterns of Colorectal Liver Metastasis by Multi-Habitat and Multi-Sequence Based Radiomics Analysis. Front. Oncol. 2020, 10, 1363. [Google Scholar] [CrossRef]
- Aleotti, F.; Nano, R.; Piemonti, L.; Falconi, M.; Balzano, G. Total pancreatectomy sequelae and quality of life: Results of islet autotransplantation as a possible mitigation strategy. Updates Surg. 2021, 73, 1237–1246. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Li, Y.; Gong, J.; Shen, X.; Li, M.; Zhang, H.; Feng, F.; Tong, T. Assessment of Primary Colorectal Cancer CT Radiomics to Predict Metachronous Liver Metastasis. Front. Oncol. 2022, 12, 861892. [Google Scholar] [CrossRef]
- Shu, Z.; Fang, S.; Ding, Z.; Mao, D.; Cai, R.; Chen, Y.; Pang, P.; Gong, X. MRI-based Radiomics nomogram to detect primary rectal cancer with synchronous liver metastases. Sci. Rep. 2019, 9, 3374. [Google Scholar] [CrossRef]
- Liang, M.; Cai, Z.; Zhang, H.; Huang, C.; Meng, Y.; Zhao, L.; Li, D.; Ma, X.; Zhao, X. Machine Learning-based Analysis of Rectal Cancer MRI Radiomics for Prediction of Metachronous Liver Metastasis. Acad. Radiol. 2019, 26, 1495–1504. [Google Scholar] [CrossRef]
- Devoto, L.; Ganeshan, B.; Keller, D.; Groves, A.; Endozo, R.; Arulampalam, T.; Chand, M. Using texture analysis in the development of a potential radiomic signature for early identification of hepatic metastasis in colorectal cancer. Eur. J. Radiol. Open 2022, 9, 100415. [Google Scholar] [CrossRef] [PubMed]
- Biglarian, A.; Bakhshi, E.; Gohari, M.R.; Khodabakhshi, R. Artificial neural network for prediction of distant metastasis in colorectal cancer. Asian Pac. J. Cancer Prev. 2012, 13, 927–930. [Google Scholar] [CrossRef] [PubMed]
- AmirHosseini, B.; Hosseini, R. An improved fuzzy-differential evolution approach applied to classification of tumors in liver CT scan images. Med. Biol. Eng. Comput. 2019, 57, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ma, X.; Shen, F.; Xia, Y.; Jia, Y.; Lu, J. MRI-based radiomics nomogram to predict synchronous liver metastasis in primary rectal cancer patients. Cancer Med. 2020, 9, 5155–5163. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choe, E.K.; Kim, S.Y.; Kim, H.S.; Park, K.J.; Kim, D. Liver imaging features by convolutional neural network to predict the metachronous liver metastasis in stage I-III colorectal cancer patients based on preoperative abdominal CT scan. BMC Bioinform. 2020, 21 (Suppl. S13), 382. [Google Scholar] [CrossRef]
- Goehler, A.; Harry Hsu, T.M.; Lacson, R.; Gujrathi, I.; Hashemi, R.; Chlebus, G.; Szolovits, P.; Khorasani, R. Three-Dimensional Neural Network to Automatically Assess Liver Tumor Burden Change on Consecutive Liver MRIs. J. Am. Coll. Radiol. 2020, 17, 1475–1484. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Guo, Y.; Miao, Z.; Liu, X.; Guo, S.; Zhang, H. Development and assessment of an individualized nomogram to predict colorectal cancer liver metastases. Quant. Imaging Med. Surg. 2020, 10, 397–414. [Google Scholar] [CrossRef]
- Lee, H.; Hong, H.; Bae, H.; Lim, J.S.; Kim, J. Classification of focal liver lesions in CT images using convolutional neural networks with lesion information augmented patches and synthetic data augmentation. Med. Phys. 2021, 48, 5029–5046. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.; Han, K.; Bae, H.; Shin, J.; Lim, J.S. Diagnostic Performance of Deep Learning-Based Lesion Detection Algorithm in CT for Detecting Hepatic Metastasis from Colorectal Cancer. Korean J. Radiol. 2021, 22, 912–921. [Google Scholar] [CrossRef]
- Vicini, S.; Bortolotto, C.; Rengo, M.; Ballerini, D.; Bellini, D.; Carbone, I.; Preda, L.; Laghi, A.; Coppola, F.; Faggioni, L. A narrative review on current imaging applications of artificial intelligence and radiomics in oncology: Focus on the three most common cancers. Radiol. Med. 2022, 127, 819–836. [Google Scholar] [CrossRef]
- De Robertis, R.; Geraci, L.; Tomaiuolo, L.; Bortoli, L.; Beleù, A.; Malleo, G.; D’Onofrio, M. Liver metastases in pancreatic ductal adenocarcinoma: A predictive model based on CT texture analysis. Radiol. Med. 2022, 127, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Palumbo, D.; De Cobelli, F.; Fiorino, C. Does radiomics play a role in the diagnosis, staging and re-staging of gastroesophageal junction adenocarcinoma? Updates Surg. 2023, 75, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, X.; Wang, Y.; Ding, X. Prognosis prediction of extremity and trunk wall soft-tissue sarcomas treated with surgical resection with radiomic analysis based on random survival forest. Updates Surg. 2022, 74, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A deep look into radiomics. Radiol. Med. 2021, 126, 1296–1311. [Google Scholar] [CrossRef]
- Gurgitano, M.; Angileri, S.A.; Rodà, G.M.; Liguori, A.; Pandolfi, M.; Ierardi, A.M.; Wood, B.J.; Carrafiello, G. Interventional Radiology ex-machina: Impact of Artificial Intelligence on practice. Radiol. Med. 2021, 126, 998–1006. [Google Scholar] [CrossRef]
- Brunese, L.; Brunese, M.C.; Carbone, M.; Ciccone, V.; Mercaldo, F.; Santone, A. Automatic PI-RADS assignment by means of formal methods. Radiol. Med. 2022, 127, 83–89. [Google Scholar] [CrossRef]
- Vigano, L.; Galvanin, J.; Poretti, D.; Del Fabbro, D.; Gentile, D.; Pedicini, V.; Solbiati, L.; Torzilli, G. Percutaneous ablation of post-surgical solitary early recurrence of colorectal liver metastases is an effective “test-of-time” approach. Updates Surg. 2021, 73, 1349–1358. [Google Scholar] [CrossRef]
- Zhou, F.; Ding, J. Prognosis and factors affecting colorectal cancer with ovarian metastasis. Updates Surg. 2021, 73, 391–398. [Google Scholar] [CrossRef]
- Loffredo, D.; Marvaso, A.; Ceraso, S.; Cinelli, N.; Rocca, A.; Vitale, M.; Rossi, M.; Genovese, E.; Amato, B.; Cinelli, M. Minimal invasive surgery in treatment of liver metastases from colorectal carcinomas: Case studies and survival rates. BMC Surg. 2013, 13 (Suppl. 2), S45. [Google Scholar] [CrossRef]
- Xu, J.M.; Zhu, D.X.; Ren, L. Surgical treatment of colorectal liver metastases. Zhonghua Wai Ke Za Zhi 2017, 55, 491–495. [Google Scholar] [CrossRef]
- Sena, G.; Picciariello, A.; Marino, F.; Goglia, M.; Rocca, A.; Meniconi, R.L.; Gallo, G. One-Stage Total Laparoscopic Treatment for Colorectal Cancer With Synchronous Metastasis. Is It Safe and Feasible? Front. Surg. 2021, 8, 752135. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Chee, Y.; Rasheed, S.; Cunningham, D.; Bhogal, R.H.; Jiao, L.; Tekkis, P.; Kontovounisios, C. Which surgical strategy for colorectal cancer with synchronous hepatic metastases provides the best outcome? A comparison between primary first, liver first and simultaneous approach. Updates Surg. 2022, 74, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Masetti, M.; Fallani, G.; Ratti, F.; Ferrero, A.; Giuliante, F.; Cillo, U.; Guglielmi, A.; Ettorre, G.M.; Torzilli, G.; Vincenti, L.; et al. Minimally invasive treatment of colorectal liver metastases: Does robotic surgery provide any technical advantages over laparoscopy? A multicenter analysis from the IGoMILS (Italian Group of Minimally Invasive Liver Surgery) registry. Updates Surg. 2022, 74, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Torzilli, G.; Sorrentino, M.; Donadon, M. Horseshoe hepatectomy: Another step pursuing the concept of parenchyma sparing major hepatectomies. Updates Surg. 2022, 74, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Rocca, A.; De Rosa, M.; Fontani, A.; Ermili, F.; Andolfi, E.; Bugiantella, W.; Levi Sandri, G.B. Minimally invasive robotic-assisted combined colorectal and liver excision surgery: Feasibility, safety and surgical technique in a pilot series. Updates Surg. 2021, 73, 1015–1022. [Google Scholar] [CrossRef]
- Wang, H.W.; Jin, K.M.; Li, J.; Wang, K.; Xing, B.C. Postoperative complications predict poor outcomes only in patients with a low modified clinical score after resection of colorectal liver metastases: A retrospective cohort study. Updates Surg. 2022, 74, 1601–1610. [Google Scholar] [CrossRef]
- Milana, F.; Galvanin, J.; Sommacale, D.; Brustia, R. Left hepatectomy and microwave ablation for bilobar colorectal metastases: Video description of a “complicated” robotic approach. Updates Surg. 2022, 74, 2019–2021. [Google Scholar] [CrossRef]
- Cillo, U.; D’Amico, F.E.; Furlanetto, A.; Perin, L.; Gringeri, E. Robotic hepatectomy and biliary reconstruction for perihilar cholangiocarcinoma: A pioneer western case series. Updates Surg. 2021, 73, 999–1006. [Google Scholar] [CrossRef]
- Ye, S.P.; Qiu, H.; Liao, S.J.; Ai, J.H.; Shi, J. Mini-invasive vs. open resection of colorectal cancer and liver metastases: A meta-analysis. World J. Gastroenterol. 2019, 25, 2819–2832. [Google Scholar] [CrossRef]
- Tsung, A.; Geller, D.A.; Sukato, D.C.; Sabbaghian, S.; Tohme, S.; Steel, J.; Marsh, W.; Reddy, S.K.; Bartlett, D.L. Robotic versus laparoscopic hepatectomy: A matched comparison. Ann. Surg. 2014, 259, 549–555. [Google Scholar] [CrossRef]
- Spampinato, M.G.; Mandalá, L.; Quarta, G.; Del Medico, P.; Baldazzi, G. One-stage, totally laparoscopic major hepatectomy and colectomy for colorectal neoplasm with synchronous liver metastasis: Safety, feasibility and short-term outcome. Surgery 2013, 153, 861–865. [Google Scholar] [CrossRef]
- Martin, J.; Petrillo, A.; Smyth, E.C.; Shaida, N.; Khwaja, S.; Cheow, H.K.; Duckworth, A.; Heister, P.; Praseedom, R.; Jah, A.; et al. Colorectal liver metastases: Current management and future perspectives. World J. Clin. Oncol. 2020, 11, 761–808. [Google Scholar] [CrossRef] [PubMed]
- Mohamedahmed, A.Y.Y.; Zaman, S.; Albendary, M.; Wright, J.; Abdalla, H.; Patel, K.; Mankotia, R.; Sillah, A.K. Laparoscopic versus open hepatectomy for malignant liver tumours in the elderly: Systematic review and meta-analysis. Updates Surg. 2021, 73, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yin, Z.; Pan, L.; Li, C.; Hu, M.; Lau, W.Y.; Liu, R. Robotic hepatic resection in postero-superior region of liver. Updates Surg. 2021, 73, 1007–1014. [Google Scholar] [CrossRef]
- Cipriani, F.; Ratti, F.; Fiorentini, G.; Reineke, R.; Aldrighetti, L. Systematic review of perioperative and oncologic outcomes of minimally-invasive surgery for hilar cholangiocarcinoma. Updates Surg. 2021, 73, 359–377. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, K.; Li, B.; Xu, H.; Wei, Y.; Liu, F. Laparoscopic versus open liver resection for resectable HCC with BCLC stage B: A propensity score-matched analysis. Updates Surg. 2022, 74, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, B.; Zhang, S.; Huang, Q.; Zhang, M.; Liu, G. Prognostic impact of tumor size on patients with metastatic colorectal cancer: A large SEER-based retrospective cohort study. Updates Surg. 2023, 75, 1135–1147. [Google Scholar] [CrossRef]
- Xia, Y.; Li, J.; Liu, G.; Wang, K.; Qian, G.; Lu, Z.; Yang, T.; Yan, Z.; Lei, Z.; Si, A.; et al. Long-term Effects of Repeat Hepatectomy vs. Percutaneous Radiofrequency Ablation Among Patients With Recurrent Hepatocellular Carcinoma: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 255–263. [Google Scholar] [CrossRef]
- Rao, S.X.; Lambregts, D.M.; Schnerr, R.S.; van Ommen, W.; van Nijnatten, T.J.; Martens, M.H.; Heijnen, L.A.; Backes, W.H.; Verhoef, C.; Zeng, M.S.; et al. Whole-liver CT texture analysis in colorectal cancer: Does the presence of liver metastases affect the texture of the remaining liver? United Eur. Gastroenterol. J. 2014, 2, 530–538. [Google Scholar] [CrossRef]
- Rojas Llimpe, F.L.; Di Fabio, F.; Ercolani, G.; Giampalma, E.; Cappelli, A.; Serra, C.; Castellucci, P.; D’Errico, A.; Golfieri, R.; Pinna, A.D.; et al. Imaging in resectable colorectal liver metastasis patients with or without preoperative chemotherapy: Results of the PROMETEO-01 study. Br. J. Cancer 2014, 111, 667–673. [Google Scholar] [CrossRef]
- Reali, C.; Bocca, G.; Lindsey, I.; Jones, O.; Cunningham, C.; Guy, R.; George, B.; Boyce, S. Influence of incorrect staging of colorectal carcinoma on oncological outcome: Are we playing safely? Updates Surg. 2022, 74, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Beckers, R.C.J.; Lambregts, D.M.J.; Schnerr, R.S.; Maas, M.; Rao, S.X.; Kessels, A.G.H.; Thywissen, T.; Beets, G.L.; Trebeschi, S.; Houwers, J.B.; et al. Whole liver CT texture analysis to predict the development of colorectal liver metastases-A multicentre study. Eur. J. Radiol. 2017, 92, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Fiz, F.; Viganò, L.; Gennaro, N.; Costa, G.; La Bella, L.; Boichuk, A.; Cavinato, L.; Sollini, M.; Politi, L.S.; Chiti, A.; et al. Radiomics of Liver Metastases: A Systematic Review. Cancers 2020, 12, 2881. [Google Scholar] [CrossRef]
- Marino, R.; Olthof, P.B.; Shi, H.J.; Tran, K.T.C.; Ijzermans, J.N.M.; Terkivatan, T. Minimally Invasive Liver Surgery: A Snapshot from a Major Dutch HPB and Transplant Center. World J. Surg. 2022, 46, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Grassi, R.; Grassi, F.; Ottaiano, A.; Nasti, G.; Tatangelo, F.; et al. Radiomics textural features by MR imaging to assess clinical outcomes following liver resection in colorectal liver metastases. Radiol. Med. 2022, 127, 461–470. [Google Scholar] [CrossRef]
- Renzulli, M.; Brandi, N.; Argalia, G.; Brocchi, S.; Farolfi, A.; Fanti, S.; Golfieri, R. Morphological, dynamic and functional characteristics of liver pseudolesions and benign lesions. Radiol. Med. 2022, 127, 129–144. [Google Scholar] [CrossRef]
- Nardone, V.; Reginelli, A.; Grassi, R.; Boldrini, L.; Vacca, G.; D’Ippolito, E.; Annunziata, S.; Farchione, A.; Belfiore, M.P.; Desideri, I.; et al. Delta radiomics: A systematic review. Radiol. Med. 2021, 126, 1571–1583. [Google Scholar] [CrossRef]
- Coppola, F.; Faggioni, L.; Regge, D.; Giovagnoni, A.; Golfieri, R.; Bibbolino, C.; Miele, V.; Neri, E.; Grassi, R. Artificial intelligence: Radiologists’ expectations and opinions gleaned from a nationwide online survey. Radiol. Med. 2021, 126, 63–71. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Belli, A.; Ottaiano, A.; Nasti, G.; La Porta, M.; Danti, G.; Cappabianca, S.; et al. Intrahepatic cholangiocarcinoma and its differential diagnosis at MRI: How radiologist should assess MR features. Radiol. Med. 2021, 126, 1584–1600. [Google Scholar] [CrossRef]
- Singh, M.; Singh, T.; Soni, S. Pre-operative Assessment of Ablation Margins for Variable Blood Perfusion Metrics in a Magnetic Resonance Imaging Based Complex Breast Tumour Anatomy: Simulation Paradigms in Thermal Therapies. Comput. Methods Programs Biomed. 2021, 198, 105781. [Google Scholar] [CrossRef]
- Grieco, M.; Lorenzon, L.; Pernazza, G.; Carlini, M.; Brescia, A.; Santoro, R.; Crucitti, A.; Palmieri, R.M.; Santoro, E.; Stipa, F.; et al. Impact of implementation of the ERAS program in colorectal surgery: A multi-center study based on the “Lazio Network” collective database. Int. J. Color. Dis. 2020, 35, 445–453. [Google Scholar] [CrossRef]
- Carlini, M.; Grieco, M.; Spoletini, D.; Menditto, R.; Napoleone, V.; Brachini, G.; Mingoli, A.; Marcellinaro, R. Implementation of the gut microbiota prevents anastomotic leaks in laparoscopic colorectal surgery for cancer:the results of the MIRACLe study. Updates Surg. 2022, 74, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Grieco, M.; Marcellinaro, R.; Spoletini, D.; Menditto, R.; Lisi, G.; Russo, G.; Napoleone, V.; Carlini, M. Laparoscopic right colectomy: Changes in surgical technique and perioperative management allow better postoperative results in a comparative series of 361 patients. Updates Surg. 2022, 74, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Marcellinaro, R.; Grieco, M.; Spoletini, D.; Troiano, R.; Avella, P.; Brachini, G.; Mingoli, A.; Carlini, M. How to reduce the colorectal anastomotic leakage? The MIRACLe protocol experience in a cohort in a single high-volume centre. Updates Surg. 2023, 75, 1559–1567. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef]
- Rotolo, S.; Di Giorgio, A.; Santullo, F.; Attalla El Halabieh, M.; Lodoli, C.; Abatini, C.; Pacelli, F. Cytoreductive surgery and mitomycin C hyperthermic intraperitoneal chemotherapy with CO2 recirculation (HIPEC-CO2) for colorectal cancer peritoneal metastases: Analysis of short-term outcomes. Updates Surg. 2021, 73, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, T.; Hayashi, H.; Miyamoto, Y.; Imai, K.; Kitano, Y.; Kaida, T.; Sawayama, H.; Beppu, T.; Yamashita, Y.I.; Baba, H. Clinical Impact of Operative Order in Laparoscopic Simultaneous Resection for Synchronous Colorectal Liver Metastases. Cancer Diagn. Progn. 2021, 1, 151–156. [Google Scholar] [CrossRef]
- Tranchart, H.; Fuks, D.; Vigano, L.; Ferretti, S.; Paye, F.; Wakabayashi, G.; Ferrero, A.; Gayet, B.; Dagher, I. Laparoscopic simultaneous resection of colorectal primary tumor and liver metastases: A propensity score matching analysis. Surg. Endosc. 2016, 30, 1853–1862. [Google Scholar] [CrossRef]
- Ratti, F.; Catena, M.; Di Palo, S.; Staudacher, C.; Aldrighetti, L. Laparoscopic Approach for Primary Colorectal Cancer Improves Outcome of Patients Undergoing Combined Open Hepatic Resection for Liver Metastases. World J. Surg. 2015, 39, 2573–2582. [Google Scholar] [CrossRef]
- Takasu, C.; Shimada, M.; Sato, H.; Miyatani, T.; Imura, S.; Morine, Y.; Ikemoto, T.; Kanamoto, M.; Kurita, N.; Eto, S.; et al. Benefits of simultaneous laparoscopic resection of primary colorectal cancer and liver metastases. Asian J. Endosc. Surg. 2014, 7, 31–37. [Google Scholar] [CrossRef]
- Ferretti, S.; Tranchart, H.; Buell, J.F.; Eretta, C.; Patriti, A.; Spampinato, M.G.; Huh, J.W.; Vigano, L.; Han, H.S.; Ettorre, G.M.; et al. Laparoscopic Simultaneous Resection of Colorectal Primary Tumor and Liver Metastases: Results of a Multicenter International Study. World J. Surg. 2015, 39, 2052–2060. [Google Scholar] [CrossRef]
- Komici, K.; Cappuccio, M.; Scacchi, A.; Vaschetti, R.; Delli Carpini, G.; Picerno, V.; Avella, P.; Brunese, M.C.; Rengo, G.; Guerra, G.; et al. The Prevalence and the Impact of Frailty in Hepato-Biliary Pancreatic Cancers: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Brunese, M.C.; Cappuccio, M.; Scacchi, A.; Martucci, G.; Buondonno, A.; Perrotta, F.M.; Quarto, G.; Avella, P.; Amato, B. Impact of Physical Activity on Disability Risk in Elderly Patients Hospitalized for Mild Acute Diverticulitis and Diverticular Bleeding Undergone Conservative Management. Medicina 2021, 57, 360. [Google Scholar] [CrossRef]
- Luciani, C.; Scacchi, A.; Vaschetti, R.; Di Marzo, G.; Fatica, I.; Cappuccio, M.; Guerra, G.; Ceccarelli, G.; Avella, P.; Rocca, A. The uniportal VATS in the treatment of stage II pleural empyema: A safe and effective approach for adults and elderly patients-a single-center experience and literature review. World J. Emerg. Surg. 2022, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Capussotti, L.; Ferrero, A.; Viganò, L.; Ribero, D.; Lo Tesoriere, R.; Polastri, R. Major liver resections synchronous with colorectal surgery. Ann. Surg. Oncol. 2007, 14, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Serenari, M.; Bonatti, C.; Zanoni, L.; Peta, G.; Tabacchi, E.; Cucchetti, A.; Ravaioli, M.; Pettinato, C.; Bagni, A.; Siniscalchi, A.; et al. The role of hepatobiliary scintigraphy combined with spect/ct in predicting severity of liver failure before major hepatectomy: A single-center pilot study. Updates Surg. 2021, 73, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Zhou, S.; Liu, S.; Liu, H.; Lu, Z. Prognostic role of preoperative albumin-bilirubin score in posthepatectomy liver failure and mortality: A systematic review and meta-analysis. Updates Surg. 2022, 74, 821–831. [Google Scholar] [CrossRef]
- Brunese, M.C.; Fantozzi, M.R.; Fusco, R.; De Muzio, F.; Gabelloni, M.; Danti, G.; Borgheresi, A.; Palumbo, P.; Bruno, F.; Gandolfo, N.; et al. Update on the Applications of Radiomics in Diagnosis, Staging, and Recurrence of Intrahepatic Cholangiocarcinoma. Diagnostics 2023, 13, 1488. [Google Scholar] [CrossRef]
- Singh, M.; Dalal, M.; Sodhi, G.S. Estimation of clinical size of breast tumour lesions using contrast enhanced magnetic resonance imaging: Delineation of tumour boundaries. In UMBC Student Collection; UMBC: Baltimore, MD, USA, 2021. [Google Scholar]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef]
- Fleischer, J.R.; Schmitt, A.M.; Haas, G.; Xu, X.; Zeisberg, E.M.; Bohnenberger, H.; Küffer, S.; Teuwen, L.A.; Karras, P.J.; Beißbarth, T.; et al. Molecular differences of angiogenic versus vessel co-opting colorectal cancer liver metastases at single-cell resolution. Mol. Cancer 2023, 22, 17. [Google Scholar] [CrossRef] [PubMed]


| Author | Year | Method | N. of Patients | Primary Tumor | Patients Division for Model Checker & Validation Set | Chemotherapy (Yes/No) | Imaging | Time of Metastases Detection from Primary Tumor Diagnosis (Months) |
|---|---|---|---|---|---|---|---|---|
| Biglarian et al. [42] | 2012 | Artificial neural networks (ANNs) & Logistic regression (LR) | 1007 (786 CC, 204 RC) | CRC | 705 patients for training set and 302 patients for validation set | NA | NA | NA |
| Shu et al. [39] | 2019 | Radiomics nomogram | 194 | RC | 135 independent cohorts for training set and 59 patients for validation set | No | MRI + CT | NA |
| Liang et al. [40] | 2019 | Machine learning: Support vector machine (SVM) & LR | 3087 | RC | NA | NA | MRI + CT | <12 months |
| AmirHosseini et al. [43] | 2019 | Neural network and Fuzzy genetic algorithm | 125 | NA | NA | NA | CT | NA |
| Liu et al. [44] | 2020 | Radiomics nomogram | 127 | RC | 88 patients for training set and 39 for validation set | NA | CT | NA |
| Han et al. [34] | 2020 | Machine learning: Classification model (Decision tree) | 107 | CRC | NA | NA | MRI | NA |
| Lee et al. [45] | 2020 | Convolution neural network (CNN) | 2019 | CRC | 1413 patients for training set and 606 for test set | NA | CT | NA |
| Goehler et al. [46] | 2020 | Deep learning model | 64 | NA | 45 patients for training set, 19 for test set, 20% of training data was split to validation set | NA | MRI | NA |
| Taghavi et al. [18] | 2020 | Machine learning based radiomics model | 91 | CRC | 70 patients for training set, 21 for validation set | NA | CT | 24 patients developed metastases before 24 months after primary staging |
| Li et al. [47] | 2020 | Radiomics intelligent analysis toolkit (RIAT) and LR | 100 | CRC | 80 patients for cross validation set and 20 for test set | NA | CT | NA |
| Lee et al. [48] | 2021 | Deep learning model | 502 | CRC | NA | NA | CT | NA |
| Kim et al. [49] | 2021 | Deep learning model | 6526 | CRC | 5129 patients for training set and 1397 for validation set | NA | CT | NA |
| Stollmayer et al. [22] | 2021 | Densely connected convolutional neural networks (DenseNets) | 69 | 14 CRC (42 Focal nodule hyperplasia and 13 HCC) | NA | NA | MRI | NA |
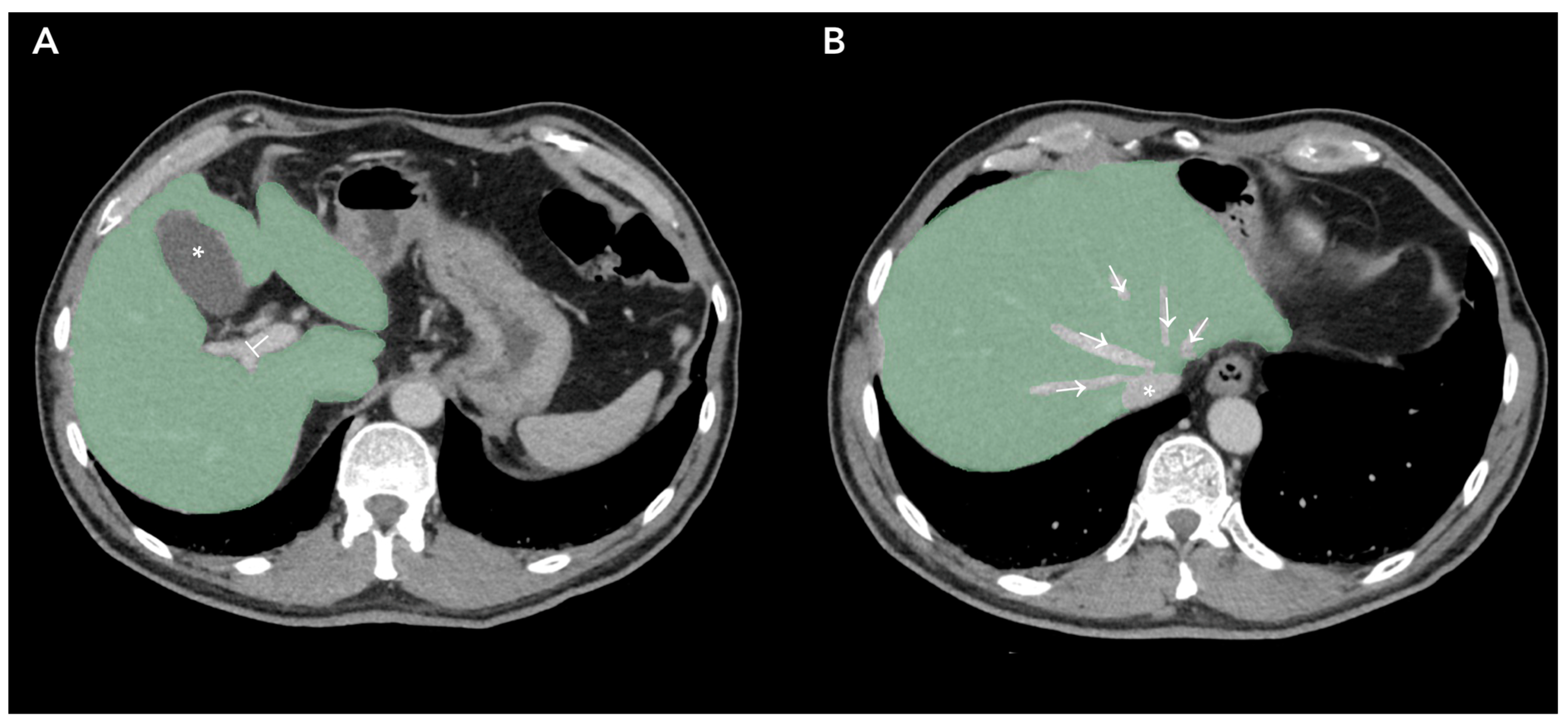
| Rocca et al. [21] | 2021 | Formal methods | 30 | CRC | NA | NA | CT | 3–48 months |
| Li et al. [38] | 2022 | Machine learning: Classification model | 323 | CRC | 171 patients for training set, 77 for internal validation set and 75 for external validation set | No | CT | NA |
| Granata et al. [29] | 2022 | Radiomics & Machine learning analysis | 81 | CRC | 51 patients for training set, 30 for external validation set | NA | MRI + CT | NA |
| Devoto et al. [41] | 2022 | Textural analysis | 23 | CRC | NA | NA | CT | <7 months |
| Author | Dataset | N. of Features | Accuracy | Specificity | Precision | Recall | AUROC | AUAFROC | CI |
|---|---|---|---|---|---|---|---|---|---|
| Biglarian et al. [42] | Training set Validation set | NA | NA | RC with ANN: 85.7%, CC with ANN: 91.4%, CC with LR: 92.3% | NA | RC with ANN: 44.4%, CC with ANN: 48.6%, CC with LR: 32.4% | NA | NA | ANN: 0.812 LR: 0.779 |
| Shu et al. [39] | Training set Validation set | 328 | Training set: 92.1%, Validation set: 91.2% | NA | NA | NA | Training set: 85.7%, Validation set: 83.4% | NA | Accuracy training set: 0.862–0.961, Accuracy validation set: 0.809–0.970 |
| Liang et al. [40] | 5-fold cross validation * | 35 | LR: 80% SVM: 72% | LR: 76% SVM: 69% | NA | LR: 83% SVM: 76% | LR: 87% SVM: 83% | NA | LR: 0.730–0.880, SVM 0.650–0.840 |
| AmirHosseini et al. [43] | Training set Test set | NA | 99.24% | Neural network: 85.7%, Fuzzy GA: 100% | NA | Neural network: 81.8%, Fuzzy GA: 96.67% | 98.32% | NA | Neural network: 0.744–0.925, Fuzzy GA: 0.885–1.000 |
| Liu et al. [44] | Training set Validation set | 866 | Validation set: 89.66% | Validation set: 93.65% | NA | Validation set: 79.17% | Validation set: 86.6% | NA | Validation set: 0.770–0.963 |
| Han et al. [34] | Training set, Internal validation set, External validation set | 182 | Internal validation set: 95.2%, External validation set: 78.8% | Internal validation set: 70%, External validation set: 34.5% | NA | Internal validation set: 95.2%, External validation set: 100% | Training set: 97.1%, Internal validation set: 90.9%, External validation set: 90.5% | NA | NA |
| Lee et al. [45] | 5-fold cross validation * | 4096 | NA | NA | NA | NA | 74.7% | NA | NA |
| Goehler et al. [46] | X-fold cross validation * | NA | 88% | 92% | 92% | 85% | NA | NA | Recall: 0.770–0.930, Specificity: 0.870–0.960 |
| Taghavi et al. [18] | Training set Validation set | 101 | NA | NA | NA | NA | Training set: 93%, Validation set: 86% | NA | Training set: 0.910–0.950, Validation set: 0.850–0.870 |
| Li et al. [47] | 5-fold cross validation * | 210 | NA | Test set: 91%, Validation set: 75%, Cross validation set: 84% | NA | Test set: 78%, Validation set: 85%, Cross validation set: 81% | Test set: 89.9%, Validation set: 86%, Cross validation set: 90.6% | NA | Test set: 0.761–1.000, Cross validation set: 0.840–0.971 |
| Lee et al. [48] | Training set Validation set | NA | 71.66% | 72.78% | NA | 70.47% | 84.10% | NA | NA |
| Kim et al. [49] | Training set and Validation set | NA | NA | 22.22% | NA | 87.50% | NA | 0.631 (0.520–0.737) | NA |
| Stollmayer et al. [22] | Training set, Test set and Validation set | NA | NA | 2D model: 100%, 3D: 95% | NA | 2D model: 80%, 3D: 70% | Test set 2D model: 96%, 3D: 90.5% | NA | Test set 2D model: 0.879–1.000, 3D: 0.789–1.000 |
| Rocca et al. [21] | NA | 22 | 93.3% | 100% | 100% | 77.8% | NA | NA | NA |
| Li et al. [38] | Training set Validation set | 1288 | Validation dataset 1: 58.4%, Validation set 2: 65.3% | Validation dataset 1: 48.2%, Validation set 2: 65.4% | NA | Validation dataset 1: 85.7%, Validation set 2: 65.2% | Validation dataset 1: 79%, Validation set 2: 72% | NA | Validation dataset 1: 0.680–0.870 Validation set 2: 0.600–0.820 |
| Granata et al. [29] | Training set Validation set | 851 | Validation set T2W SPACE: 86%, Arterial Phase: 89%, Portal Phase: 91%, EOB Phase: 80% | Validation set T2W SPACE: 86%, Arterial Phase: 91%, Portal Phase: 96%, EOB Phase: 100% | NA | Validation set T2W SPACE: 86%, Arterial Phase: 85%, Portal Phase: 81%, EOB Phase: 67% | Validation set T2W SPACE: 88%, Arterial Phase: 96%, Portal Phase: 99%, EOB Phase: 95% | NA | NA |
| Devoto et al. [41] | NA | NA | NA | 63.6% | NA | 83.3% | 75% | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avella, P.; Cappuccio, M.; Cappuccio, T.; Rotondo, M.; Fumarulo, D.; Guerra, G.; Sciaudone, G.; Santone, A.; Cammilleri, F.; Bianco, P.; et al. Artificial Intelligence to Early Predict Liver Metastases in Patients with Colorectal Cancer: Current Status and Future Prospectives. Life 2023, 13, 2027. https://doi.org/10.3390/life13102027
Avella P, Cappuccio M, Cappuccio T, Rotondo M, Fumarulo D, Guerra G, Sciaudone G, Santone A, Cammilleri F, Bianco P, et al. Artificial Intelligence to Early Predict Liver Metastases in Patients with Colorectal Cancer: Current Status and Future Prospectives. Life. 2023; 13(10):2027. https://doi.org/10.3390/life13102027
Chicago/Turabian StyleAvella, Pasquale, Micaela Cappuccio, Teresa Cappuccio, Marco Rotondo, Daniela Fumarulo, Germano Guerra, Guido Sciaudone, Antonella Santone, Francesco Cammilleri, Paolo Bianco, and et al. 2023. "Artificial Intelligence to Early Predict Liver Metastases in Patients with Colorectal Cancer: Current Status and Future Prospectives" Life 13, no. 10: 2027. https://doi.org/10.3390/life13102027
APA StyleAvella, P., Cappuccio, M., Cappuccio, T., Rotondo, M., Fumarulo, D., Guerra, G., Sciaudone, G., Santone, A., Cammilleri, F., Bianco, P., & Brunese, M. C. (2023). Artificial Intelligence to Early Predict Liver Metastases in Patients with Colorectal Cancer: Current Status and Future Prospectives. Life, 13(10), 2027. https://doi.org/10.3390/life13102027







