Abstract
New innovations within spine surgery continue to propel the field forward. These technologies improve surgeons’ understanding of their patients and allow them to optimize treatment planning both in the operating room and clinic. Additionally, changes in the implants and surgeon practice habits continue to evolve secondary to emerging biomaterials and device design. With ongoing advancements, patients can expect enhanced preoperative decision-making, improved patient outcomes, and better intraoperative execution. Additionally, these changes may decrease many of the most common complications following spine surgery in order to reduce morbidity, mortality, and the need for reoperation. This article reviews some of these technological advancements and how they are projected to impact the field. As the field continues to advance, it is vital that practitioners remain knowledgeable of these changes in order to provide the most effective treatment possible.
1. Introduction
New innovations within spine surgery continue to propel the field forward. These technologies improve surgeons’ understanding of their patients and allow them to optimize treatment planning both in the operating room and clinic. Additionally, changes in the implants and procedures continue to evolve secondary to emerging biomaterials and device design. This article reviews some of these technological advancements and how they are projected to impact the field.
2. Methodology
Topics related to emerging technologies within spine surgery were chosen based on recent trends in the published literature, as well as popular themes seen at national and international conferences. The chosen topics included robotics, navigation, virtual reality, sagittal alignment parameters, artificial intelligence, biomaterials, motion preservation devices, and surgical training. Search queries were performed for these topics through the PubMed database. After initial screening and evaluation, additional pertinent citations were extracted from these studies’ bibliographies.
4. Sagittal Parameters
Disorders of the spine may be caused or exacerbated by atypical alignment. Consequently, understanding the parameters of spinal alignment, especially in the sagittal plane, has become an important component of operative planning. The concept of the ideal alignment is controversial and highly debated among spine surgeons. Numerous metrics are considered, including lordosis and kyphosis measures, overall spinal and local sagittal vertical axes, and the spine’s relation to the pelvis. One of the central metrics evaluated for overall sagittal spinal alignment is the sagittal vertical axis (SVA). This is measured as the distance from a C7 plumb line to the posterosuperior S1 body. Optimal values are less than 5 cm. However, clinically acceptable values for SVA likely increase with age.
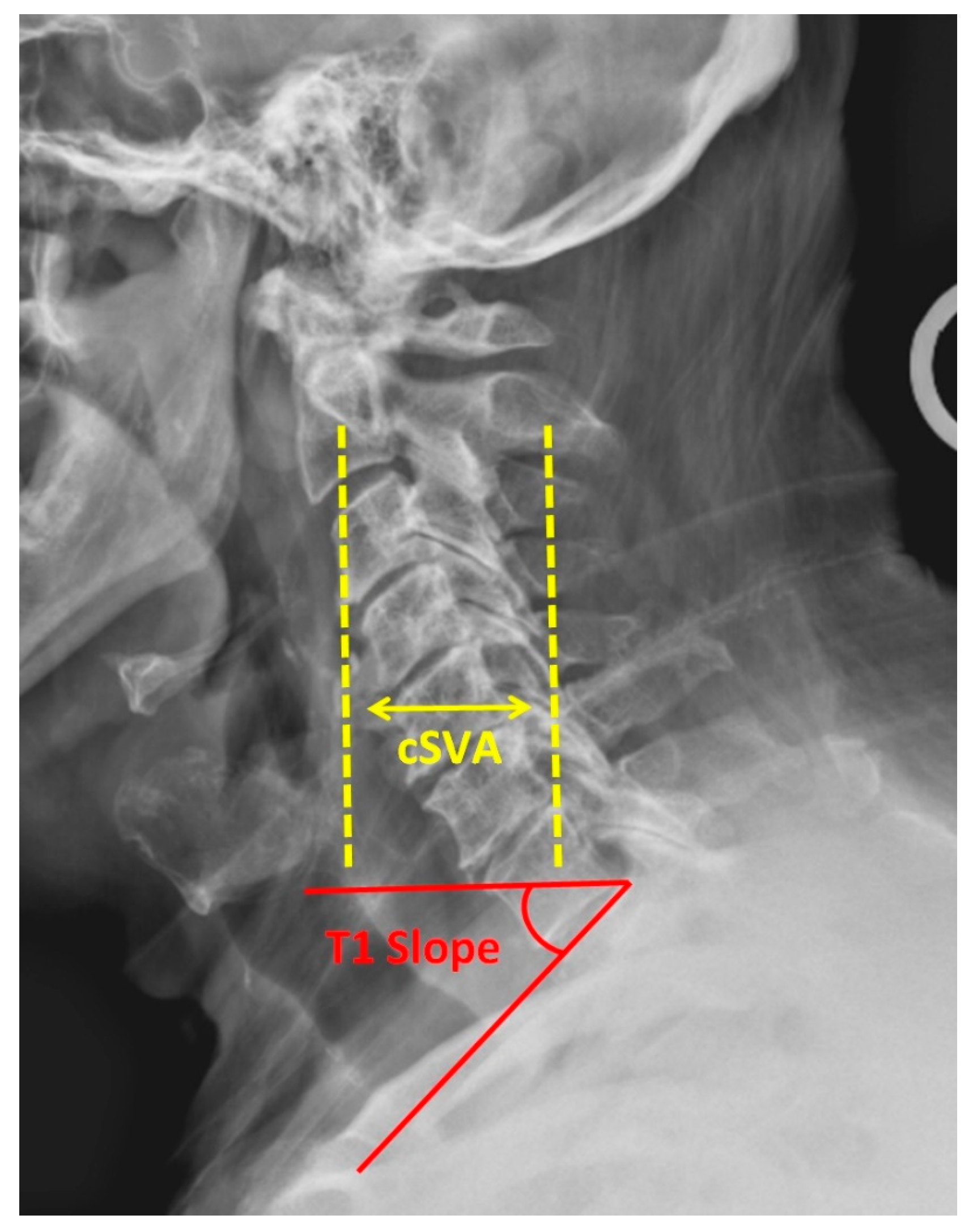
The cervical spine is considered a super-structure that is reliant on the positioning and alignment of the thoracic, lumbar, and pelvic structures. Normal cervical lordosis is 20–40 degrees. Significant effort has been put into defining the variants of cervical morphology [16]. Among the various metrics studied, the T1 slope and cervical sagittal vertical axis (cSVA) have been shown to be clinically important (Figure 1). The T1 slope is the angle between the horizontal and the superior endplate of T1. cSVA is the distance from a C2 plumb line to the posterosuperior corner of C7. Good clinical outcomes are associated with a T1 slope at or below 40° and a cSVA less than 40 mm [17]. In a large meta-analysis conducted by Azimi et al., patients with cervical pathology had increased T1 slope (healthy 24.5°, symptomatic 25.7°) and cSVA (healthy 18.7°, symptomatic 22.7°) relative to an asymptomatic control group [18]. Additionally, an elevated T1 slope and cSVA predict postoperative kyphotic deformity as the center of the head mass is anteriorly pushed [19,20]. A similar metric, the center gravity of the head to C7 SVA (CGH-C7SVA), is also associated with increased risk of postoperative kyphotic failure following laminoplasty [20]. Lastly, the occipital cervical angle (OCI) is a critical measurement in one’s ability to maintain a normal gaze with increased OCI associated with the presence and progression of ASD [21]. This measurement is the angle formed between a line drawn from the posterior border of the C4 body and McGregor’s line.
Figure 1.
Lateral cervical spine radiograph demonstrating cervical sagittal vertical axis (cSVA) and T1 slope.
The regions of the spine are highly interdependent with a high prevalence (53%) of cervical deformity and concomitant thoracolumbar deformity [22]. Normal thoracic kyphosis (TK) and lumbar lordosis (LL) are 20–50° and 20–80°, respectively. Pelvic incidence (PI) is commonly considered when planning for surgical correction of spinal malalignment. This value is the sum of an individual’s sacral slope (the Cobb angle from the superior endplate L1 to the inferior endplate L5) and pelvic tilt (the angle formed by a line from the midpoint of the sacral endplate to the center of the femoral head and a vertical reference line). This value is considered normal when within nine degrees of lumbar lordosis. Odland et al. demonstrated that the mean PI is approximately 50° (24–69°) in asymptomatic individuals [23]. Low PI, low LL, and high pelvic tilt are associated with increased ASD incidence [24]. The correction of these parameters to within normal limits with expandable cages or hyperlordotic implants may improve patient-reported outcome measures (PROMs) [25,26,27,28].
5. Artificial Intelligence
Artificial intelligence (AI) refers to technologies that interpret and analyze data in ways that mimic human cognitive function. Machine learning is a subset of AI that is able to interpret huge quantities of data and makes predictions about the sampled population. This technology’s development has recently accelerated in healthcare following the development of large datasets, the availability of cloud computing, affordable high processing power computers, and open source software development [29]. Proponents claim improved patient experiences and health, healthcare-related cost reductions, and better work-life quality for providers as hopeful benefits. AI has been extensively used in general medical practice, but its utilization in spine surgery lags behind many other fields [30]. It has demonstrated value in clinical and radiographic imaging interpretation, genomic analysis, disease progression prediction, electrocardiogram pattern recognition, and numerous other unique medical applications [31].
AI’s role in automating image interpretation appears to have its largest current clinical application in spine surgery. It is able to calculate spinopelvic parameters and automatically segment vertebrae for its end users [32]. These measurements have excellent reliability and accuracy compared to expert human measurements [33,34,35,36]. Because AI is able to analyze advanced imaging on a pixel level, it can make granular assumptions about the data and modify the original image. This results in less image data input required to complete scans, decreased hardware-related distortions and motion artifacts, and MRI osseous structure analysis equivalent to that seen on CT scans [37]. Carson et al. demonstrated, in an animal model, that nerves passing through the psoas muscle may be visualized with AI for use in lateral lumbar interbody fusion. The accuracy of nerve localization in this model was greater than 95% [38].
AI has the ability to both classify imaging using existing schemes as well as form new systems based on self-directed dataset learning. It has been used to accurately grade spinal stenosis and classify intervertebral disc degeneration using MRI [39,40]. Burns et al. used machine learning to locate and classify thoracolumbar fractures based on the Denis system [41]. In one study, an unsupervised artificial neural network clustered six patient adult spinal deformity types and determined their associations with sagittal vertebral alignment and proximal junctional kyphosis [42]. Another study by Ames et al. created a four-cluster classification system for spinal deformity that predicted associated complication profiles [43].
Finding consensus for the treatment of spinal conditions is frequently difficult to obtain. A recent survey revealed that 69% of providers differed in their treatment of recurrent disc herniation [44]. AI has the potential to improve this process by guiding preoperative risk stratification and making procedure recommendations in regard to its generated outcome and complication profile [37]. Mourad et al. created a hybrid AI model that evaluated clinical symptoms, MRI, and demographic factors to determine surgical candidacy for lumbar spinal surgery. Their model performed in a similar way to a multidisciplinary team, which included five fellowship-trained spine surgeons [45]. A separate retrospective study had 86% agreement in its surgical plan, with the actual treatment course using demographics, medical history, patient-reported outcome measures, and radiographic parameters as guidance [46]. Machine learning may also be particularly useful in creating patient risk profiles, predicting clinical outcomes, and making treatment recommendations when deployed in large data registries [32].
The majority of current forecasting models have been used to predict postoperative outcomes and complications. Spine surgery-specific AI has examined satisfaction, quality of life, mJOA, mortality, hospital readmission, opioid utilization, transfusion requirements, proximal junctional kyphosis, pseudoarthrosis, infection, and other postoperative complications [43,47,48,49,50,51,52,53,54,55,56,57,58]. Khan et al. noted that newer ML-based models perform at least as well as traditional regression modeling in the prediction of postoperative outcomes following spinal cord injury [59]. Additionally, Merali demonstrated the superiority of their model over traditional statistical models for predicting outcomes following surgery for cervical myelopathy [60]. These models have shown satisfactory accuracy in a study by Scheer et al., predicting pseudoarthrosis with 91% accuracy following adult spinal deformity correction at the two-year follow-up [61].
AI has the ability to enhance spine surgery-related research. It uses multiple complex models in parallel to examine indirect relationships and generate accurate predictions. It will expand our considered measures into previously unexplored areas such as biomarkers and epigenetics [62]. However, concerns regarding the utilization of AI within spine surgery exist. Most existing studies are retrospective with curated populations examined against expert performance and do not involve real-time decisions [31]. Other concerns exist regarding bias against subgroups of samples, privacy, accidental fitting to confounders, population characteristic shifts, and the future of clinical training. Ultimately, this is a field with great potential but which remains in its infancy with low clinical conversion efficiency.
6. Implant Materials
Since the advent of spine surgery, practitioners have sought to find the ideal materials to promote fusion and improve alignment. High rates of complications such as implant migration and subsidence continue to motivate surgeons in this pursuit [63]. A variety of materials have been proposed as solutions to these problems, including metals and their alloys, ceramics, polymers, and composites. These materials are frequently combined in an attempt to modulate their properties in a process called “doping”. Additionally, biologic supplements such as recombinant bone morphogenic protein-2 (rh-BMP2) are used to promote the body’s innate processes toward improved fusion and healing.
6.1. Metals
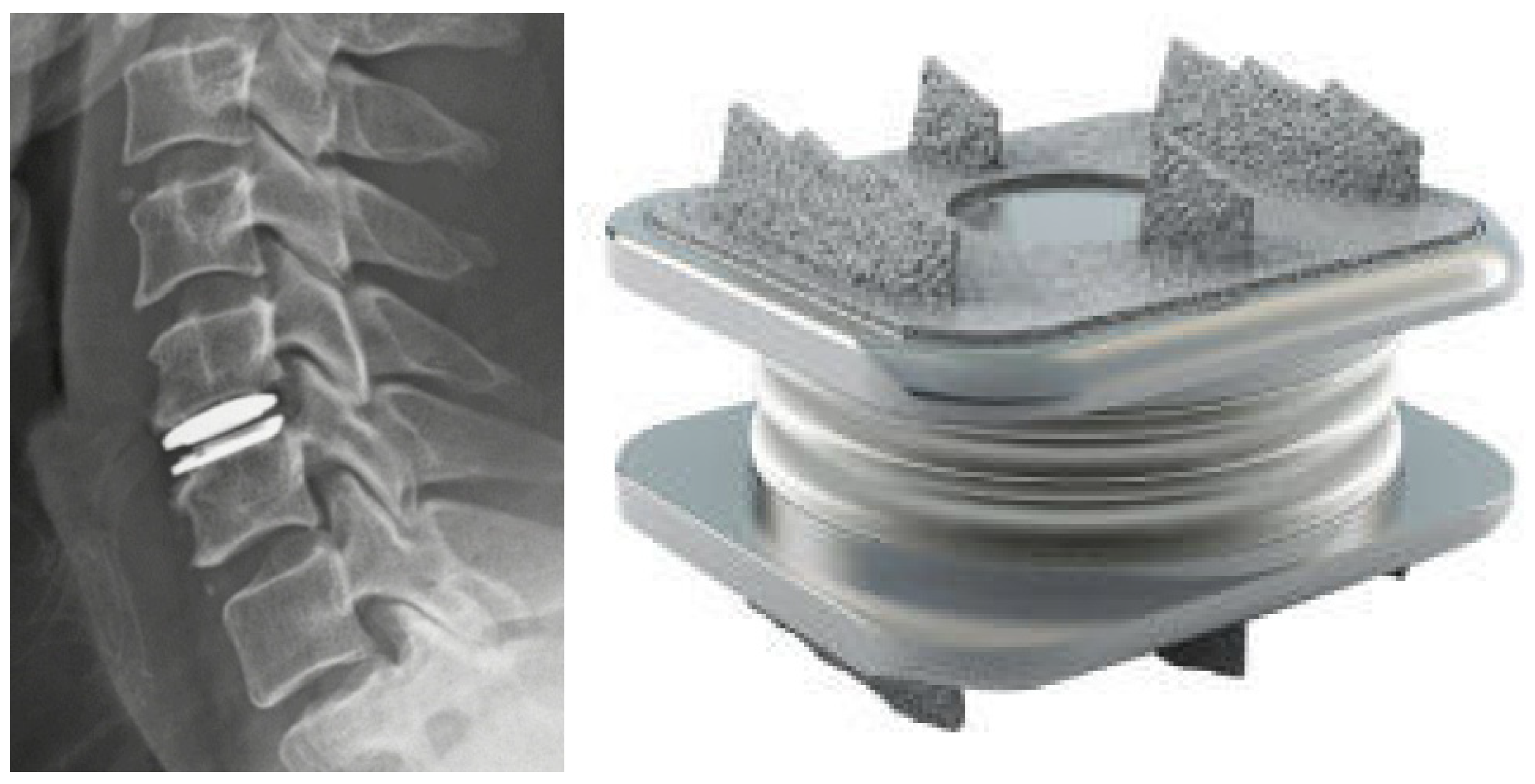
Titanium (Ti) and its alloys are widely utilized due to their biocompatibility, toughness, and fracture resistance [64]. This metal has excellent corrosion resistance and a more similar modulus of elasticity (MoE) to bone than other commonly used metals in spinal instrumentation. Adding a porous coating to the implant surface allows for osseous integration. While Ti is relatively expensive compared to stainless steel and polyetheretherketone (PEEK), it has less image artifact than many alloys. Ti implants may have, at worst, non-inferior fusion rates when compared to PEEK (Figure 2) [63,65,66]. However, multiple studies have demonstrated increased rates of subsidence in relation to PEEK and ceramics [64,67,68]. Stainless steel (FeCrNi) and cobalt-chrome (CoCr) are two other common alloys employed for spinal instrumentation. Stainless steel rods may cause pedicle stress shielding due to excessive stiffness related to its higher MoE. For this reason, PEEK and Ti rods are generally preferred. Stainless steel is notch-resistant but has substantial susceptibility to corrosion. CoCr is frequently used in scoliosis surgery as the stiffer rods maintain intraoperative correction, though with more image artifacts than Ti.

Figure 2.
Titanium (left) and PEEK (right) lumbar interbody implants are demonstrated.
Tantalum and nitinol (50% Ti, 50% nickel) are occasionally used in spinal implants. These are more expensive than other materials such as Ti, stainless steel, and PEEK. Porous Tantalum is similar in structure to cancellous bone, has a low MoE, and a high friction coefficient, which provides excellent interface characteristics. These qualities enable load sharing, which may prevent stress shielding. Tantalum has improved pullout strength and greater osteoblast proliferation induction than Ti [69]. The current literature demonstrates in vivo animal models with early improvements in bony ingrowth versus PEEK and improved fusion rates relative to autologous bone [70]. Nitinol is a notch-sensitive metal that produces a minimal inflammatory reaction when placed in the spinal canal. It has shape memory elastic properties, which make it an optimal rod material in scoliosis corrective surgery.
6.2. PEEK
PEEK is the most researched biomaterial used in lumbar and cervical fusions [63]. This semi-rigid plastic is hydrophobic and unable to adhere to bone. This property may be responsible for the higher rates of implant migration and subsidence relative to Ti [71]. Proponents of its utilization point to its similar MoE as cancellous bone, reduced image artifact, and radiolucency. Much of the stress shielding seen with stiff metallic rods is reduced with PEEK rods, which allow for more axial and bending ranges of motion and anterior column load sharing [72]. Increased bending may prevent the accelerated ASD seen with stiffer metal constructs. Though this material has 2.5 times less compressive strength than Ti, PEEK interbody implants have shown better maintenance of Cobb angles than Ti cages when used for anterior cervical discectomy and fusion. PEEK has also demonstrated non-inferior clinical outcomes following spinal fusion versus Ti and ABG implants [66,72]. Overall, these three materials demonstrate similar complication profiles.
Reinforcing PEEK with carbon fiber (CFR-PEEK) or doping with Ti (TiPEEK) allows for a mixing of attributes to suit unique purposes. The use of CFR-PEEK constructs has shown potential in the field of spinal tumors. This modified plastic has excellent radiographic characteristics with no noted differences in outcomes or complications from traditional Ti or PEEK [73]. CFR-PEEK may enhance early detection of tumor recurrence and radiation dosing accuracy, though limited clinical data currently exist [74]. When PEEK is doped with Ti, it may show improved subsidence rates relative to PEEK alone. However, most studies have demonstrated similar clinical and radiographic outcomes, including ultimate fusion rates [63,66,75].
6.3. Hydroxyapatite
Hydroxyapatite (HA) is a phosphate mineral that may be surfaced on an implant to improve bony integration. HA-coated pedicle screws have increased pullout force and bone-to-implant contact relative to uncoated screws [76,77]. This material is frequently indicated in patients with decreased bone mineral density due to these properties. Studies have shown shorter operative times and blood loss in procedures utilizing HA versus autogenous bone graft, but no difference in final outcomes or complications [78,79].
6.4. rhBMP2
rhBMP2 is an alternative to an iliac crest bone graft (ICBG) applied to avoid donor site morbidity and increased wound-related complications seen with autograft harvest. rhBMP2 has superior spinal fusion rates relative to ICBG, local autograft, allograft, BMP-7, and demineralized bone graft (DMB) when used alone [63,80]. The addition of BMP2 addition to PEEK in lumbar fusion increases fusion rates and may improve PROMs relative to PEEK HA+tricalcium phosphate. It has also been shown to improve fusion rates in ACDF, but may cause potentially life-threatening prevertebral edema [65]. In rat models, rhBMP2 caused more soft tissue edema and higher inflammatory marker levels than HA-DBM [81].
6.5. Ceramics
Ceramics are currently being used within spinal surgery to promote fusion. Silicon nitride is a brittle, semi-radiolucent ceramic with high thermal and bacterial resistance. Because its particles are able to be resorbed, they do not contribute to particle load and subsequent osteolysis. Costs of production and subsidence rates are comparable with PEEK [68,70]. The U.S. Federal Drug Administration reported an adverse effect rate of 0.07% in human applications. Tricalcium phosphate (TCP) is another osteoinductive ceramic bone graft substitute. A randomized, controlled trial investigating ICBG versus TCP in lumbar interbody fusion showed no difference in complications or clinical outcomes, with excellent fusion rates in both treatment groups [82]. Silicon-substituted calcium phosphate is a modified form of TCP with a 93% reported radiographic fusion rate similar to that seen in rhBMP2-impregnated grafts. In one clinical study, the use of this material in interbody fusion resulted in a significant postoperative improvement in clinical outcomes [83]. Osteoinductive factors may be added to ceramics to improve their fusion rates. Stromal vascular fraction (SVF) is a component of adipose tissue that is isolated and used in conjunction with ceramics in lumbar interbody procedures. A case series evaluating TCP in lumbar interbody fusion with and without SVF showed a statistically significant improvement in fusion grade with the use of SVF at 6 months, though these differences were not maintained at subsequent follow-up [84].
6.6. Bioabsorbable Materials
Bioactive glass is a semi-crystalline, gel material that has antimicrobial and osteoinductive properties. Like silicon nitride, it is soluble and resorbed by the body. The remaining ions deposit Ca and Phosphorus to form hydroxyapatite. In regards to fusion, it behaves with similar efficacy to iliac crest autograft [70]. Adequate clinical studies of this biomaterial are still lacking and have yet to establish its non-inferiority [85]. Other bioabsorbable materials, such as modified polymer interbody cages and annulus fibrosis-repairing biogels, have been used with limited success [86,87]. There has been, overall, poor quality of evidence and significant bias present in most studies evaluating composite bone substitutes and factors versus autograft (ICBG) [88].
7. Motion Preservation
7.1. Cervical Disc Arthroplasty
Anterior cervical discectomy and fusion is considered the gold standard for the surgical treatment of cervical radiculopathy and is a commonly used option for cervical myelopathy. As the number of fused levels increases, pseudoarthrosis and failure rates increase [89,90]. Additionally, the loss of motion at the index level is thought to cause abnormal compensatory motion and stress transfer to the adjacent levels, leading to adjacent segment degeneration (ASD) [91]. CDA is designed to maintain ROM at the index and adjacent levels. There are currently nine FDA-approved CDA implants, with numerous others in IDE trials (Figure 3). Of these, three have received FDA approval for two-level usage. The inclusion criteria include one- or two-level cervical degenerative disc disease levels causing intractable radiculopathy or myelopathy [92]. The most common designs of arthroplasty implants consist of either a core sandwiched between two metal baseplates or a “ball-in-trough” configuration. Plastics such as ultra-high molecular weight polyethylene and elastic composites have been utilized in the core component. Early implants used metal-on-metal bearings, but this design has largely been abandoned.
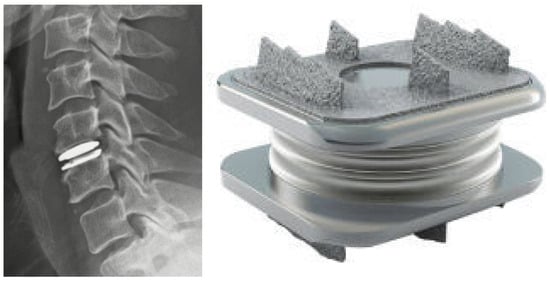
Figure 3.
Lateral radiograph of the cervical spine following cervical disc arthroplasty at C5/6 (left). The M6-CTM Artificial Cervical Disc (right).
Single-level CDA reliably improves neck disability index (NDI) and visual analog scale (VAS) scores. Versus ACDF, CDA results in at least non-inferior overall satisfaction and success rates, with the majority of investigations indicating its superiority [93,94,95,96,97]. CDA also demonstrates greater improvements in NDI and VAS relative to ACDF [95,96,98]. Both one-level and two-level CDA boast high rates of successful outcomes at 86% versus 96% at 1 year, respectively [99]. No difference in patient-reported outcomes measures (PROMs) has been noted between one- and two-level procedures [100,101]. However, higher overall success and satisfaction rates have been seen with two-level CDA versus two-level ACDF [92,102,103]. PROMs similarly improve in both ACDF and CDA for two-level procedures, though CDA has been demonstrated to be equivalent to superior short- and long-term improvements versus ACDF [92,100,102,104,105,106,107,108,109].
Biomechanical studies have compared ROM among single and multilevel fusion, arthroplasty, and hybrid constructs. The index level and total cervical ROM both decrease following single-level ACDF. Two-level ACDF increases adjacent-level ROM at both levels, arthroplasty has no significant change in adjacent ROM from preoperative values, and hybrid surgery increases ROM adjacent to the fused level [110,111,112]. In hybrid constructs, ROM at the arthroplasty level also increases [112]. These findings have been replicated in clinical studies with significantly less ROM at adjacent levels in hybrid constructs than in multilevel ACDF [113]. Following CDA, index-level ROM improves and is maintained at long-term follow-up [94,97,103,104,106,108,114,115,116,117]. This preservation of native ROM at the index and adjacent levels may reduce future ASD development.
Complications following CDA and ACDF include ASD, reoperation, dysphagia, and heterotopic ossification (HO). Overall, lower complication rates have been seen following CDA than following ACDF, with no significant difference in serious adverse events. Less ASD at both superior and inferior levels has been seen following arthroplasty versus fusion in one- and two-level procedures [92,95,104,116,117,118,119]. Superior adjacent-level disc height (an inversely related surrogate to ASD) decreased in both CDA and ACDF at 7 years with no significant difference between groups [120]. Jawahar et al. noted no difference in ASD between one- and two-level CDA, indicating that the presence of CDA does not affect overall ASD rates [121]. Like ASD, no difference in reoperation rates has been noted between one- and two-level CDA [101]. One- and two-level CDA demonstrated lower reoperation rates at both the index and adjacent levels versus ACDF [92,94,97,98,100,103,104,106,117,122,123]. Similarly, dysphagia following single-level CDA was demonstrated to occur at lower rates compared to ACDF at 7-year follow-up [124]. HO formation following CDA is a known potential outcome, with variable incidence rates in the literature ranging from 0% to 75% [100,108,115,125]. Its clinical significance has yet to be established [126]. Increased HO development has been seen in two-level CDA versus one-level CDA [100,101,126]. Higher rates of adjacent level ossification development (ALOD) have also been seen with ACDF than with CDA [127].
The currently available data suggest the statistically significant superiority of two-level CDA over two-level ACDF in long-term outcome measures and reoperation rates. Much of the existing data supporting multilevel CDA come from industry-funded IDE trials, with varying degrees of risk of bias [128]. Providers should consider the potential for conflicts of interest and bias when interpreting these results.
7.2. Laminoplasty
Laminoplasty (LP) is a procedure that, like CDA, preserves cervical ROM in an effort to reduce ASD [129]. LP has been indicated to treat myelopathy secondary to spondylosis or ossification of the posterior longitudinal ligament (OPLL). During the open door technique, an opening trough is made at the junction of the lamina and lateral mass. A second trough is made of the contralateral side with only the dorsal lamina being violated. The posterior arch is then hinged open and fixed in place using a small plate with screws, a suture, a suture anchor, and/or bone. Alternatively, the French door approach may be used with bilateral hinges and an opening through the midline. While the latter procedure potentially produces less blood loss, an open door LP is generally preferred due to greater improvements in PROMs, the maintenance of ROM, and a favorable complication profile [130]. LP is ideal for use in the lordotic spine, though the existing literature indicates it may be successful for patients with up to 13° of kyphotic alignment. Many surgeons prefer at a least neutral sagittal balance, as some loss of lordosis (approximately 5°) is expected after LP. By retaining the posterior spinous structures, the prevention of catastrophic kyphotic failure seen after laminectomy may be avoided. Most patients can expect a moderate improvement in PROMs after LP. Equal or superior PROM improvements are seen after LP versus ACDF or laminectomy [131,132,133]. An equivalent or decreased rate of complications is seen with LP than other treatments for cervical myelopathy. Posterior cervical procedures, including LP, have more wound complications, including infections, than anterior procedures. However, fewer infections are seen in LP than in a laminectomy. LP has similar postop JOA and neurological improvement, lower complications, and worse alignment correction than ACDF [134]. LP also has lower rates of C5 palsy than both ACDF and laminectomy [132,133].
8. Developments in Surgical Training
The foundation of spine surgery training remains hands-on, supervised learning with graduated autonomy applied in vivo. While studies have shown that this is an overall effective and safe method of education, training programs desire to provide translatable skills in consequence-free environments. Waisbrod et al. demonstrated no difference in outcomes or complications with single-level lumbar procedures performed by faculty surgeons or by trainees under supervision [135]. However, given that residents obtain varied instruction in their training and are limited by workhour regulations, innovative methodologies including synthetic and simulation models are being used to complement traditional surgical practice [136].
A key component of most curricula is bioskills training. This consists of task-oriented goals performed outside of the traditional operating room setting. Directed bioskills training improves trainee technical performance and decreases in-lab operating errors [137]. The most commonly used method of bioskills training is the utilization of human cadaveric models [138]. This established tool provides trainees with faithful exposure to anatomy and tissue [139]. Its use is limited by tissue degradation and expense concerns. Animal models also have potential benefits, particularly for minimally invasive surgical (MIS) training. Gotfryd et al. demonstrated that a swine model for MIS decompression and pedicle instrumentation training was similar and translatable to human procedures. Its value in intradiscal procedures was more limited [140]. Synthetic modeling with products, such as Sawbones, is another current practice [138,141]. It is a cheap alternative that provides haptic feedback but with significantly less tissue fidelity. Consequently, researchers are working to improve synthetic tissue biomechanical characteristics. For instance, Gragnaniello et al. developed a polymer that can be injected into animal or human cadaveric models in order to mimic spinal pathology [142]. Mixed reviews exist regarding perceptions of bioskills training efficacy. Instructing surgeons have reported that performance by their trainees in the bioskills laboratory would moderately encourage them to advance their trainees’ participation in the operating room [138].
While the utilization of surgical simulations is increasing in training programs, a deficiency in quality systems exists [141]. Examples of simulators include virtual reality, augmented reality, and synthetic models with adapted imaging systems. Furst et al. created an electromagnetic tracking system using a synthetic patient model that simulates fluoroscopic thoracolumbar pedicle screw placement [143]. These systems are valuable for practicing intraoperative tasks, such as pedicle screw instrumentation or dural repairs. Other skills, such as those specific to MIS procedures, have steep learning curves and are thus excellent candidates for pre-patient training. Simulators target surgical skill improvement but are also evaluation tools. A study by Wang et al. was able to differentiate novice, intermediate, and expert MIS operators by evaluating the task completion time, the instrument movement distance, and the number of movements [139]. Virtual reality simulators allow for full immersion and may be useful for training team-oriented tasks [144]. These systems do not provide haptic feedback, are expensive, and their effectiveness has not yet been validated.
Preoperative surgical planning software frequently accompanies robotic surgical systems. It allows surgeons to see the impact of planned interventions such as osteotomies, rod curvature, screw positioning, and various interbody implants. This may be utilized as a teaching tool for residents and fellows when developing preoperative plans, particularly in complex deformity correction. However, concerns exist regarding the use of robotics in training, leading to overreliance, lower anatomic knowledge, and less tactile skill development [145].
9. Conclusions
Emerging technologies within spine surgery have transformed the way that surgeons practice. Advancements allow for enhanced preoperative decision-making, improved patient outcomes, and better intraoperative execution. Changes in implant materials and device options also allow for fewer potential complications, such as subsidence, ASD, loss of alignment correction, and need for reoperation. As the field continues to advance, it is vital that practitioners remain knowledgeable of these changes in order to provide the most effective treatment possible.
Author Contributions
All authors contributed to the writing, editing, and review of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Diltz, Z.R.; Sheffer, B.J. Intraoperative Navigation and Robotics in Pediatric Spinal Deformity. Orthop. Clin. N. Am. 2023, 54, 201–207. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Wang, C.C.; Zhang, R.J.; Zhou, L.P.; Jia, C.Y.; Ge, P.; Shen, C.L. Predictors of accurate intrapedicular screw placement in single-level lumbar (L4-5) fusion: Robot-assisted pedicle screw, traditional pedicle screw, and cortical bone trajectory screw insertion. BMC Surg. 2022, 22, 284. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.D.; Kadiyala, M.; Talwar, D.; Sankar, W.N.; Flynn, J.J.M.; Anari, J.B. Does intraoperative CT navigation increase the accuracy of pedicle screw placement in pediatric spinal deformity surgery? A systematic review and meta-analysis. Spine Deform. 2022, 10, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Matur, A.V.; Palmisciano, P.; Duah, H.O.; Chilakapati, S.S.; Cheng, J.S.; Adogwa, O. Robotic and navigated pedicle screws are safer and more accurate than fluoroscopic freehand screws: A systematic review and meta-analysis. Spine J. 2023, 23, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Ringel, F.; Stuer, C.; Reinke, A.; Preuss, A.; Behr, M.; Auer, F.; Stoffel, M.; Meyer, B. Accuracy of robot-assisted placement of lumbar and sacral pedicle screws: A prospective randomized comparison to conventional freehand screw implantation. Spine 2012, 37, E496–E501. [Google Scholar] [CrossRef]
- Oba, H.; Uehara, M.; Ikegami, S.; Hatakenaka, T.; Kamanaka, T.; Miyaoka, Y.; Kurogouchi, D.; Fukuzawa, T.; Mimura, T.; Tanikawa, Y.; et al. Tips and pitfalls to improve accuracy and reduce radiation exposure in intraoperative CT navigation for pediatric scoliosis: A systematic review. Spine J. 2023, 23, 183–196. [Google Scholar] [CrossRef]
- Laine, T.; Lund, T.; Ylikoski, M.; Lohikoski, J.; Schlenzka, D. Accuracy of pedicle screw insertion with and without computer assistance: A randomised controlled clinical study in 100 consecutive patients. Eur. Spine J. 2000, 9, 235–240. [Google Scholar] [CrossRef]
- Mendelsohn, D.; Strelzow, J.; Dea, N.; Ford, N.L.; Batke, J.; Pennington, A.; Yang, K.; Ailon, T.; Boyd, M.; Dvorak, M.; et al. Patient and surgeon radiation exposure during spinal instrumentation using intraoperative computed tomography-based navigation. Spine J. 2016, 16, 343–354. [Google Scholar] [CrossRef]
- Bratschitsch, G.; Leitner, L.; Stucklschweiger, G.; Guss, H.; Sadoghi, P.; Puchwein, P.; Leithner, A.; Radl, R. Radiation Exposure of Patient and Operating Room Personnel by Fluoroscopy and Navigation during Spinal Surgery. Sci. Rep. 2019, 9, 17652. [Google Scholar] [CrossRef]
- McAfee, P.C. Spinal Navigation and Robotics Are More Accurate, More Precise, and More Minimally Invasive. Glob. Spine J. 2022, 12, 4S–6S. [Google Scholar] [CrossRef]
- Crawford, A.M.; Striano, B.M.; Lightsey, H.M.t.; Gong, J.; Simpson, A.K.; Schoenfeld, A.J. Intraoperative CT for lumbar fusion is not associated with improved short- or long-term complication profiles. Spine J. 2023, 23, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Sielatycki, J.A.; Mitchell, K.; Leung, E.; Lehman, R.A. State of the art review of new technologies in spine deformity surgery-robotics and navigation. Spine Deform. 2022, 10, 5–17. [Google Scholar] [CrossRef]
- Dominy, C.L.; Tang, J.E.; Arvind, V.; Cho, B.H.; Selverian, S.; Shah, K.C.; Kim, J.S.; Cho, S.K. Trends in the Charges and Utilization of Computer-Assisted Navigation in Cervical and Thoracolumbar Spinal Surgery. Asian Spine J. 2022, 16, 625–633. [Google Scholar] [CrossRef]
- Ghaednia, H.; Fourman, M.S.; Lans, A.; Detels, K.; Dijkstra, H.; Lloyd, S.; Sweeney, A.; Oosterhoff, J.H.F.; Schwab, J.H. Augmented and virtual reality in spine surgery, current applications and future potentials. Spine J. 2021, 21, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.S.; Yoon, J.W. Intra-operative wearable visualization in spine surgery: Past, present, and future. J. Spine Surg. 2022, 8, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Virk, S.; Lafage, R.; Elysee, J.; Louie, P.; Kim, H.J.; Albert, T.; Lenke, L.G.; Schwab, F.; Lafage, V. The 3 Sagittal Morphotypes That Define the Normal Cervical Spine: A Systematic Review of the Literature and an Analysis of Asymptomatic Volunteers. J. Bone Jt. Surg. Am. 2020, 102, e109. [Google Scholar] [CrossRef]
- Ling, F.P.; Chevillotte, T.; Leglise, A.; Thompson, W.; Bouthors, C.; Le Huec, J.C. Which parameters are relevant in sagittal balance analysis of the cervical spine? A literature review. Eur. Spine J. 2018, 27, 8–15. [Google Scholar] [CrossRef]
- Azimi, P.; Yazdanian, T.; Benzel, E.C.; Hai, Y.; Montazeri, A. Sagittal balance of the cervical spine: A systematic review and meta-analysis. Eur. Spine J. 2021, 30, 1411–1439. [Google Scholar] [CrossRef]
- Passias, P.G.; Vasquez-Montes, D.; Poorman, G.W.; Protopsaltis, T.; Horn, S.R.; Bortz, C.A.; Segreto, F.; Diebo, B.; Ames, C.; Smith, J.; et al. Predictive model for distal junctional kyphosis after cervical deformity surgery. Spine J. 2018, 18, 2187–2194. [Google Scholar] [CrossRef]
- Pettersson, S.D.; Skrzypkowska, P.; Ali, S.; Szmuda, T.; Krakowiak, M.; Pocivavsek, T.; Sunesson, F.; Fercho, J.; Miekisiak, G. Predictors for cervical kyphotic deformity following laminoplasty: A systematic review and meta-analysis. J. Neurosurg. Spine 2023, 38, 4–13. [Google Scholar] [CrossRef]
- Yang, X.; Bartels, R.; Donk, R.; Arts, M.P.; Goedmakers, C.M.W.; Vleggeert-Lankamp, C.L.A. The association of cervical sagittal alignment with adjacent segment degeneration. Eur. Spine J. 2020, 29, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Lafage, V.; Schwab, F.J.; Shaffrey, C.I.; Protopsaltis, T.; Klineberg, E.; Gupta, M.; Scheer, J.K.; Fu, K.M.; Mundis, G.; et al. Prevalence and type of cervical deformity among 470 adults with thoracolumbar deformity. Spine 2014, 39, E1001–E1009. [Google Scholar] [CrossRef] [PubMed]
- Odland, K.; Yson, S.; Polly, D.W., Jr. Wide anatomical variability of PI normative values within an asymptomatic population: A systematic review. Spine Deform. 2023, 11, 559–566. [Google Scholar] [CrossRef]
- Wang, T.; Ding, W. Risk factors for adjacent segment degeneration after posterior lumbar fusion surgery in treatment for degenerative lumbar disorders: A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 582. [Google Scholar] [CrossRef] [PubMed]
- Formica, M.; Quarto, E.; Zanirato, A.; Mosconi, L.; Lontaro-Baracchini, M.; Alessio-Mazzola, M.; Felli, L. ALIF in the correction of spinal sagittal misalignment. A systematic review of literature. Eur. Spine J. 2021, 30, 50–62. [Google Scholar] [CrossRef]
- Ochtman, A.E.A.; Kruyt, M.C.; Jacobs, W.C.H.; Kersten, R.; le Huec, J.C.; Oner, F.C.; van Gaalen, S.M. Surgical Restoration of Sagittal Alignment of the Spine: Correlation with Improved Patient-Reported Outcomes: A Systematic Review and Meta-Analysis. JBJS Rev. 2020, 8, e1900100. [Google Scholar] [CrossRef]
- Eun, I.S.; Son, S.M.; Goh, T.S.; Lee, J.S. Sagittal spinopelvic alignment after spinal fusion in degenerative lumbar scoliosis: A meta-analysis. Br. J. Neurosurg. 2020, 34, 176–180. [Google Scholar] [CrossRef]
- Wan, S.H.; Wong, D.L.; To, S.C.; Meng, N.; Zhang, T.; Cheung, J.P. Patient and surgical predictors of 3D correction in posterior spinal fusion: A systematic review. Eur. Spine J. 2023, 32, 1927–1946. [Google Scholar] [CrossRef]
- Katsuura, Y.; Colon, L.F.; Perez, A.A.; Albert, T.J.; Qureshi, S.A. A Primer on the Use of Artificial Intelligence in Spine Surgery. Clin. Spine Surg. 2021, 34, 316–321. [Google Scholar] [CrossRef]
- Gutman, M.J.; Schroeder, G.D.; Murphy, H.; Flanders, A.E.; Vaccaro, A.R. Artificial Intelligence in Spine Care. Clin. Spine Surg. 2021, 34, 121–124. [Google Scholar] [CrossRef]
- Kelly, C.J.; Karthikesalingam, A.; Suleyman, M.; Corrado, G.; King, D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 2019, 17, 195. [Google Scholar] [CrossRef]
- Charles, Y.P.; Lamas, V.; Ntilikina, Y. Artificial intelligence and treatment algorithms in spine surgery. Orthop. Traumatol. Surg. Res. 2023, 109, 103456. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.; Siebenwirth, J.; Caspari, C.; Drange, S.; Dreischarf, M.; Le Huec, J.C.; Putzier, M.; Franke, J. Can artificial intelligence support or even replace physicians in measuring sagittal balance? A validation study on preoperative and postoperative full spine images of 170 patients. Eur. Spine J. 2022, 31, 1943–1951. [Google Scholar] [CrossRef]
- Wu, H.; Bailey, C.; Rasoulinejad, P.; Li, S. Automated comprehensive Adolescent Idiopathic Scoliosis assessment using MVC-Net. Med. Image Anal. 2018, 48, 1–11. [Google Scholar] [CrossRef]
- Korez, R.; Putzier, M.; Vrtovec, T. A deep learning tool for fully automated measurements of sagittal spinopelvic balance from X-ray images: Performance evaluation. Eur. Spine J. 2020, 29, 2295–2305. [Google Scholar] [CrossRef]
- Weng, C.H.; Wang, C.L.; Huang, Y.J.; Yeh, Y.C.; Fu, C.J.; Yeh, C.Y.; Tsai, T.T. Artificial Intelligence for Automatic Measurement of Sagittal Vertical Axis Using ResUNet Framework. J. Clin. Med. 2019, 8, 1826. [Google Scholar] [CrossRef]
- Rasouli, J.J.; Shao, J.; Neifert, S.; Gibbs, W.N.; Habboub, G.; Steinmetz, M.P.; Benzel, E.; Mroz, T.E. Artificial Intelligence and Robotics in Spine Surgery. Glob. Spine J. 2021, 11, 556–564. [Google Scholar] [CrossRef]
- Carson, T.; Ghoshal, G.; Cornwall, G.B.; Tobias, R.; Schwartz, D.G.; Foley, K.T. Artificial Intelligence-enabled, Real-time Intraoperative Ultrasound Imaging of Neural Structures within the Psoas: Validation in a Porcine Spine Model. Spine 2021, 46, E146–E152. [Google Scholar] [CrossRef]
- Won, D.; Lee, H.J.; Lee, S.J.; Park, S.H. Spinal Stenosis Grading in Magnetic Resonance Imaging Using Deep Convolutional Neural Networks. Spine 2020, 45, 804–812. [Google Scholar] [CrossRef]
- Jamaludin, A.; Lootus, M.; Kadir, T.; Zisserman, A.; Urban, J.; Battie, M.C.; Fairbank, J.; McCall, I.; Genodisc, C. ISSLS PRIZE IN BIOENGINEERING SCIENCE 2017: Automation of reading of radiological features from magnetic resonance images (MRIs) of the lumbar spine without human intervention is comparable with an expert radiologist. Eur. Spine J. 2017, 26, 1374–1383. [Google Scholar] [CrossRef]
- Burns, J.E.; Yao, J.; Munoz, H.; Summers, R.M. Automated Detection, Localization, and Classification of Traumatic Vertebral Body Fractures in the Thoracic and Lumbar Spine at CT. Radiology 2016, 278, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Durand, W.M.; Lafage, R.; Hamilton, D.K.; Passias, P.G.; Kim, H.J.; Protopsaltis, T.; Lafage, V.; Smith, J.S.; Shaffrey, C.; Gupta, M.; et al. Artificial intelligence clustering of adult spinal deformity sagittal plane morphology predicts surgical characteristics, alignment, and outcomes. Eur. Spine J. 2021, 30, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Ames, C.P.; Smith, J.S.; Pellise, F.; Kelly, M.; Alanay, A.; Acaroglu, E.; Perez-Grueso, F.J.S.; Kleinstuck, F.; Obeid, I.; Vila-Casademunt, A.; et al. Artificial Intelligence Based Hierarchical Clustering of Patient Types and Intervention Categories in Adult Spinal Deformity Surgery: Towards a New Classification Scheme that Predicts Quality and Value. Spine 2019, 44, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Mroz, T.E.; Lubelski, D.; Williams, S.K.; O’Rourke, C.; Obuchowski, N.A.; Wang, J.C.; Steinmetz, M.P.; Melillo, A.J.; Benzel, E.C.; Modic, M.T.; et al. Differences in the surgical treatment of recurrent lumbar disc herniation among spine surgeons in the United States. Spine J. 2014, 14, 2334–2343. [Google Scholar] [CrossRef] [PubMed]
- Mourad, R.; Kolisnyk, S.; Baiun, Y.; Falk, A.; Yuriy, T.; Valerii, F.; Kopeev, A.; Suldina, O.; Pospelov, A.; Kim, J.; et al. Performance of hybrid artificial intelligence in determining candidacy for lumbar stenosis surgery. Eur. Spine J. 2022, 31, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Durand, W.M.; Daniels, A.H.; Hamilton, D.K.; Passias, P.; Kim, H.J.; Protopsaltis, T.; LaFage, V.; Smith, J.S.; Shaffrey, C.; Gupta, M.; et al. Artificial Intelligence Models Predict Operative Versus Nonoperative Management of Patients with Adult Spinal Deformity with 86% Accuracy. World Neurosurg. 2020, 141, e239–e253. [Google Scholar] [CrossRef]
- Pellise, F.; Serra-Burriel, M.; Smith, J.S.; Haddad, S.; Kelly, M.P.; Vila-Casademunt, A.; Sanchez Perez-Grueso, F.J.; Bess, S.; Gum, J.L.; Burton, D.C.; et al. Development and validation of risk stratification models for adult spinal deformity surgery. J. Neurosurg. Spine 2019, 31, 587–599. [Google Scholar] [CrossRef]
- Ames, C.P.; Smith, J.S.; Pellise, F.; Kelly, M.; Gum, J.L.; Alanay, A.; Acaroglu, E.; Perez-Grueso, F.J.S.; Kleinstuck, F.S.; Obeid, I.; et al. Development of predictive models for all individual questions of SRS-22R after adult spinal deformity surgery: A step toward individualized medicine. Eur. Spine J. 2019, 28, 1998–2011. [Google Scholar] [CrossRef]
- Ames, C.P.; Smith, J.S.; Pellise, F.; Kelly, M.P.; Gum, J.L.; Alanay, A.; Acaroglu, E.; Perez-Grueso, F.J.S.; Kleinstuck, F.S.; Obeid, I.; et al. Development of Deployable Predictive Models for Minimal Clinically Important Difference Achievement Across the Commonly Used Health-related Quality of Life Instruments in Adult Spinal Deformity Surgery. Spine 2019, 44, 1144–1153. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, F.; Sun, Y.; Chen, X.; Diao, Y.; Zhao, Y.; Huang, H.; Fan, X.; Zhang, G.; Li, X. The application of artificial intelligence in spine surgery. Front. Surg. 2022, 9, 885599. [Google Scholar] [CrossRef]
- Kuris, E.O.; Veeramani, A.; McDonald, C.L.; DiSilvestro, K.J.; Zhang, A.S.; Cohen, E.M.; Daniels, A.H. Predicting Readmission After Anterior, Posterior, and Posterior Interbody Lumbar Spinal Fusion: A Neural Network Machine Learning Approach. World Neurosurg. 2021, 151, e19–e27. [Google Scholar] [CrossRef] [PubMed]
- Azimi, P.; Benzel, E.C.; Shahzadi, S.; Azhari, S.; Mohammadi, H.R. Use of artificial neural networks to predict surgical satisfaction in patients with lumbar spinal canal stenosis: Clinical article. J. Neurosurg. Spine 2014, 20, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Durand, W.M.; DePasse, J.M.; Daniels, A.H. Predictive Modeling for Blood Transfusion after Adult Spinal Deformity Surgery: A Tree-Based Machine Learning Approach. Spine 2018, 43, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Staartjes, V.E.; de Wispelaere, M.P.; Vandertop, W.P.; Schroder, M.L. Deep learning-based preoperative predictive analytics for patient-reported outcomes following lumbar discectomy: Feasibility of center-specific modeling. Spine J. 2019, 19, 853–861. [Google Scholar] [CrossRef]
- Joshi, R.S.; Lau, D.; Ames, C.P. Artificial intelligence for adult spinal deformity: Current state and future directions. Spine J. 2021, 21, 1626–1634. [Google Scholar] [CrossRef]
- Kim, J.S.; Merrill, R.K.; Arvind, V.; Kaji, D.; Pasik, S.D.; Nwachukwu, C.C.; Vargas, L.; Osman, N.S.; Oermann, E.K.; Caridi, J.M.; et al. Examining the Ability of Artificial Neural Networks Machine Learning Models to Accurately Predict Complications Following Posterior Lumbar Spine Fusion. Spine 2018, 43, 853–860. [Google Scholar] [CrossRef]
- Kim, J.S.; Arvind, V.; Oermann, E.K.; Kaji, D.; Ranson, W.; Ukogu, C.; Hussain, A.K.; Caridi, J.; Cho, S.K. Predicting Surgical Complications in Patients Undergoing Elective Adult Spinal Deformity Procedures Using Machine Learning. Spine Deform. 2018, 6, 762–770. [Google Scholar] [CrossRef]
- Hopkins, B.S.; Mazmudar, A.; Driscoll, C.; Svet, M.; Goergen, J.; Kelsten, M.; Shlobin, N.A.; Kesavabhotla, K.; Smith, Z.A.; Dahdaleh, N.S. Using artificial intelligence (AI) to predict postoperative surgical site infection: A retrospective cohort of 4046 posterior spinal fusions. Clin. Neurol. Neurosurg. 2020, 192, 105718. [Google Scholar] [CrossRef]
- Khan, O.; Badhiwala, J.H.; Wilson, J.R.F.; Jiang, F.; Martin, A.R.; Fehlings, M.G. Predictive Modeling of Outcomes After Traumatic and Nontraumatic Spinal Cord Injury Using Machine Learning: Review of Current Progress and Future Directions. Neurospine 2019, 16, 678–685. [Google Scholar] [CrossRef]
- Merali, Z.G.; Witiw, C.D.; Badhiwala, J.H.; Wilson, J.R.; Fehlings, M.G. Using a machine learning approach to predict outcome after surgery for degenerative cervical myelopathy. PLoS ONE 2019, 14, e0215133. [Google Scholar] [CrossRef]
- Scheer, J.K.; Oh, T.; Smith, J.S.; Shaffrey, C.I.; Daniels, A.H.; Sciubba, D.M.; Hamilton, D.K.; Protopsaltis, T.S.; Passias, P.G.; Hart, R.A.; et al. Development of a validated computer-based preoperative predictive model for pseudarthrosis with 91% accuracy in 336 adult spinal deformity patients. Neurosurg. Focus 2018, 45, E11. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.; Pizones, J.; Raganato, R.; Safaee, M.M.; Scheer, J.K.; Pellise, F.; Ames, C.P. Future Data Points to Implement in Adult Spinal Deformity Assessment for Artificial Intelligence Modeling Prediction: The Importance of the Biological Dimension. Int. J. Spine Surg. 2023, 17, S34–S44. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Sartori, M.; Griffoni, C.; Valacco, M.; Tedesco, G.; Davassi, P.F.; Gasbarrini, A.; Fini, M.; Barbanti Brodano, G. Complications in Spinal Fusion Surgery: A Systematic Review of Clinically Used Cages. J. Clin. Med. 2022, 11, 6279. [Google Scholar] [CrossRef]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101–110. [Google Scholar] [CrossRef]
- Iunes, E.A.; Barletta, E.A.; Barba Belsuzarri, T.A.; Onishi, F.J.; Cavalheiro, S.; Joaquim, A.F. Correlation Between Different Interbody Grafts and Pseudarthrosis After Anterior Cervical Discectomy and Fusion Compared with Control Group: Systematic Review. World Neurosurg. 2020, 134, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.T.; Xu, Y.; Cao, B.; Dai, J.; Zhang, S.Y.; Huang, J.M.; Liang, S.; Jiang, F.X. Titanium-coated PEEK Versus Uncoated PEEK Cages in Lumbar Interbody Fusion: A Systematic Review and Meta-analysis of Randomized Controlled Trial. Clin. Spine Surg. 2023, 36, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Seaman, S.; Kerezoudis, P.; Bydon, M.; Torner, J.C.; Hitchon, P.W. Titanium vs. polyetheretherketone (PEEK) interbody fusion: Meta-analysis and review of the literature. J. Clin. Neurosci. 2017, 44, 23–29. [Google Scholar] [CrossRef]
- Ament, J.D.; Vokshoor, A.; Yee, R.; Johnson, J.P. A Systematic Review and Meta-Analysis of Silicon Nitride and Biomaterial Modulus as it Relates to Subsidence Risk in Spinal Fusion Surgery. N. Am. Spine Soc. J. 2022, 12, 100168. [Google Scholar] [CrossRef]
- Shi, L.Y.; Wang, A.; Zang, F.Z.; Wang, J.X.; Pan, X.W.; Chen, H.J. Tantalum-coated pedicle screws enhance implant integration. Colloids Surf. B Biointerfaces 2017, 160, 22–32. [Google Scholar] [CrossRef]
- Fiani, B.; Jarrah, R.; Shields, J.; Sekhon, M. Enhanced biomaterials: Systematic review of alternatives to supplement spine fusion including silicon nitride, bioactive glass, amino peptide bone graft, and tantalum. Neurosurg. Focus 2021, 50, E10. [Google Scholar] [CrossRef]
- Tan, J.H.; Cheong, C.K.; Hey, H.W.D. Titanium (Ti) cages may be superior to polyetheretherketone (PEEK) cages in lumbar interbody fusion: A systematic review and meta-analysis of clinical and radiological outcomes of spinal interbody fusions using Ti versus PEEK cages. Eur. Spine J. 2021, 30, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, H.; Li, C.; Liu, T.; Guan, J.; Yang, Y.; Yu, X. Polyetheretherketone (PEEK) rods versus titanium rods for posterior lumbar fusion surgery: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2023, 18, 348. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, A.; Siddiqi, I.; Ghanchi, H.; Lischalk, J.; Vrionis, F.; Ratliff, J.; Bilsky, M.; Hariri, O.R. Radiolucent Carbon Fiber-Reinforced Implants for Treatment of Spinal Tumors-Clinical, Radiographic, and Dosimetric Considerations. World Neurosurg. 2021, 152, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Perez-Roman, R.J.; Boddu, J.V.; Bashti, M.; Bryant, J.P.; Amadasu, E.; Gyedu, J.S.; Wang, M.Y. The Use of Carbon Fiber-Reinforced Instrumentation in Patients with Spinal Oncologic Tumors: A Systematic Review of Literature and Future Directions. World Neurosurg. 2023, 173, 13–22. [Google Scholar] [CrossRef]
- Singhatanadgige, W.; Tangchitcharoen, N.; Kerr, S.J.; Tanasansomboon, T.; Yingsakmongkol, W.; Kotheeranurak, V.; Limthongkul, W. A Comparison of Polyetheretherketone and Titanium-Coated Polyetheretherketone in Minimally Invasive Transforaminal Lumbar Interbody Fusion: A Randomized Clinical Trial. World Neurosurg. 2022, 168, e471–e479. [Google Scholar] [CrossRef]
- Hasegawa, T.; Inufusa, A.; Imai, Y.; Mikawa, Y.; Lim, T.H.; An, H.S. Hydroxyapatite-coating of pedicle screws improves resistance against pull-out force in the osteoporotic canine lumbar spine model: A pilot study. Spine J. 2005, 5, 239–243. [Google Scholar] [CrossRef]
- Sanden, B.; Olerud, C.; Johansson, C.; Larsson, S. Improved bone-screw interface with hydroxyapatite coating: An in vivo study of loaded pedicle screws in sheep. Spine 2001, 26, 2673–2678. [Google Scholar] [CrossRef]
- Liu, X.J.; Zhu, Q.S.; Sun, H.F.; Song, X.J.; Wang, C.L.; Wu, Y.T.; Ma, Y.H. The clinical efficacy of hydroxyapatite and its composites in spinal reconstruction: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4614–4624. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, Y.; Wang, W. Application of nano-hydroxyapatite matrix graft in inter-vertebral fusion therapy: A meta-analysis. BMC Musculoskelet. Disord. 2023, 24, 427. [Google Scholar] [CrossRef]
- Feng, J.T.; Yang, X.G.; Wang, F.; He, X.; Hu, Y.C. Efficacy and safety of bone substitutes in lumbar spinal fusion: A systematic review and network meta-analysis of randomized controlled trials. Eur. Spine J. 2020, 29, 1261–1276. [Google Scholar] [CrossRef]
- Plantz, M.; Lyons, J.; Yamaguchi, J.T.; Greene, A.C.; Ellenbogen, D.J.; Hallman, M.J.; Shah, V.; Yun, C.; Jakus, A.E.; Procissi, D.; et al. Preclinical Safety of a 3D-Printed Hydroxyapatite-Demineralized Bone Matrix Scaffold for Spinal Fusion. Spine 2022, 47, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Menezes, C.M.; Lacerda, G.C.; do Valle, G.S.O.; de Oliveira Arruda, A.; Menezes, E.G. Ceramic bone graft substitute vs autograft in XLIF: A prospective randomized single-center evaluation of radiographic and clinical outcomes. Eur. Spine J. 2022, 31, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Cottrill, E.; Premananthan, C.; Pennington, Z.; Ehresman, J.; Theodore, N.; Sciubba, D.M.; Witham, T. Radiographic and clinical outcomes of silicate-substituted calcium phosphate (SiCaP) bone grafts in spinal fusion: Systematic review and meta-analysis. J. Clin. Neurosci. 2020, 81, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.Y.; Kim, K.T.; Kim, K.G.; Lim, S.H.; Kim, Y.J.; Sohn, S.; Sheen, S.H.; Heo, C.Y.; Han, I. Safety and Tolerability of Stromal Vascular Fraction Combined with beta-Tricalcium Phosphate in Posterior Lumbar Interbody Fusion: Phase I Clinical Trial. Cells 2020, 9, 2250. [Google Scholar] [CrossRef]
- Kwon, B.T.; Kim, H.J.; Lee, S.; Park, S.M.; Ham, D.W.; Park, H.J.; Kwon, O.; Yeom, J.S. Feasibility and safety of a CaO-SiO2-P2O5-B2O3 bioactive glass ceramic spacer in posterior lumbar interbody fusion compared with polyetheretherketone cage: A prospective randomized controlled trial. Acta Neurochir. 2023, 165, 135–144. [Google Scholar] [CrossRef]
- Frost, A.; Bagouri, E.; Brown, M.; Jasani, V. Osteolysis following resorbable poly-L-lactide-co-D, L-lactide PLIF cage use: A review of cases. Eur. Spine J. 2012, 21, 449–454. [Google Scholar] [CrossRef]
- DiStefano, T.J.; Shmukler, J.O.; Danias, G.; Iatridis, J.C. The Functional Role of Interface Tissue Engineering in Annulus Fibrosus Repair: Bridging Mechanisms of Hydrogel Integration with Regenerative Outcomes. ACS Biomater. Sci. Eng. 2020, 6, 6556–6586. [Google Scholar] [CrossRef]
- Salamanna, F.; Tschon, M.; Borsari, V.; Pagani, S.; Martini, L.; Fini, M. Spinal fusion procedures in the adult and young population: A systematic review on allogenic bone and synthetic grafts when compared to autologous bone. J. Mater. Sci. Mater. Med. 2020, 31, 51. [Google Scholar] [CrossRef]
- Swank, M.L.; Lowery, G.L.; Bhat, A.L.; McDonough, R.F. Anterior cervical allograft arthrodesis and instrumentation: Multilevel interbody grafting or strut graft reconstruction. Eur. Spine J. 1997, 6, 138–143. [Google Scholar] [CrossRef]
- Lowery, G.L.; McDonough, R.F. The significance of hardware failure in anterior cervical plate fixation. Patients with 2- to 7-year follow-up. Spine 1998, 23, 181–186, discussion 186–187. [Google Scholar] [CrossRef]
- Lopez-Espina, C.G.; Amirouche, F.; Havalad, V. Multilevel cervical fusion and its effect on disc degeneration and osteophyte formation. Spine 2006, 31, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Radcliff, K.; Davis, R.J.; Hisey, M.S.; Nunley, P.D.; Hoffman, G.A.; Jackson, R.J.; Bae, H.W.; Albert, T.; Coric, D. Long-term Evaluation of Cervical Disc Arthroplasty with the Mobi-C(c) Cervical Disc: A Randomized, Prospective, Multicenter Clinical Trial with Seven-Year Follow-up. Int. J. Spine Surg. 2017, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Vleggeert-Lankamp, C.L.A.; Janssen, T.M.H.; van Zwet, E.; Goedmakers, C.M.W.; Bosscher, L.; Peul, W.; Arts, M.P. The NECK trial: Effectiveness of anterior cervical discectomy with or without interbody fusion and arthroplasty in the treatment of cervical disc herniation; a double-blinded randomized controlled trial. Spine J. 2019, 19, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, G.M.; Lavelle, W.F.; Florman, J.E.; Riew, K.D.; Levi, A.D. Symptomatic Adjacent Level Disease Requiring Surgery: Analysis of 10-Year Results from a Prospective, Randomized, Clinical Trial Comparing Cervical Disc Arthroplasty to Anterior Cervical Fusion. Neurosurgery 2019, 84, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Phillips, F.M.; Geisler, F.H.; Gilder, K.M.; Reah, C.; Howell, K.M.; McAfee, P.C. Long-term Outcomes of the US FDA IDE Prospective, Randomized Controlled Clinical Trial Comparing PCM Cervical Disc Arthroplasty with Anterior Cervical Discectomy and Fusion. Spine 2015, 40, 674–683. [Google Scholar] [CrossRef]
- Loumeau, T.P.; Darden, B.V.; Kesman, T.J.; Odum, S.M.; Van Doren, B.A.; Laxer, E.B.; Murrey, D.B. A RCT comparing 7-year clinical outcomes of one level symptomatic cervical disc disease (SCDD) following ProDisc-C total disc arthroplasty (TDA) versus anterior cervical discectomy and fusion (ACDF). Eur. Spine J. 2016, 25, 2263–2270. [Google Scholar] [CrossRef]
- Lavelle, W.F.; Riew, K.D.; Levi, A.D.; Florman, J.E. Ten-year Outcomes of Cervical Disc Replacement with the BRYAN Cervical Disc: Results from a Prospective, Randomized, Controlled Clinical Trial. Spine 2019, 44, 601–608. [Google Scholar] [CrossRef]
- Sasso, W.R.; Smucker, J.D.; Sasso, M.P.; Sasso, R.C. Long-term Clinical Outcomes of Cervical Disc Arthroplasty: A Prospective, Randomized, Controlled Trial. Spine 2017, 42, 209–216. [Google Scholar] [CrossRef]
- Goffin, J.; Van Calenbergh, F.; van Loon, J.; Casey, A.; Kehr, P.; Liebig, K.; Lind, B.; Logroscino, C.; Sgrambiglia, R.; Pointillart, V. Intermediate follow-up after treatment of degenerative disc disease with the Bryan Cervical Disc Prosthesis: Single-level and bi-level. Spine 2003, 28, 2673–2678. [Google Scholar] [CrossRef]
- Alvin, M.D.; Mroz, T.E. The Mobi-C cervical disc for one-level and two-level cervical disc replacement: A review of the literature. Med. Devices 2014, 7, 397–403. [Google Scholar] [CrossRef]
- Huppert, J.; Beaurain, J.; Steib, J.P.; Bernard, P.; Dufour, T.; Hovorka, I.; Stecken, J.; Dam-Hieu, P.; Fuentes, J.M.; Vital, J.M.; et al. Comparison between single- and multi-level patients: Clinical and radiological outcomes 2 years after cervical disc replacement. Eur. Spine J. 2011, 20, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Gornet, M.F.; Lanman, T.H.; Burkus, J.K.; Hodges, S.D.; McConnell, J.R.; Dryer, R.F.; Copay, A.G.; Nian, H.; Harrell, F.E., Jr. Cervical disc arthroplasty with the Prestige LP disc versus anterior cervical discectomy and fusion, at 2 levels: Results of a prospective, multicenter randomized controlled clinical trial at 24 months. J. Neurosurg. Spine 2017, 26, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Coric, D.; Guyer, R.D.; Bae, H.; Nunley, P.D.; Strenge, K.B.; Peloza, J.H.; Boltes, M.O.; Ohnmeiss, D.D. Prospective, multicenter study of 2-level cervical arthroplasty with a PEEK-on-ceramic artificial disc. J. Neurosurg. Spine 2022, 37, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J.; Kim, K.D.; Hisey, M.S.; Hoffman, G.A.; Bae, H.W.; Gaede, S.E.; Rashbaum, R.F.; Nunley, P.D.; Peterson, D.L.; Stokes, J.K. Cervical total disc replacement with the Mobi-C cervical artificial disc compared with anterior discectomy and fusion for treatment of 2-level symptomatic degenerative disc disease: A prospective, randomized, controlled multicenter clinical trial: Clinical article. J. Neurosurg. Spine 2013, 19, 532–545. [Google Scholar] [CrossRef]
- Cheng, L.; Nie, L.; Zhang, L.; Hou, Y. Fusion versus Bryan Cervical Disc in two-level cervical disc disease: A prospective, randomised study. Int. Orthop. 2009, 33, 1347–1351. [Google Scholar] [CrossRef]
- Gornet, M.F.; Lanman, T.H.; Burkus, J.K.; Dryer, R.F.; McConnell, J.R.; Hodges, S.D.; Schranck, F.W. Two-level cervical disc arthroplasty versus anterior cervical discectomy and fusion: 10-year outcomes of a prospective, randomized investigational device exemption clinical trial. J. Neurosurg. Spine 2019, 31, 508–518. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, Y.; Yuan, W.; Wang, X.; Chen, H.; Yang, L.; Zhang, Y. Cervical kinematics and radiological changes after Discover artificial disc replacement versus fusion. Spine J. 2014, 14, 867–877. [Google Scholar] [CrossRef]
- Kim, K.; Hoffman, G.; Bae, H.; Redmond, A.; Hisey, M.; Nunley, P.; Jackson, R.; Tahernia, D.; Araghi, A. Ten-Year Outcomes of 1- and 2-Level Cervical Disc Arthroplasty from the Mobi-C Investigational Device Exemption Clinical Trial. Neurosurgery 2021, 88, 497–505. [Google Scholar] [CrossRef]
- Kim, S.W.; Shin, J.H.; Arbatin, J.J.; Park, M.S.; Chung, Y.K.; McAfee, P.C. Effects of a cervical disc prosthesis on maintaining sagittal alignment of the functional spinal unit and overall sagittal balance of the cervical spine. Eur. Spine J. 2008, 17, 20–29. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, Z.; Hoof, T.V.; Kalala, J.P.; Liu, Z.; Wu, B. Comparison of hybrid constructs with 2-level artificial disc replacement and 2-level anterior cervical discectomy and fusion for surgical reconstruction of the cervical spine: A kinematic study in whole cadavers. Med. Sci. Monit. 2015, 21, 1031–1037. [Google Scholar] [CrossRef]
- Gandhi, A.A.; Kode, S.; DeVries, N.A.; Grosland, N.M.; Smucker, J.D.; Fredericks, D.C. Biomechanical Analysis of Cervical Disc Replacement and Fusion Using Single Level, Two Level, and Hybrid Constructs. Spine 2015, 40, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, Y.; Feng, R.; Han, S. Study on biomechanical analysis of two-level cervical Mobi-C and arthrodesis. Am. J. Transl. Res. 2021, 13, 12714–12723. [Google Scholar] [PubMed]
- Zhang, J.; Meng, F.; Ding, Y.; Li, J.; Han, J.; Zhang, X.; Dong, W. Comprehensive Analysis of Hybrid Surgery and Anterior Cervical Discectomy and Fusion in Cervical Diseases: A Meta-Analysis. Medicine 2020, 99, e19055. [Google Scholar] [CrossRef]
- Coric, D.; Guyer, R.D.; Nunley, P.D.; Musante, D.; Carmody, C.; Gordon, C.; Lauryssen, C.; Boltes, M.O.; Ohnmeiss, D.D. Prospective, randomized multicenter study of cervical arthroplasty versus anterior cervical discectomy and fusion: 5-year results with a metal-on-metal artificial disc. J. Neurosurg. Spine 2018, 28, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.J.; Kim, K.R.; Son, D.W.; Shin, D.A.; Yi, S.; Kim, K.N.; Yoon, D.H.; Ha, Y. Radiological Changes in Adjacent and Index Levels after Cervical Disc Arthroplasty. Yonsei Med. J. 2022, 63, 72–81. [Google Scholar] [CrossRef]
- Wu, T.K.; Wang, B.Y.; Meng, Y.; Ding, C.; Yang, Y.; Lou, J.G.; Liu, H. Multilevel cervical disc replacement versus multilevel anterior discectomy and fusion: A meta-analysis. Medicine 2017, 96, e6503. [Google Scholar] [CrossRef]
- Li, Y.; Shen, H.; Khan, K.Z.; Fang, S.; Liao, Z.; Liu, W. Comparison of Multilevel Cervical Disc Replacement and Multilevel Anterior Discectomy and Fusion: A Systematic Review of Biomechanical and Clinical Evidence. World Neurosurg. 2018, 116, 94–104. [Google Scholar] [CrossRef]
- Loidolt, T.; Kurra, S.; Riew, K.D.; Levi, A.D.; Florman, J.; Lavelle, W.F. Comparison of adverse events between cervical disc arthroplasty and anterior cervical discectomy and fusion: A 10-year follow-up. Spine J. 2021, 21, 253–264. [Google Scholar] [CrossRef]
- Satin, A.M.; Rogers-LaVanne, M.P.; Derman, P.B. Cervical Disk Arthroplasty and Range of Motion at 7 Years: Impact on Adjacent Level Degeneration. Clin. Spine Surg. 2023, 36, 83–89. [Google Scholar] [CrossRef]
- Miller, J.; Sasso, R.; Anderson, P.; Riew, K.D.; McPhilamy, A.; Gianaris, T. Adjacent Level Degeneration: Bryan Total Disc Arthroplasty Versus Anterior Cervical Discectomy and Fusion. Clin. Spine Surg. 2018, 31, E98–E101. [Google Scholar] [CrossRef]
- Jawahar, A.; Cavanaugh, D.A.; Kerr, E.J., 3rd; Birdsong, E.M.; Nunley, P.D. Total disc arthroplasty does not affect the incidence of adjacent segment degeneration in cervical spine: Results of 93 patients in three prospective randomized clinical trials. Spine J. 2010, 10, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J.; Davis, R.J.; Hoffman, G.A.; Bae, H.W.; Hisey, M.S.; Kim, K.D.; Gaede, S.E.; Nunley, P.D. Subsequent surgery rates after cervical total disc replacement using a Mobi-C Cervical Disc Prosthesis versus anterior cervical discectomy and fusion: A prospective randomized clinical trial with 5-year follow-up. J. Neurosurg. Spine 2016, 24, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.E.; Zigler, J.E.; Spivak, J.M.; Delamarter, R.B.; Darden, B.V., 2nd; Kopjar, B. ProDisc-C Total Disc Replacement Versus Anterior Cervical Discectomy and Fusion for Single-Level Symptomatic Cervical Disc Disease: Seven-Year Follow-up of the Prospective Randomized U.S. Food and Drug Administration Investigational Device Exemption Study. J. Bone Joint Surg. Am. 2015, 97, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Smucker, J.D.; Bassuener, S.R.; Sasso, R.C.; Riew, K.D. Comparison of Long-term Differences in Dysphagia: Cervical Arthroplasty and Anterior Cervical Fusion. Clin. Spine Surg. 2017, 30, E1160–E1164. [Google Scholar] [CrossRef]
- Zarkadis, N.J.; Cleveland, A.W.; Kusnezov, N.A.; Dunn, J.C.; Caram, P.M.; Herzog, J.P. Outcomes Following Multilevel Cervical Disc Arthroplasty in the Young Active Population. Mil. Med. 2017, 182, e1790–e1794. [Google Scholar] [CrossRef]
- Mehren, C.; Suchomel, P.; Grochulla, F.; Barsa, P.; Sourkova, P.; Hradil, J.; Korge, A.; Mayer, H.M. Heterotopic ossification in total cervical artificial disc replacement. Spine 2006, 31, 2802–2806. [Google Scholar] [CrossRef]
- Boody, B.S.; Lee, E.N.; Sasso, W.R.; Vinayek, S.; Demeter, J.M.; Sasso, R.C.; Smucker, J.D. Functional Outcomes Associated With Adjacent-level Ossification Disease 10 Years After Cervical Disc Arthroplasty or ACDF. Clin. Spine Surg. 2020, 33, E420–E425. [Google Scholar] [CrossRef]
- Xie, L.; Liu, M.; Ding, F.; Li, P.; Ma, D. Cervical disc arthroplasty (CDA) versus anterior cervical discectomy and fusion (ACDF) in symptomatic cervical degenerative disc diseases (CDDDs): An updated meta-analysis of prospective randomized controlled trials (RCTs). Springerplus 2016, 5, 1188. [Google Scholar] [CrossRef]
- Paracino, R.; Fasinella, M.R.; Mancini, F.; Marini, A.; Dobran, M. Review of laminoplasty versus laminectomy in the surgical management of cervical spondylotic myelopathy. Surg. Neurol. Int. 2021, 12, 44. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Welle, K.; Gathen, M.; Zhang, L.; Xiao, J.; Kabir, K. Comparative Effectiveness and Safety of Open-Door Laminoplasty, French-Door Laminoplasty, Laminectomy and Fusion, and Laminectomy Alone for Multilevel Degenerative Cervical Myelopathy: A Bayesian Network Analysis. Adv. Ther. 2022, 39, 117–139. [Google Scholar] [CrossRef]
- Weinberg, D.S.; Rhee, J.M. Cervical laminoplasty: Indication, technique, complications. J. Spine Surg. 2020, 6, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wo, J.; Wen, J.; Zhang, L.; Xu, W.; Wang, X. Laminoplasty versus laminectomy with fusion for treatment of multilevel cervical compressive myelopathy: An updated meta-analysis. Postgrad. Med. J. 2022, 98, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ren, R.; Ma, W.; Xu, S.; Peng, L.; Zhong, Z.; Zheng, Y. Comparison of Laminoplasty vs. Laminectomy for Cervical Spondylotic Myelopathy: A Systematic Review and Meta-Analysis. Front. Surg. 2021, 8, 790593. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Egawa, S.; Chikuda, H.; Wakao, N.; Furuya, T.; Kanchiku, T.; Nagoshi, N.; Fujiwara, Y.; Yoshida, M.; Taguchi, T.; et al. A systematic review and meta-analysis comparing anterior decompression with fusion and posterior laminoplasty for cervical spondylotic myelopathy. J. Orthop. Sci. 2021, 26, 116–122. [Google Scholar] [CrossRef]
- Waisbrod, G.; Mannion, A.F.; Fekete, T.F.; Kleinstueck, F.; Jeszenszky, D.; Haschtmann, D. Surgical training in spine surgery: Safety and patient-rated outcome. Eur. Spine J. 2019, 28, 807–816. [Google Scholar] [CrossRef]
- Pham, M.H.; Jakoi, A.M.; Wali, A.R.; Lenke, L.G. Trends in Spine Surgery Training During Neurological and Orthopaedic Surgery Residency: A 10-Year Analysis of ACGME Case Log Data. J. Bone Jt. Surg. Am. 2019, 101, e122. [Google Scholar] [CrossRef]
- Boody, B.S.; Hashmi, S.Z.; Rosenthal, B.D.; Maslak, J.P.; McCarthy, M.H.; Patel, A.A.; Savage, J.W.; Hsu, W.K. The Effectiveness of Bioskills Training for Simulated Lumbar Pedicle Screw Placement. Glob. Spine J. 2018, 8, 557–562. [Google Scholar] [CrossRef]
- McCarthy, M.H.; Boody, B.S.; Swiatek, P.R.; Rosenthal, B.D.; Savage, J.; Hsu, W.K.; Patel, A.A. The perceived efficacy and utility of spine bioskills curricula for resident and fellow education. J. Orthop. 2020, 20, 87–91. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, J. Simulation Training in Spine Surgery. J. Am. Acad. Orthop. Surg. 2022, 30, 400–408. [Google Scholar] [CrossRef]
- Gotfryd, A.O.; Paula, F.C.; Sauma, M.L.; Iutaka, A.S.; Rodrigues, L.M.R.; Meyer, G.P.C.; Teivelis, M.P.; Poetscher, A.W.; Del Curto, D.; Kang, D.W.W.; et al. Minimally invasive swine spine surgery training: Technical aspects, benefits, and anatomical limitations. Einstein 2022, 20, eAO6318. [Google Scholar] [CrossRef]
- Sayari, A.J.; Chen, O.; Harada, G.K.; Lopez, G.D. Success of Surgical Simulation in Orthopedic Training and Applications in Spine Surgery. Clin. Spine Surg. 2021, 34, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Gragnaniello, C.; Abou-Hamden, A.; Mortini, P.; Colombo, E.V.; Bailo, M.; Seex, K.A.; Litvack, Z.; Caputy, A.J.; Gagliardi, F. Complex Spine Pathology Simulator: An Innovative Tool for Advanced Spine Surgery Training. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2016, 77, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Furst, D.; Hollensteiner, M.; Gabauer, S.; Esterer, B.; Trieb, K.; Eckstein, F.; Schrempf, A. Transpedicular Approach on a Novel Spine Simulator: A Validation Study. J. Surg. Educ. 2018, 75, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Stefan, P.; Pfandler, M.; Wucherer, P.; Habert, S.; Furmetz, J.; Weidert, S.; Euler, E.; Eck, U.; Lazarovici, M.; Weigl, M.; et al. Team training and assessment in mixed reality-based simulated operating room: Current state of research in the field of simulation in spine surgery exemplified by the ATMEOS project. Unfallchirurg 2018, 121, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Liounakos, J.I.; Chenin, L.; Theodore, N.; Wang, M.Y. Robotics in Spine Surgery and Spine Surgery Training. Oper. Neurosurg. 2021, 21, 35–40. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).