An Alkaline Foregut Protects Herbivores from Latex in Forage, but Increases Their Susceptibility to Bt Endotoxin
Abstract
1. Introduction
1.1. Latex as a Herbivory Deterrent
1.2. Divergent Digestive Morphology of Foregut- and Hindgut-Fermenters
1.3. Alkaline Guts Show Susceptibility to Bacillus thuringiensis and Related δ-Endotoxins
1.4. Latex in Forage May Have Shaped the Morphology of Animal Digestive Systems
- Areas with abundant latex-containing plants correlate with the origins of foregut-fermenting animals. This correlation indicates that latex may have exerted a selective pressure for gut alkalinity.
- Foregut-fermenting (often polygastric) animals have higher first-chamber pH than hindgut-fermenting (monogastric) animals. This allows the former to tolerate small amounts of latex in the diet, whereas the latter have to avoid latex-containing forage. Awareness of this correlation is important anywhere captive animals are provided with forage, such as in farming operations or zoos.
- The presence of an alkaline gut pH makes foregut-fermenting vertebrates, metamorphosing tadpoles, and certain orders of insects susceptible to gut damage from Bt δ-endotoxin and related insecticidal toxins. Due to their perceived target-specific nature, these toxins have been widely disseminated and may pose a health threat to foregut-fermenting animals in the wild, as well as to domesticated livestock.
2. Materials and Methods
2.1. Literature Search
2.2. Search Categories
- Stomach pHs of foregut- and hindgut-fermenting herbivores.
- 2.
- Plant deterrents against herbivory, including latex.
- 3.
- Geography and evolution of foregut and hindgut fermenters.
- 4.
- The action of Bacillus thuringiensis and related toxins in animals with alkaline gut pH.
- 5.
- Occasionally, search terms were used to answer specific questions that arose while writing the paper. They were: stomach pH (wild animals by name); hindgut foregut digestion Cretaceous; evolution Artiodactyla Cretaceous; latex levels mature young leaves; Bt effect tadpoles; horses unripe apples; and cattle silvopasture.
2.3. Data Analysis
3. Results and Discussion
- Areas with abundant latex-containing plants coincide with the regions of origin of foregut-fermenting animals.
- 2.
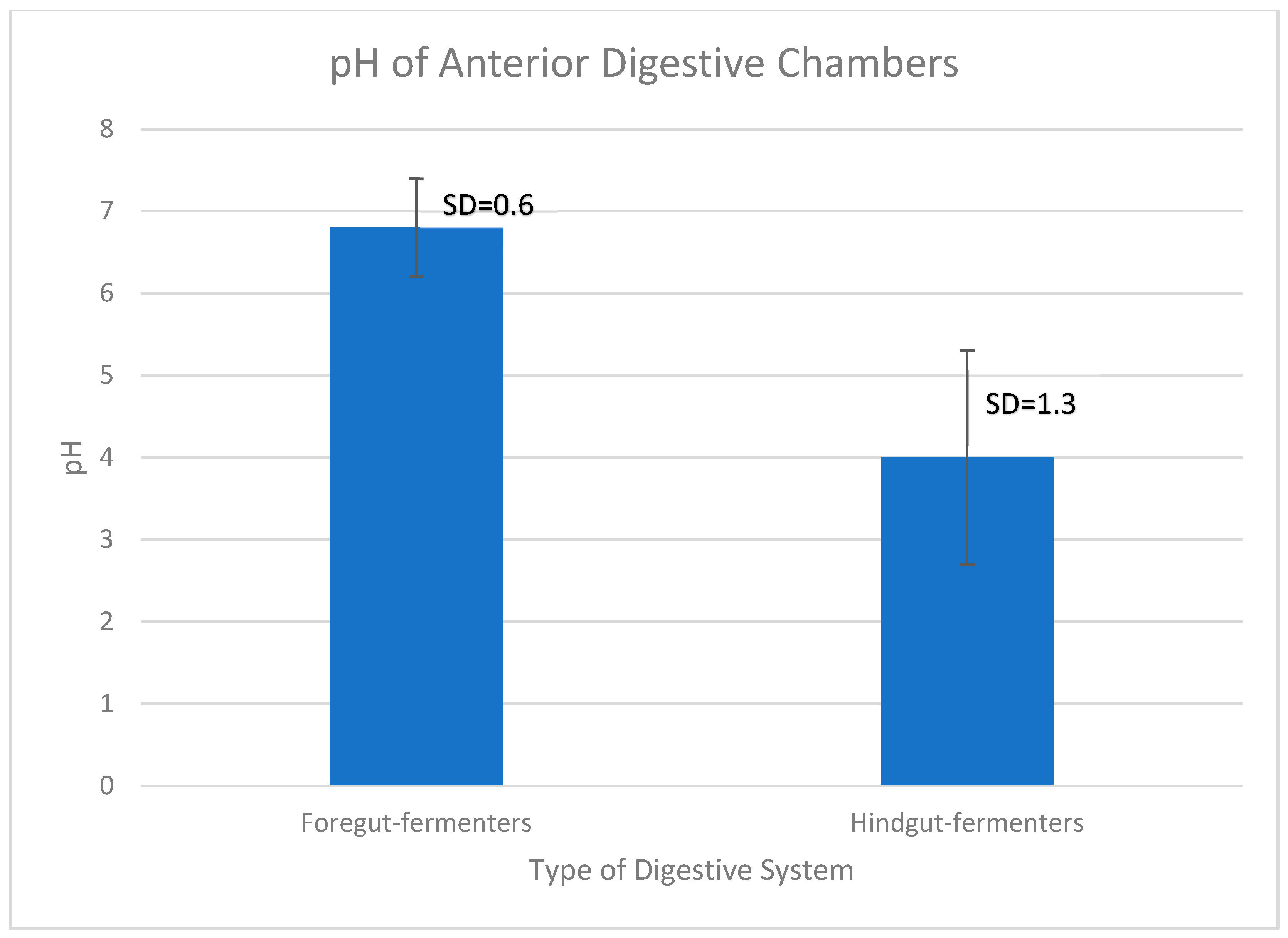
- Foregut-fermenting browsers have higher first-chamber pH, predictive of higher latex tolerance, than hindgut fermenters.
| Common Name | Binomial Name | Anterior Chamber pH | Reference |
|---|---|---|---|
| Ox | Bos sp. | 6 | [37] |
| Hippo | Hippopotamus amphibius | 5.7 | [38] |
| Sheep | Ovis aries | 6.4 | [37] |
| Brocket deer | Mazama sp. | 6.5 | [39] |
| Langur monkey | Presbytis cristatus | 5.9 | [40] |
| Collared peccary | Tayassu pecari | 6.3 | [39] |
| Cows | Bos taurus | 6.1 | [41] |
| Colobus monkey | Colobus polykomos | 6.8 | [42] |
| Camel | Camelus sp. | 6.4 | [43] |
| Hoatzin bird * | Opistocomus hoazin | 6.4 | [44] |
| Macropodid kangaroo | Macropodidae | 6.9 | [45] |
| Guanaco | Lama guanicoe f. glama | 6 | [46] |
| Sloth | Choloepus sp. | 7.4 | [45] |
| Quokka ** | Setonix brachyurus | 6.8 | [47] |
| Flathead grey mullet | Mugil cephalus, M.cerama | 7.8 | [48] |
| Common Name | Binomial Name | Anterior Chamber (Stomach) pH | Reference |
|---|---|---|---|
| Beaver | Castor canadensis | 1.6 | [51] |
| Rabbit | Oryctolagus cuniculus | 1.9 | [37] |
| Elephant | Loxodonta africana | 3.1 | [38] |
| Rhino | Diceros bicornis | 4.5 | [38] |
| Southern hairy nosed wombat | Lasiorhinus latifrons | 3.3 | [52] |
| Guinea pig | Cavia porcellus | 4.5 | [37] |
| Horse | Equus ferus caballus | 5.4 | [37] |
| Howler monkey | Alouatta palliata | 4.5 | [53] |
| New world porcupine | Erethizon dorsatum | 4.5 | [54] |
| Gerbil | Gerbillinae | 5.5 | [37] |
| Golden hamster | Mesocricetus auratus | 5.1 | [55] |
- 3.
- The presence of an alkaline gut pH makes foregut-fermenting mammals, metamorphosing tadpoles, and certain orders of insects susceptible to gut damage by Bt and related insecticidal toxins.
4. Implications
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoener, T.W. Theory of feeding strategies. Annu. Rev. Ecol. Syst. 1971, 2, 369–404. Available online: https://www.annualreviews.org/doi/abs/10.1146%2Fannurev.es.02.110171.002101 (accessed on 28 September 2023). [CrossRef]
- Allan, E.; Crawley, M.J. Contrasting effects of insect and molluscan herbivores on plant diversity in a long-term field experiment. Ecol. Lett. 2011, 14, 1246–1253. [Google Scholar] [CrossRef]
- Rivera-Vega, L.J.; Acevedo, F.E.; Felton, G.W. Genomics of Lepidoptera Saliva Reveals Function in Herbivory. Curr. Opin. Insect Sci. 2017, 19, 61–69. [Google Scholar] [CrossRef]
- Furness, J.B.; Cottrell, J.J.; Bravo, D.M. Comparative Gut Physiology Symposium: Comparative physiology of digestion. J. Anim. Sci. 2015, 93, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A.; Hastings, A.P. Plant Defense by Latex: Ecological Genetics of Inducibility in the Milkweeds and a General Review of Mechanisms, Evolution, and Implications for Agriculture. J. Chem. Ecol. 2019, 45, 1004–1018. [Google Scholar] [CrossRef] [PubMed]
- Warowicka, A.; Nawrot, R.; Goździcka-Józefiak, A. Pharmacologically active compounds from latex-bearing plants. Adv. Bot. Res. 2020, 93, 119–151. [Google Scholar] [CrossRef]
- Kerche-Silva, L.E.; Cavalcante, D.G.S.M.; Job, A.E. Natural rubber latex biomaterials in bone regenerative medicine. Biomater. Regen. Med. 2018, 1, 13. [Google Scholar] [CrossRef]
- Gracz-Bernaciak, J.; Mazur, O.; Nawrot, R. Functional Studies of Plant Latex as a Rich Source of Bioactive Compounds: Focus on Proteins and Alkaloids. Int. J. Mol. Sci. 2021, 22, 12427. [Google Scholar] [CrossRef]
- Abarca, L.F.S.; Kinkhamer, P.G.L.; Choi, Y.H. Plant Latex, from Ecological Interests to Bioactive Chemical Resources. Planta Med. 2019, 85, 856–868. [Google Scholar] [CrossRef]
- Yasuyuki, H. Production of natural rubber from Para rubber tree. Plant Biotechnol. 2009, 26, 67–70. [Google Scholar] [CrossRef]
- Yohanna, C.T.; Onaji, A.I.; Nyam, M.A.; Azila, J.J. Suitability of Latex-Producing Plant Species as Bio-security for some Landed Properties in Jos South, Jos, Plateau State. Int. J. Biol. Sci. 2023, 6, 01–15. [Google Scholar]
- Gordon, I.J.; Prins, H.H. (Eds.) The Ecology of Browsing and Grazing II; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; Volume 239, Available online: https://www.researchgate.net/profile/Rene-Van-Der-Wal/publication/337202219_The_Ecology_of_Browsing_and_Grazing_in_Other_Vertebrate_Taxa/links/5edde7df4585152945445f41/The-Ecology-of-Browsing-and-Grazing-in-Other-Vertebrate-Taxa.pdf#page=409 (accessed on 15 April 2022).
- Shipley, L.A. Grazers and browsers: How digestive morphology affects diet selection. Grazing Behav. Livest. Wildl. 1999, 70, 20–27. Available online: https://www.webpages.uidaho.edu/range456/readings/shipley.pdf (accessed on 25 March 2022).
- Agrawal, A.A.; Konno, K. Latex: A model for understanding mechanisms, ecology, and evolution of plant defense against herbivory. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 311–331. [Google Scholar] [CrossRef]
- Konno, K.; Hirayama, C.; Nakamura, M.; Tateishi, K.; Tamura, Y.; Hattori, M.; Kohno, K. Papain protects papaya trees from herbivorous insects: Role of cysteine protease in latex. Plant J. 2004, 37, 370–378. [Google Scholar] [CrossRef]
- Salazar, D.; Marquis, R.J. Herbivore Pressure Increases toward the Equator. Proc. Natl. Acad. Sci. USA 2012, 109, 12616–12620. [Google Scholar] [CrossRef]
- Friis, E.M.; Pedersen, K.R.; Crane, P.R. When Earth started blooming: Insights from the fossil record. Curr. Opin. Plant Biol. 2005, 8, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Epping, J.; Schulze Gronover, C.; Fricke, J.; Aziz, Z.; Brillatz, T.; Swyers, M.; Köllner, T.G.; Vogel, H.; Hammerbacher, A.; et al. A latex metabolite benefits plant fitness under root herbivore attack. PLoS Biol. 2016, 14, e1002332. [Google Scholar] [CrossRef]
- Angiosperm-Classification. (n.d.) Encyclopedia Britannica. Available online: https://www.britannica.com/plant/angiosperm/Classification (accessed on 24 August 2023).
- Ahrestani, F.S.; Heitkönig, I.M.; Matsubayashi, H.; Prins, H.H. Grazing and Browsing by Large Herbivores in South and Southeast Asia. In The Ecology of Large Herbivores in South and Southeast Asia; Ahrestani, F., Sankaran, M., Eds.; Springer: Dordrecht, The Netherlands, 2016; Volume 225. [Google Scholar] [CrossRef]
- Caccia, S.; Casartelli, M.; Tettamanti, G. The amazing complexity of insect midgut cells: Types, peculiarities, and functions. Cell Tissue Res. 2019, 377, 505–525. [Google Scholar] [CrossRef]
- McNamara, S.; Wlizla, M.; Horb, M.E. Husbandry, general care, and transportation of Xenopus laevis and Xenopus tropicalis. In Xenopus: Methods and Protocols; Humana Press: New York, NY, USA, 2018; pp. 1–17. [Google Scholar] [CrossRef]
- Chalmers, A.D.; Slack, J.M. The Xenopus tadpole gut: Fate maps and morphogenetic movements. Development 2000, 127, 381–392. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=baf7c5d4eab41df3d3b1f1c6435b5a90dc9f6aa9 (accessed on 28 October 2023). [CrossRef]
- Kohl, K.D.; Cary, T.L.; Karasov, W.H.; Dearing, M.D. Restructuring of the amphibian gut microbiota through metamorphosis. Env Microbiol Rep. 2013, 5, 899–903. [Google Scholar] [CrossRef]
- Berry, C. The bacterium, Lysinibacillus sphaericus, as an insect pathogen. J. Invertebr. Pathol. 2012, 109, 1–10. [Google Scholar] [CrossRef]
- Baranek, J.; Pogodziński, B.; Szipluk, N.; Zielezinski, A. TOXiTAXi: A web resource for toxicity of Bacillus thuringiensis protein compositions towards species of various taxonomic groups. Sci. Rep. 2020, 10, 19767. [Google Scholar] [CrossRef] [PubMed]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst. Rev. 2021, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, D.; Bacon, C.D.; Ding, W.; Zhang, Q.; Donoghue, P.C.; Antonelli, A.; Xing, Y. Fossil data support a pre-Cretaceous origin of flowering plants. Nat. Ecol. Evol. 2021, 5, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Amnh.org. Evolution Perissodactyl. 2014. Available online: https://research.amnh.org/paleontology/perissodactyl/evolution/intro (accessed on 14 October 2023).
- Cassini, G.H.; Muñoz, N.A.; Merino, M.L.; Agnolin, F.L.; Lio, G.L.; Brisson-Egli, F.; Chimento, N.R.; Novas, F.E. Evolutionary history of South American Artiodactyla. Hist. Evol. Paleobiogeográfica Vertebr. América Sur. Contrib. MACN 2016, 6, 673–689. Available online: https://www.researchgate.net/profile/Guillermo-Cassini/publication/331071363_Evolutionary_History_of_South_American_Artiodactyla/links/5c641ed845851582c3e5aeb0/Evolutionary-History-of-South-American-Artiodactyla.pdf (accessed on 15 October 2023).
- Julliot, C.; Sabatier, D. Diet of the red howler monkey (Alouatta seniculus) in French Guiana. Int. J. Primatol. 1993, 14, 527–550. [Google Scholar] [CrossRef]
- Herrel, A.; Huyghe, K.; Vanhooydonck, B.; Backeljau, T.; Breugelmans, K.; Grbac, I.; Van Damme, R.; Irschick, D.J. Rapid large-scale evolutionary divergence in morphology and performance associated with exploitation of a different dietary resource. Proc. Natl. Acad. Sci. USA 2008, 105, 4792–4795. [Google Scholar] [CrossRef]
- Yong, E. The Giant Panda Is a Closet Carnivore. The Atlantic. 2019. Available online: https://www.theatlantic.com/science/archive/2019/05/giant-panda-closet-carnivore/588553 (accessed on 15 October 2023).
- Ng, J.W.; Othman, N.; Yusof, N.H. Various Coagulation Techniques and Their Impacts towards the Properties of Natural Rubber Latex from Hevea Brasiliensis—A Comprehensive Review Related to Tyre Application. Ind. Crops Prod. 2022, 181, 114835. [Google Scholar] [CrossRef]
- Sarkar, B.; Mandal, S. Gordonia sp. BSTG01 isolated from Hevea brasiliensis plantation efficiently degrades polyisoprene (rubber). 3 Biotech 2021, 11, 508. [Google Scholar] [CrossRef]
- Smith, H.W. Observations on the flora of the alimentary tract of animals and factors affecting its composition. J. Pathol. 1965, 89, 95–122. [Google Scholar] [CrossRef]
- Clemens, E.T.; Maloiy, G.M.O. The digestive physiology of three East African herbivores: The elephant, rhinoceros and hippopotamus. J. Zool. 1982, 198, 141–156. Available online: http://www.rhinoresourcecenter.com/pdf_files/151/1519596941.pdf (accessed on 3 November 2023). [CrossRef]
- Bodmer, R.E. Frugivory in Amazonian Artiodactyla: Evidence for the evolution of the ruminant stomach. J. Zool 1989, 219, 457–467. [Google Scholar] [CrossRef]
- Bauchop, T.; Martucci, R.W. Ruminant-like digestion of the langur monkey. Science 1968, 161, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Duffield, T.; Plaizier, J.C.; Fairfield, A.; Bagg, R.; Vessie, G.; Dick, P.; Wilson, J.; Aramini, J.; McBride, B. Comparison of techniques for measurement of rumen pH in lactating dairy cows. J. Dairy Sci. 2004, 87, 59–66. [Google Scholar] [CrossRef]
- Kay, R.N.B.; Hoppe, P.; Maloiy, G.M.O. Fermentative digestion of food in the colobus monkey, Colobus polykomos. Experientia 1976, 32, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.J. Rumen function in the camel. Nature 1963, 4873, 1221. Available online: https://www.nature.com/articles/1971221a0.pdf (accessed on 3 November 2023). [CrossRef]
- Grajal, A. Structure and Function of the Digestive Tract of the Hoatzin (Opisthocomus hoazin): A Folivorous Bird with Foregut Fermentation. Auk 1995, 112, 20–28. [Google Scholar] [CrossRef]
- Dehority, B.A. Foregut fermentation. In Gastrointestinal Microbiology: Volume 1 Gastrointestinal Ecosystems and Fermentations; Springer: Boston, MA, USA, 1997; pp. 39–83. Available online: https://link.springer.com/content/pdf/10.1007/978-1-4615-4111-0_3.pdf?pdf=inline%20link (accessed on 13 July 2023).
- Heller, R.; Gregory, P.C.; Engelhardt, W.v. Pattern of motility and flow of digesta in the forestomach of the llama (Lama guanacoe f. glama). J. Comp. Physiol. B 1984, 154, 529–533. [Google Scholar] [CrossRef]
- Moir, R.J.; Somers, M.; Waring, H. Studies on marsupial nutrition I. Ruminant-like digestion in a herbivorous marsupial (Setonix brachyurus Quoy & Gaimard). Aus. J. Biol. Sci. 1956, 9, 293–304. Available online: https://www.publish.csiro.au/bi/pdf/bi9560293 (accessed on 3 November 2023).
- Payne, A.I. Gut ph and digestive strategies in estuarine grey mullet (Mugilidae) and tilapia (Cichlidae). J. Fish Biol. 1978, 13, 627–629. [Google Scholar] [CrossRef]
- Mureb, L.S.; Rocha-Santos, L.; Cassano, C.R.; da Silva Lopes, G.; Rosa, B.; Miranda, F.R.; Miranda, C.R.R.; Giné, G.A.F. Tree diversity mediates individual diet specialization of the maned sloth (Bradypus torquatus). Mamm. Biol. 2023, 103, 145–159. [Google Scholar] [CrossRef]
- Harris, T.R.; Chapman, C.A. Variation in diet and ranging of black and white colobus monkeys in Kibale National Park, Uganda. Primates 2007, 48, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Kitts, W.D.; Bose, R.J.; Wood, A.J.; Cowan, I.M. Preliminary observations on the digestive enzyme system of the beaver (Castor canadensis). Can. J. Zool. 1957, 35, 449–452. [Google Scholar] [CrossRef]
- Hume, I.D. (Ed.) Herbivorous marsupials—The non-macropodids. In Digestive Physiology and Nutrition of Marsupials; Press Syndicate of the University of Cambridge: Cambridge, UK, 1982; pp. 69–110. Available online: https://archive.org/details/digestivephysiol0000hume (accessed on 3 November 2023).
- Milton, K.; McBee, R.H. Rates of fermentative digestion in the howler monkey, Alouatta palliata (Primates: Ceboidea). Comp Comp. Biochem. Physiol. Part A Physiol. 1983, 74, 29–31. [Google Scholar] [CrossRef]
- Beasley, D.E.; Koltz, A.M.; Lambert, J.E.; Fierer, N.; Dunn, R.R. The evolution of stomach acidity and its relevance to the human microbiome. PLoS ONE 2015, 10, e0134116. [Google Scholar] [CrossRef]
- Williams, P.J.; Taylor, T.G. A comparative study of phytate hydrolysis in the gastrointestinal tract of the golden hamster (Mesocricetus auratus) and the laboratory rat. Brit. J. Nutr. 1985, 54, 429–435. Available online: https://www.cambridge.org/core/services/aop-cambridge-core/content/view/A8C80DC8946FDA1D4FF515011C871C86/S0007114585000472a.pdf/div-class-title-a-comparative-study-of-phytate-hydrolysis-in-the-gastrointestinal-tract-of-the-golden-hamster-span-class-italic-mesocricetus-auratus-span-and-the-laboratory-rat-div.pdf (accessed on 15 July 2023). [CrossRef]
- Thirunavukkarasu, K.; Yoheswaran, K. Coagulation of rubber latex in the stomach. Br. Med. J. 1967, 4, 484. [Google Scholar] [CrossRef][Green Version]
- Cohen, Z.P. Bacillus thuringiensis, bio-pesticide. Cornell.edu. 2015. Available online: https://biocontrol.entomology.cornell.edu/pathogens/bacillus.php (accessed on 24 August 2023).
- De Maagd, R.A.; Bravo, A.; Crickmore, N. How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. TRENDS Genet. 2001, 17, 193–199. [Google Scholar] [CrossRef]
- Glöckner, G.; Séralini, G.É. Pathology reports on the first cows fed with Bt176 maize (1997–2002). Sch. J. Agric. Sci. 2016, 6, 1–8. Available online: https://jeffreydachmd.com/wp-content/uploads/2016/07/Pathology-reports-cows-fed-Bt176-maize-Gl%C3%B6ckner-and-S%C3%A9ralini-2016.pdf (accessed on 15 July 2023).
- Ramdas, S.R. Bt Cotton and Livestock: Health Impacts, Bio-Safety Concerns and the Legitimacy of Public Scientific Research Institutions. National Workshop on Genetically Modified Crops/Foods and Heath Impacts. Available online: http://indiaenvironmentportal.org.in/files/bt-cotton-and-livestock-health-impacts-dr-sagari-r-ramdas.pdf (accessed on 15 July 2023).
- Hashim, M.A.; ElObied, G.H.; Adawi, I.A. Respondents Evolution of the Effect of Grazing on Bt-cotton Crop Residues by Ruminants on Health and Milk Characteristics in Gezira State, Sudan. Int. J. Res. Agric. Sci. 2017, 4, 304–309. Available online: http://www.ijras.org/administrator/components/com_jresearch/files/publications/IJRAS_610_FINAL.pdf (accessed on 15 July 2023).
- Rubio-Infante, N.; Moreno-Fierros, L. An overview of the safety and biological effects of Bacillus thuringiensis Cry toxins in mammals. J. Appl. Toxicol. 2016, 36, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Folmer, J.D.; Grant, R.J.; Milton, C.T.; Beck, J. Utilization of Bt corn residues by grazing beef steers and Bt corn silage and grain by growing beef cattle and lactating dairy cows. J. Anim. Sci. 2002, 80, 1352–1361. [Google Scholar] [CrossRef]
- Faust, M.; Smith, B.; Rice, D.; Owens, F.; Hinds, M.; Dana, G.; Hunst, P. Performance of lactating dairy cows fed silage and grain from a maize hybrid with the cry1F trait versus its nonbiotech counterpart. J. Dairy Sci. 2007, 90, 5706–5713. [Google Scholar] [CrossRef]
- Lajmanovich, R.C.; Junges, C.M.; Cabagna-Zenklusen, M.C.; Attademo, A.M.; Peltzer, P.M.; Maglianese, M.; Márquez, V.E.; Beccaria, A.J. Toxicity of Bacillus thuringiensis var. israelensis in aqueous suspension on the South American common frog Leptodactylus latrans (Anura: Leptodactylidae) tadpoles. Environ. Res. 2015, 136, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Bjorndal, K.A. Fermentation in Reptiles and Amphibians. In Gastrointestinal Microbiology; Mackie, R.I., White, B.A., Eds.; Chapman & Hall Microbiology Series; Springer: Boston, MA, USA, 1997; pp. 199–230. [Google Scholar] [CrossRef]
- Zaayman, J.L. Bt Maize and Frogs: An Investigation into Possible Adverse Effects of Bt Toxin Exposure to Amphibian Larvae. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2012. Available online: https://repository.nwu.ac.za/bitstream/handle/10394/9869/Zaayman_JL.pdf?sequence=1 (accessed on 10 October 2023).
- Spilatro, S.R.; Mahlberg, P.G. Latex and laticifer starch content of developing leaves of Euphorbia pulcherrima. Am. J. Bot. 1986, 73, 1312–1318. [Google Scholar] [CrossRef]
- Crissey, S. The complexity of formulating diets for zoo animals: A matrix. Int. Zoo Yearbook 2005, 39, 36–43. [Google Scholar] [CrossRef]
- Anonymous. Can Horses Eat Apples?—National Equine. 2022. Available online: https://www.nationalequine.org/feeding/horses-eat-apples/ (accessed on 16 October 2023).
- Jose, S.; Dollinger, J. Silvopasture: A sustainable livestock production system. Agroforest Syst. 2019, 93, 1–9. [Google Scholar] [CrossRef]
- Raymond, B.; Federici, B.A. In defence of Bacillus thuringiensis, the safest and most successful microbial insecticide available to humanity—A response to EFSA. FEMS Microbiol. Ecol. 2017, 93, fix084. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.; Johnson, C.; Perani, M. The safety of Bacillus thuringiensis to mammals investigated by oral and subcutaneous dosage. World J. Microbiol. Biot. 1999, 15, 375–380. [Google Scholar] [CrossRef]
- Webster, C.C.; Paardekooper, E.C. The botany of the rubber tree. In Rubber Tropical Agriculture Series; Webster, C.C., Baulkwill, W.J., Eds.; Longman Scientific & Technical/John Wiley & Sons, Inc.: New York, NY, USA, 1989. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajan, V. An Alkaline Foregut Protects Herbivores from Latex in Forage, but Increases Their Susceptibility to Bt Endotoxin. Life 2023, 13, 2195. https://doi.org/10.3390/life13112195
Rajan V. An Alkaline Foregut Protects Herbivores from Latex in Forage, but Increases Their Susceptibility to Bt Endotoxin. Life. 2023; 13(11):2195. https://doi.org/10.3390/life13112195
Chicago/Turabian StyleRajan, Vidya. 2023. "An Alkaline Foregut Protects Herbivores from Latex in Forage, but Increases Their Susceptibility to Bt Endotoxin" Life 13, no. 11: 2195. https://doi.org/10.3390/life13112195
APA StyleRajan, V. (2023). An Alkaline Foregut Protects Herbivores from Latex in Forage, but Increases Their Susceptibility to Bt Endotoxin. Life, 13(11), 2195. https://doi.org/10.3390/life13112195






