Host–Parasitoid Phenology, Distribution, and Biological Control under Climate Change
Abstract
1. Introduction
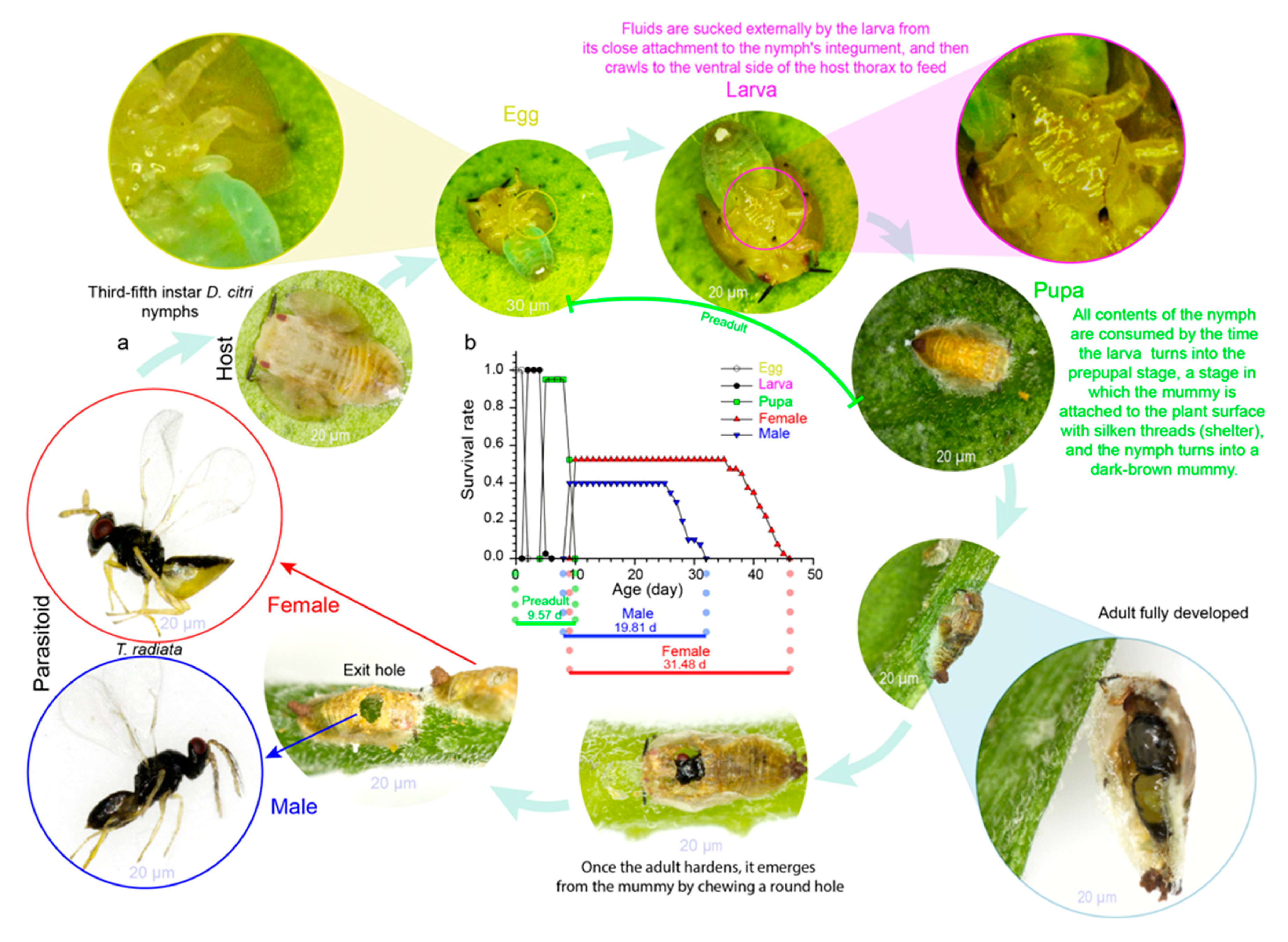
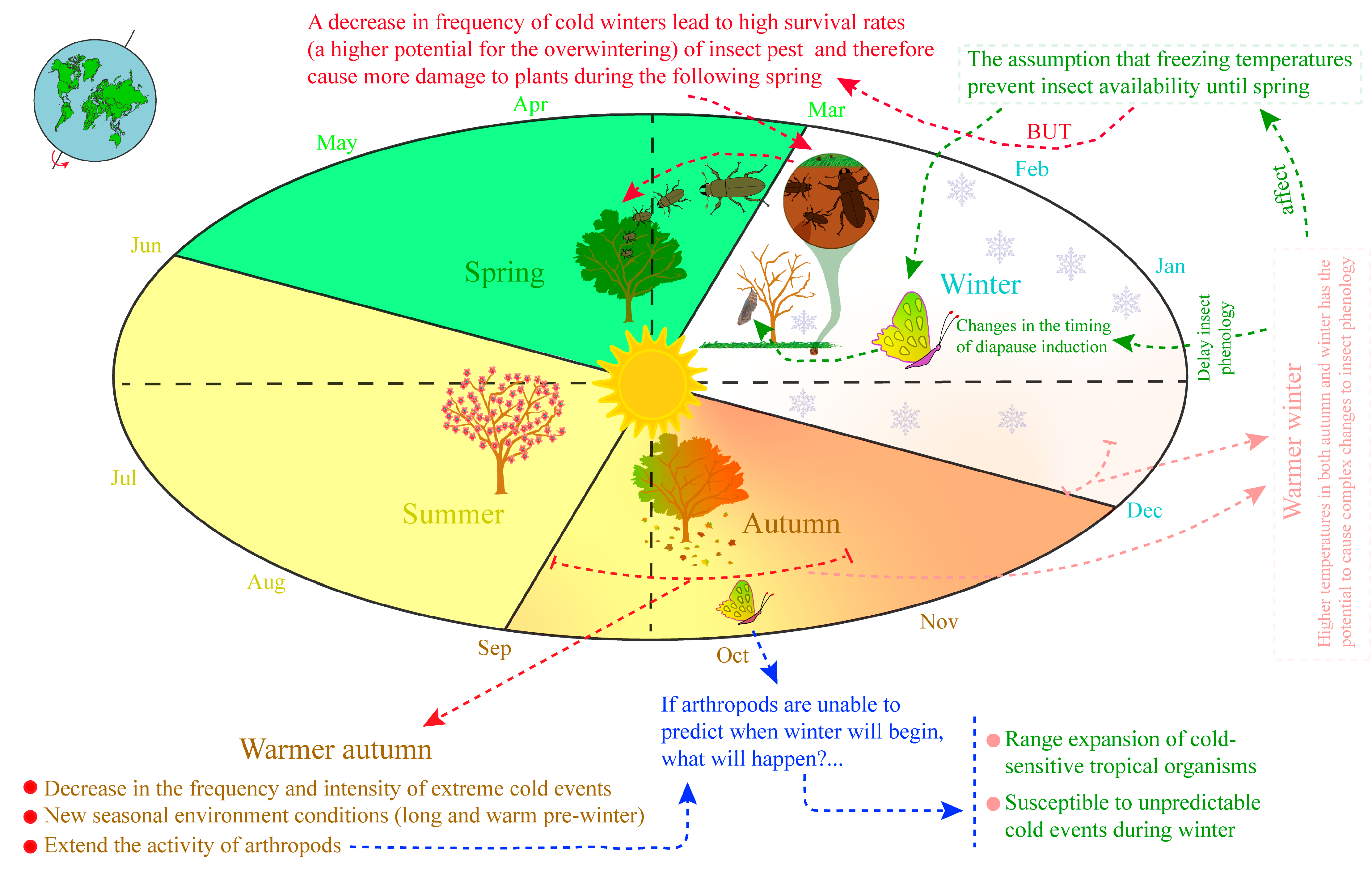
2. Arthropods’ Phenology and Climate Relationship
3. Host–Parasitoid Geographical Distribution under Climate Change
4. Warmer Winter Effects on Host–Parasitoid Interactions
5. Temperature Tolerance Ranges and Implications for Biocontrol Efficacy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romshoo, S.A.; Bashir, J.; Rashid, I. Twenty-first century-end climate scenario of Jammu and Kashmir Himalaya, India, using ensemble climate models. Clim. Chang. 2020, 162, 1473–1491. [Google Scholar] [CrossRef]
- Liu, P.R.; Raftery, A.E. Country-based rate of emissions reductions should increase by 80% beyond nationally determined contributions to meet the 2 °C target. Commun. Earth Environ. 2021, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Schewe, J.; Gosling, S.N.; Reyer, C.; Zhao, F.; Ciais, P.; Elliott, J.; Francois, L.; Huber, V.; Lotze, H.K.; Seneviratne, S.I.; et al. State-of-the-art global models underestimate impacts from climate extremes. Nat. Commun. 2019, 10, 1005. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.-O.; Roberts, D.C.; Adams, H.; Adler, C.; Aldunce, P.; Ali, E.; Begum, R.A.; Betts, R.; Kerr, R.B.; Biesbroek, R. Climate Change 2022: Impacts, Adaptation and Vulnerability; IPCC: Geneva, Switzerland, 2022. [Google Scholar]
- Abarca, M.; Spahn, R. Direct and indirect effects of altered temperature regimes and phenological mismatches on insect populations. Curr. Opin. Insect Sci. 2021, 47, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Postema, E.G.; Hayes, T.E.; Lippey, M.K.; MacArthur-Waltz, D.J. The complexity of global change and its effects on insects. Curr. Opin. Insect Sci. 2021, 47, 90–102. [Google Scholar] [CrossRef]
- Antão, L.H.; Bates, A.E.; Blowes, S.A.; Waldock, C.; Supp, S.R.; Magurran, A.E.; Dornelas, M.; Schipper, A.M. Temperature-related biodiversity change across temperate marine and terrestrial systems. Nat. Ecol. Evol. 2020, 4, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef]
- Manes, S.; Costello, M.J.; Beckett, H.; Debnath, A.; Devenish-Nelson, E.; Grey, K.-A.; Jenkins, R.; Khan, T.M.; Kiessling, W.; Krause, C.; et al. Endemism increases species’ climate change risk in areas of global biodiversity importance. Biol. Conserv. 2021, 257, 109070. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Eggleton, P. The state of the world’s insects. Annu. Rev. Environ. Resour. 2020, 45, 61–82. [Google Scholar] [CrossRef]
- Kellermann, V.; van Heerwaarden, B. Terrestrial insects and climate change: Adaptive responses in key traits. Physiol. Entomol. 2019, 44, 99–115. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Eikeset, A.M.; McCauley, D.J.; Payne, J.L.; Sunday, J.M. Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature 2019, 569, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Heinen, R.; Gols, R.; Thakur, M.P. Climate change-mediated temperature extremes and insects: From outbreaks to breakdowns. Glob. Chang. Biol. 2020, 26, 6685–6701. [Google Scholar] [CrossRef]
- Schmitt, M.; Telusma, A.; Bigeard, E.; Guillou, L.; Alves-de-Souza, C. Temperature Affects the Biological Control of Dinoflagellates by the Generalist Parasitoid Parvilucifera rostrata. Microorganisms 2022, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Damien, M.; Tougeron, K. Prey–predator phenological mismatch under climate change. Curr. Opin. Insect Sci. 2019, 35, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K.; Himanen, S.J.; Poppy, G.M. Climate Change and its Effects on the Chemical Ecology of Insect Parasitoids. In Chemical Ecology of Insect Parasitoids; WILEY: Hoboken, NJ, USA, 2013; pp. 168–190. [Google Scholar] [CrossRef][Green Version]
- Theodorou, P.; Radzevičiūtė, R.; Lentendu, G.; Kahnt, B.; Husemann, M.; Bleidorn, C.; Settele, J.; Schweiger, O.; Grosse, I.; Wubet, T.; et al. Urban areas as hotspots for bees and pollination but not a panacea for all insects. Nat. Commun. 2020, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- Grab, H.; Branstetter, M.G.; Amon, N.; Urban-Mead, K.R.; Park, M.G.; Gibbs, J.; Blitzer, E.J.; Poveda, K.; Loeb, G.; Danforth, B.N. Agriculturally dominated landscapes reduce bee phylogenetic diversity and pollination services. Science 2019, 363, 282–284. [Google Scholar] [CrossRef]
- Ganuza, C.; Redlich, S.; Uhler, J.; Tobisch, C.; Rojas-Botero, S.; Peters, M.K.; Zhang, J.; Benjamin, C.S.; Englmeier, J.; Ewald, J. Interactive effects of climate and land use on pollinator diversity differ among taxa and scales. Sci. Adv. 2022, 8, eabm9359. [Google Scholar] [CrossRef]
- Jonsson, M.; Buckley, H.L.; Case, B.S.; Wratten, S.D.; Hale, R.J.; Didham, R.K. Agricultural intensification drives landscape-context effects on host–parasitoid interactions in agroecosystems. J. Appl. Ecol. 2012, 49, 706–714. [Google Scholar] [CrossRef]
- Nelson, A.E.; Forbes, A.A. Urban Land Use Decouples Plant-Herbivore-Parasitoid Interactions at Multiple Spatial Scales. PLoS ONE 2014, 9, e102127. [Google Scholar] [CrossRef] [PubMed]
- Marino, P.C.; Landis, D.A. Effect of Landscape Structure on Parasitoid Diversity and Parasitism in Agroecosystems. Ecol. Appl. 1996, 6, 276–284. [Google Scholar] [CrossRef]
- Ulina, E.S.; Rizali, A.; Manuwoto, S.; Pudjianto; Buchori, D. Does composition of tropical agricultural landscape affect parasitoid diversity and their host–parasitoid interactions? Agric. For. Entomol. 2019, 21, 318–325. [Google Scholar] [CrossRef]
- Menalled, F.D.; Marino, P.C.; Gage, S.H.; Landis, D.A. Does agricultural landscape structure affect parasitism and parasitoid diversity? Ecol. Appl. 1999, 9, 634–641. [Google Scholar] [CrossRef]
- Thies, C.; Tscharntke, T. Landscape Structure and Biological Control in Agroecosystems. Science 1999, 285, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Z.; Ouyang, F.; Men, X.; Zhang, K.; Liu, M.; Guo, W.; Zhu, C.; Zhao, W.; Reddy, G.V.P.; et al. Flower strips promote natural enemies, provide efficient aphid biocontrol, and reduce insecticide requirement in cotton crops. Entomol. Gen. 2022, 43, 421–432. [Google Scholar] [CrossRef]
- Albrecht, M.; Kleijn, D.; Williams, N.M.; Tschumi, M.; Blaauw, B.; Bommarco, R.; Campbell, A.J.; Dainese, M.; Drummond, F.A.; Entling, M.H.; et al. The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: A quantitative synthesis. Ecol. Lett. 2020, 23, 1488–1498. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.; Jacot, K.A. Tailored flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 2016, 53, 1169–1176. [Google Scholar] [CrossRef]
- Fiedler, A.K.; Landis, D.A.; Wratten, S.D. Maximizing ecosystem services from conservation biological control: The role of habitat management. Biol. Control 2008, 45, 254–271. [Google Scholar] [CrossRef]
- Jonsson, M.; Wratten, S.D.; Robinson, K.A.; Sam, S.A. The impact of floral resources and omnivory on a four trophic level food web. Bull. Entomol. Res. 2009, 99, 275–285. [Google Scholar] [CrossRef]
- Dangles, O.; Casas, J. Ecosystem services provided by insects for achieving sustainable development goals. Ecosyst. Serv. 2019, 35, 109–115. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.C. Climate Change Effects on Insects: Implications for Crop Protection and Food Security. J. Crop Improv. 2014, 28, 229–259. [Google Scholar] [CrossRef]
- Forbes, A.A.; Bagley, R.K.; Beer, M.A.; Hippee, A.C.; Widmayer, H.A. Quantifying the unquantifiable: Why Hymenoptera, not Coleoptera, is the most speciose animal order. BMC Ecol. 2018, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Dainese, M.; Martin, E.A.; Aizen, M.A.; Albrecht, M.; Bartomeus, I.; Bommarco, R.; Carvalheiro, L.G.; Chaplin-Kramer, R.; Gagic, V.; Garibaldi, L.A. A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 2019, 5, eaax0121. [Google Scholar] [CrossRef] [PubMed]
- Chidawanyika, F.; Mudavanhu, P.; Nyamukondiwa, C. Global climate change as a driver of bottom-up and top-down factors in agricultural landscapes and the fate of host-parasitoid interactions. Front. Ecol. Evol. 2019, 7, 80. [Google Scholar] [CrossRef]
- Ferracini, C.; Pogolotti, C.; Alma, A. A mismatch in the emergence of Torymus sinensis may affect the effectiveness of this biocontrol agent? Biol. Control 2022, 174, 105029. [Google Scholar] [CrossRef]
- Rosenheim, J.A. Higher-order predators and the regulation of insect herbivore populations. Annu. Rev. Entomol. 1998, 43, 421–447. [Google Scholar] [CrossRef]
- Dicke, M.; Cusumano, A.; Poelman, E.H. Microbial symbionts of parasitoids. Annu. Rev. Entomol. 2020, 65, 171–190. [Google Scholar] [CrossRef]
- Monticelli, L.S.; Bishop, J.; Desneux, N.; Gurr, G.M.; Jaworski, C.C.; McLean, A.H.C.; Thomine, E.; Vanbergen, A.J. Chapter Six —Multiple global change impacts on parasitism and biocontrol services in future agricultural landscapes. In Advances in Ecological Research; Bohan, D.A., Dumbrell, A.J., Vanbergen, A.J., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 65, pp. 245–304. [Google Scholar]
- Ramos Aguila, L.C.; Hussain, M.; Huang, W.; Lei, L.; Bamisile, B.S.; Wang, F.; Chi, H.; Wang, L. Temperature-Dependent Demography and Population Projection of Tamarixia radiata (Hymenoptera: Eulophidea) reared on Diaphorina citri (Hemiptera: Liviidae). J. Econ. Entomol. 2020, 113, 55–63. [Google Scholar] [CrossRef]
- Cañedo, V.; Dávila, W.; Carhuapoma, P.; Kroschel, J.; Kreuze, J. A temperature-dependent phenology model for Apanteles subandinus Blanchard, parasitoid of Phthorimaea operculella Zeller and Symmetrischema tangolias (Gyen). J. Appl. Entomol. 2022, 146, 424–439. [Google Scholar] [CrossRef]
- Fitzgerald, J.L.; Stuble, K.L.; Nichols, L.M.; Diamond, S.E.; Wentworth, T.R.; Pelini, S.L.; Gotelli, N.J.; Sanders, N.J.; Dunn, R.R.; Penick, C.A. Abundance of spring-and winter-active arthropods declines with warming. Ecosphere 2021, 12, e03473. [Google Scholar] [CrossRef]
- Zittis, G.; Almazroui, M.; Alpert, P.; Ciais, P.; Cramer, W.; Dahdal, Y.; Fnais, M.; Francis, D.; Hadjinicolaou, P.; Howari, F.; et al. Climate Change and Weather Extremes in the Eastern Mediterranean and Middle East. Rev. Geophys. 2022, 60, e2021RG000762. [Google Scholar] [CrossRef]
- Visser, M.E.; Gienapp, P. Evolutionary and demographic consequences of phenological mismatches. Nat. Ecol. Evol. 2019, 3, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Lajeunesse, M.J.; Rohr, J.R. A global synthesis of animal phenological responses to climate change. Nat. Clim. Chang. 2018, 8, 224–228. [Google Scholar] [CrossRef]
- Kong, J.D.; Hoffmann, A.A.; Kearney, M.R. Linking thermal adaptation and life-history theory explains latitudinal patterns of voltinism. Philos. Trans. R. Soc. B 2019, 374, 20180547. [Google Scholar] [CrossRef]
- Mirth, C.K.; Saunders, T.E.; Amourda, C. Growing up in a changing world: Environmental regulation of development in insects. Annu. Rev. Entomol. 2021, 66, 81–99. [Google Scholar] [CrossRef]
- Buckley, L.B. Temperature-sensitive development shapes insect phenological responses to climate change. Curr. Opin. Insect Sci. 2022, 52, 100897. [Google Scholar] [CrossRef]
- Shah, A.A.; Dillon, M.E.; Hotaling, S.; Woods, H.A. High elevation insect communities face shifting ecological and evolutionary landscapes. Curr. Opin. Insect Sci. 2020, 41, 1–6. [Google Scholar] [CrossRef]
- Yang, L.H. Toward a more temporally explicit framework for community ecology. Ecol. Res. 2020, 35, 445–462. [Google Scholar] [CrossRef]
- Nanga, S.N.; Kekeunou, S.; Kuate, A.F.; Fiaboe, K.K.M.; Kenfak, M.A.D.; Tonnang, H.E.; Gnanvossou, D.; Djiéto-Lordon, C.; Hanna, R. Temperature-dependent phenology of the parasitoid Fopius arisanus on the host Bactrocera dorsalis. J. Therm. Biol. 2021, 100, 103031. [Google Scholar] [CrossRef] [PubMed]
- Macgregor, C.J.; Thomas, C.D.; Roy, D.B.; Beaumont, M.A.; Bell, J.R.; Brereton, T.; Bridle, J.R.; Dytham, C.; Fox, R.; Gotthard, K.; et al. Climate-induced phenology shifts linked to range expansions in species with multiple reproductive cycles per year. Nat. Commun. 2019, 10, 4455. [Google Scholar] [CrossRef] [PubMed]
- Teder, T. Phenological responses to climate warming in temperate moths and butterflies: Species traits predict future changes in voltinism. Oikos 2020, 129, 1051–1060. [Google Scholar] [CrossRef]
- Neven, L.G. Physiological responses of insects to heat. Postharvest Biol. Technol. 2000, 21, 103–111. [Google Scholar] [CrossRef]
- Govindan, B.N.; Hutchison, W.D. Influence of Temperature on Age-Stage, Two-Sex Life Tables for a Minnesota-Acclimated Population of the Brown Marmorated Stink Bug (Halyomorpha halys). Insects 2020, 11, 108. [Google Scholar] [CrossRef]
- Du Plessis, H.; Schlemmer, M.L.; Van den Berg, J. The Effect of Temperature on the Development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2020, 11, 228. [Google Scholar] [CrossRef]
- Burraco, P.; Orizaola, G.; Monaghan, P.; Metcalfe, N.B. Climate change and ageing in ectotherms. Glob. Chang. Biol. 2020, 26, 5371–5381. [Google Scholar] [CrossRef]
- Huey, R.B.; Kingsolver, J.G. Climate warming, resource availability, and the metabolic meltdown of ectotherms. Am. Nat. 2019, 194, E140–E150. [Google Scholar] [CrossRef]
- Hill, S.J.; Silcocks, S.C.; Andrew, N.R. Impacts of temperature on metabolic rates of adult Extatosoma tiaratum reared on different host plant species. Physiol. Entomol. 2020, 45, 7–15. [Google Scholar] [CrossRef]
- Braz, É.C.; Bueno, A.d.F.; Colombo, F.C.; de Queiroz, A.P. Temperature impact on Telenomus podisi emergence in field releases of unprotected and encapsulated parasitoid pupae. Neotrop. Entomol. 2021, 50, 462–469. [Google Scholar] [CrossRef]
- Ramos Aguila, L.C.; Atlihan, R.; Ashraf, H.J.; Keppanan, R.; Lei, L.; Bamisile, B.S.; Cerda, H.; Wang, L. Temperature-Dependent Biological Control Effectiveness of Tamarixia radiata (Hymenoptera: Eulophidea) under Laboratory Conditions. J. Econ. Entomol. 2021, 114, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Zhang, G.F.; Liu, W.X.; Wan, F.H. Variable temperatures across different stages have novel effects on behavioral response and population viability in a host-feeding parasitoid. Sci. Rep. 2019, 9, 2202. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, P.; Ammunét, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemelä, P.; Terblanche, J.S. Complex responses of global insect pests to climate warming. Front. Ecol. Environ. 2020, 18, 141–150. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Wilson, R.J. Intra-and interspecific variation in the responses of insect phenology to climate. J. Anim. Ecol. 2021, 90, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Kharouba, H.M.; Ehrlén, J.; Gelman, A.; Bolmgren, K.; Allen, J.M.; Travers, S.E.; Wolkovich, E.M. Global shifts in the phenological synchrony of species interactions over recent decades. Proc. Natl. Acad. Sci. USA 2018, 115, 5211–5216. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.A.; Grundel, R.; Dzurisin, J.D.; Knutson, R.L.; Hellmann, J.J. Evidence of an extreme weather-induced phenological mismatch and a local extirpation of the endangered Karner blue butterfly. Conserv. Sci. Pract. 2020, 2, e147. [Google Scholar] [CrossRef]
- Moore, M.E.; Hill, C.A.; Kingsolver, J.G. Developmental timing of extreme temperature events (heat waves) disrupts host–parasitoid interactions. Ecol. Evol. 2022, 12, e8618. [Google Scholar] [CrossRef]
- Wetherington, M.T.; Jennings, D.E.; Shrewsbury, P.M.; Duan, J.J. Climate variation alters the synchrony of host–parasitoid interactions. Ecol. Evol. 2017, 7, 8578–8587. [Google Scholar] [CrossRef]
- Garretson, A.C.; Feldsine, N.; Napoli, M.; Long, E.C.; Forkner, R.E. Networks of Phenological Synchrony Reveal a Highly Interconnected Ecosystem and Potential Vulnerability to Climate-Driven Mismatches. bioRxiv 2022. [Google Scholar] [CrossRef]
- de Sassi, C.; Tylianakis, J.M. Climate Change Disproportionately Increases Herbivore over Plant or Parasitoid Biomass. PLoS ONE 2012, 7, e40557. [Google Scholar] [CrossRef]
- Evans, E.W.; Carlile, N.R.; Innes, M.B.; Pitigala, N. Warm springs reduce parasitism of the cereal leaf beetle through phenological mismatch. J. Appl. Entomol. 2013, 137, 383–391. [Google Scholar] [CrossRef]
- Duan, J.J.; Jennings, D.E.; Williams, D.C.; Larson, K.M. Patterns of parasitoid host utilization and development across a range of temperatures: Implications for biological control of an invasive forest pest. BioControl 2014, 59, 659–669. [Google Scholar] [CrossRef]
- Klapwijk, M.J.; GrÖBler, B.C.; Ward, K.; Wheeler, D.; Lewis, O.T. Influence of experimental warming and shading on host–parasitoid synchrony. Glob. Chang. Biol. 2010, 16, 102–112. [Google Scholar] [CrossRef]
- Johansson, F.; Orizaola, G.; Nilsson-Örtman, V. Temperate insects with narrow seasonal activity periods can be as vulnerable to climate change as tropical insect species. Sci. Rep. 2020, 10, 8822. [Google Scholar] [CrossRef] [PubMed]
- Rajpurohit, S.; Solanki, P.S.; Mayekar, H.V.; Arya, H.; Aradhya, R.; Suravajhala, P.; Loeschcke, V. Tropical high-altitude insects show limited capacity to handle high temperatures. bioRxiv 2022. [Google Scholar] [CrossRef]
- Parr, C.L.; Bishop, T.R. The response of ants to climate change. Glob. Chang. Biol. 2022, 28, 3188–3205. [Google Scholar] [CrossRef] [PubMed]
- Both, C.; Van Asch, M.; Bijlsma, R.G.; Van Den Burg, A.B.; Visser, M.E. Climate change and unequal phenological changes across four trophic levels: Constraints or adaptations? J. Anim. Ecol. 2009, 78, 73–83. [Google Scholar] [CrossRef]
- Thomson, L.J.; Macfadyen, S.; Hoffmann, A.A. Predicting the effects of climate change on natural enemies of agricultural pests. Biol. Control 2010, 52, 296–306. [Google Scholar] [CrossRef]
- Lo Pinto, M.; Guarino, S.; Agrò, A. Evidence of Seasonal Variation in Body Color in Adults of the Parasitoid Cirrospilus pictus (Hymenoptera: Eulophidae) in Sicily, Italy. Insects 2023, 14, 90. [Google Scholar] [CrossRef]
- Nice, C.C.; Forister, M.L.; Harrison, J.G.; Gompert, Z.; Fordyce, J.A.; Thorne, J.H.; Waetjen, D.P.; Shapiro, A.M. Extreme heterogeneity of population response to climatic variation and the limits of prediction. Glob. Chang. Biol. 2019, 25, 2127–2136. [Google Scholar] [CrossRef]
- Nufio, C.R.; Buckley, L.B. Grasshopper phenological responses to climate gradients, variability, and change. Ecosphere 2019, 10, e02866. [Google Scholar] [CrossRef]
- Moghadam, N.N.; Ketola, T.; Pertoldi, C.; Bahrndorff, S.; Kristensen, T.N. Heat hardening capacity in Drosophila melanogaster is life stage-specific and juveniles show the highest plasticity. Biol. Lett. 2019, 15, 20180628. [Google Scholar] [CrossRef] [PubMed]
- Kingsolver, J.G.; Buckley, L.B. Ontogenetic variation in thermal sensitivity shapes insect ecological responses to climate change. Curr. Opin. Insect Sci. 2020, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bartomeus, I.; Ascher, J.S.; Wagner, D.; Danforth, B.N.; Colla, S.; Kornbluth, S.; Winfree, R. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc. Natl. Acad. Sci. USA 2011, 108, 20645–20649. [Google Scholar] [CrossRef] [PubMed]
- Burkle, L.A.; Marlin, J.C.; Knight, T.M. Plant-Pollinator Interactions over 120 Years: Loss of Species, Co-Occurrence, and Function. Science 2013, 339, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Radchuk, V.; Reed, T.; Teplitsky, C.; Van De Pol, M.; Charmantier, A.; Hassall, C.; Adamík, P.; Adriaensen, F.; Ahola, M.P.; Arcese, P. Adaptive responses of animals to climate change are most likely insufficient. Nat. Commun. 2019, 10, 3109. [Google Scholar] [CrossRef] [PubMed]
- Senior, V.L.; Evans, L.C.; Leather, S.R.; Oliver, T.H.; Evans, K.L. Phenological responses in a sycamore–aphid–parasitoid system and consequences for aphid population dynamics: A 20 year case study. Glob. Chang. Biol. 2020, 26, 2814–2828. [Google Scholar] [CrossRef] [PubMed]
- Abarca, M.; Lill, J.T. Latitudinal variation in the phenological responses of eastern tent caterpillars and their egg parasitoids. Ecol. Entomol. 2019, 44, 50–61. [Google Scholar] [CrossRef]
- Des Marteaux, L.; Xi, J.; Mano, G.; Goto, S.G. Circadian clock outputs regulating insect photoperiodism: A potential role for glutamate transporter. Biochem. Biophys. Res. Commun. 2022, 590, 186. [Google Scholar] [CrossRef]
- Petrice, T.R.; Bauer, L.S.; Miller, D.L.; Poland, T.M.; Ravlin, F.W. A Phenology Model for Simulating Oobius agrili (Hymenoptera: Encyrtidae) Seasonal Voltinism and Synchrony with Emerald Ash Borer Oviposition. Environ. Entomol. 2020, 50, 280–292. [Google Scholar] [CrossRef]
- Goto, S.G. Photoperiodic time measurement, photoreception, and circadian clocks in insect photoperiodism. Appl. Entomol. Zool. 2022, 57, 193–212. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Buckley, L.B. How do phenology, plasticity, and evolution determine the fitness consequences of climate change for montane butterflies? Evol. Appl. 2018, 11, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.S.; Zohner, C.M. Climate change and phenological mismatch in trophic interactions among plants, insects, and vertebrates. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 165–182. [Google Scholar] [CrossRef]
- Schenk, M.; Krauss, J.; Holzschuh, A. Desynchronizations in bee–plant interactions cause severe fitness losses in solitary bees. J. Anim. Ecol. 2018, 87, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, R.C.; Cruse, D.; Sanders, D.; Gaston, K.J.; van Veen, F.F. Shifting daylength regimes associated with range shifts alter aphid-parasitoid community dynamics. Ecol. Evol. 2018, 8, 8761–8769. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, R.; Sanders, D.; Cruse, D.; Silk, M.; Gaston, K.J.; Bridle, J.R.; van Veen, F. Longer photoperiods through range shifts and artificial light lead to a destabilizing increase in host–parasitoid interaction strength. J. Anim. Ecol. 2020, 89, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Tougeron, K.; Brodeur, J.; Le Lann, C.; van Baaren, J. How climate change affects the seasonal ecology of insect parasitoids. Ecol. Entomol. 2020, 45, 167–181. [Google Scholar] [CrossRef]
- Iler, A.M.; CaraDonna, P.J.; Forrest, J.R.; Post, E. Demographic consequences of phenological shifts in response to climate change. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 221–245. [Google Scholar] [CrossRef]
- McCain, C.M.; Garfinkel, C.F. Climate change and elevational range shifts in insects. Curr. Opin. Insect Sci. 2021, 47, 111–118. [Google Scholar] [CrossRef]
- Vitasse, Y.; Ursenbacher, S.; Klein, G.; Bohnenstengel, T.; Chittaro, Y.; Delestrade, A.; Monnerat, C.; Rebetez, M.; Rixen, C.; Strebel, N. Phenological and elevational shifts of plants, animals and fungi under climate change in the European Alps. Biol. Rev. 2021, 96, 1816–1835. [Google Scholar] [CrossRef]
- Jeffs, C.T.; Lewis, O.T. Effects of climate warming on host–parasitoid interactions. Ecol. Entomol. 2013, 38, 209–218. [Google Scholar] [CrossRef]
- Schneider, L.; Rebetez, M.; Rasmann, S. The effect of climate change on invasive crop pests across biomes. Curr. Opin. Insect Sci. 2022, 50, 100895. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.F.; Aukema, B.H.; Fei, S.; Liebhold, A.M. Warm temperatures increase population growth of a nonnative defoliator and inhibit demographic responses by parasitoids. Ecology 2020, 101, e03156. [Google Scholar] [CrossRef] [PubMed]
- Osland, M.J.; Stevens, P.W.; Lamont, M.M.; Brusca, R.C.; Hart, K.M.; Waddle, J.H.; Langtimm, C.A.; Williams, C.M.; Keim, B.D.; Terando, A.J. Tropicalization of temperate ecosystems in North America: The northward range expansion of tropical organisms in response to warming winter temperatures. Glob. Chang. Biol. 2021, 27, 3009–3034. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.G.; Diamond, S.E.; Kelly, M.W. Mechanistic species distribution modelling as a link between physiology and conservation. Conserv. Physiol. 2015, 3, cov056. [Google Scholar] [CrossRef] [PubMed]
- Porfirio, L.L.; Harris, R.M.B.; Lefroy, E.C.; Hugh, S.; Gould, S.F.; Lee, G.; Bindoff, N.L.; Mackey, B. Improving the Use of Species Distribution Models in Conservation Planning and Management under Climate Change. PLoS ONE 2014, 9, e113749. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.L.; Bohlmann, J.; Carroll, A.L. Consequences of distributional asymmetry in a warming environment: Invasion of novel forests by the mountain pine beetle. Ecosphere 2017, 8, e01778. [Google Scholar] [CrossRef]
- Keret, N.M.; Mutanen, M.J.; Orell, M.I.; Itämies, J.H.; Välimäki, P.M. Climate change-driven elevational changes among boreal nocturnal moths. Oecologia 2020, 192, 1085–1098. [Google Scholar] [CrossRef]
- Wang, R.; Yang, H.; Wang, M.; Zhang, Z.; Huang, T.; Wen, G.; Li, Q. Predictions of potential geographical distribution of Diaphorina citri (Kuwayama) in China under climate change scenarios. Sci. Rep. 2020, 10, 9202. [Google Scholar] [CrossRef]
- Souza, P.G.C.; Aidoo, O.F.; Farnezi, P.K.B.; Heve, W.K.; Júnior, P.A.S.; Picanço, M.C.; Ninsin, K.D.; Ablormeti, F.K.; Shah, M.A.; Siddiqui, S.A.; et al. Tamarixia radiata global distribution to current and future climate using the climate change experiment (CLIMEX) model. Sci. Rep. 2023, 13, 1823. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Souza, P.G.C.; Silva, R.S.; Júnior, P.A.S.; Picanço, M.C.; Heve, W.K.; Duker, R.Q.; Ablormeti, F.K.; Sétamou, M.; Borgemeister, C. Modeling climate change impacts on potential global distribution of Tamarixia radiata Waterston (Hymenoptera: Eulophidae). Sci. Total Environ. 2023, 864, 160962. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Z.; Liu, Z.; Yang, Y.; Khoso, A.G.; Wang, L.; Liu, D. Climate change simulations revealed potentially drastic shifts in insect community structure and crop yields in China’s farmland. J. Pest Sci. 2022, 96, 55–69. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Wang, J.G.; Lei, Y.H. Predicting Distribution of the Asian Longhorned Beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae) and Its Natural Enemies in China. Insects 2022, 13, 687. [Google Scholar] [CrossRef] [PubMed]
- Furlong, M.J.; Zalucki, M.P. Climate change and biological control: The consequences of increasing temperatures on host–parasitoid interactions. Curr. Opin. Insect Sci. 2017, 20, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Hódar, J.A.; Cayuela, L.; Heras, D.; Pérez-Luque, A.J.; Torres-Muros, L. Expansion of elevational range in a forest pest: Can parasitoids track their hosts? Ecosphere 2021, 12, e03476. [Google Scholar] [CrossRef]
- Fernandez Goya, L.; Lanteri, A.A.; Confalonieri, V.A.; Rodriguero, M.S. New host-parasitoid interactions in Naupactus cervinus (Coleoptera, Curculionidae) raise the question of Wolbachia horizontal transmission. Symbiosis 2022, 86, 325–336. [Google Scholar] [CrossRef]
- Cornell, H.V.; Hawkins, B.A. Accumulation of Native Parasitoid Species on Introduced Herbivores: A Comparison of Hosts as Natives and Hosts as Invaders. Am. Nat. 1993, 141, 847–865. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; David, J.L.A.; Nash, D.R.; Lawton, J.H. The Recruitment of Parasitoid Species to Two Invading Herbivores. J. Anim. Ecol. 1995, 64, 393–402. [Google Scholar] [CrossRef]
- Grabenweger, G.; Kehrli, P.; Zweimüller, I.; Augustin, S.; Avtzis, N.; Bacher, S.; Freise, J.; Girardoz, S.; Guichard, S.; Heitland, W.; et al. Temporal and spatial variations in the parasitoid complex of the horse chestnut leafminer during its invasion of Europe. Biol. Invasions 2010, 12, 2797–2813. [Google Scholar] [CrossRef]
- Badillo-Montaño, R.; Amancio, G.; Falcón-Brindis, A.; León-Cortés, J.L.; Von Thaden, J.; Dzul-Cauich, F. Trophic host-parasitoid interactions of two Neotropical butterfly species in southeastern Mexico. Int. J. Trop. Insect Sci. 2022, 42, 1865–1875. [Google Scholar] [CrossRef]
- Opedal, Ø.H.; Ovaskainen, O.; Saastamoinen, M.; Laine, A.L.; van Nouhuys, S. Host-plant availability drives the spatiotemporal dynamics of interacting metapopulations across a fragmented landscape. Ecology 2020, 101, e03186. [Google Scholar] [CrossRef] [PubMed]
- Hoddle, M.S.; Hoddle, C.D. Classical biological control of Asian citrus psyllid with Tamarixia radiata in urban Southern California. Citrograph 2013, 4, 52–58. [Google Scholar]
- Massa, B.; Rizzo, M.; Caleca, V. Natural alternative hosts of Eulophidae (Hymenoptera Chalcidoidea) parasitoids of the Citrus Leafminer Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae) in the Mediterranean Basin. J. Hym. Res. 2001, 10, 91–100. [Google Scholar]
- Lumbierres, B.; Starý, P.; Pons, X. Seasonal parasitism of cereal aphids in a Mediterranean arable crop system. J. Pest Sci. 2007, 80, 125–130. [Google Scholar] [CrossRef]
- Kos, K.; Lacković, N.; Melika, G.; Matošević, D. Diversity and surge in abundance of native parasitoid communities prior to the onset of Torymus sinensis on the Asian chestnut gall wasp (Dryocosmus kuriphilus) in Slovenia, Croatia and Hungary. J. For. Res. 2021, 32, 1327–1336. [Google Scholar] [CrossRef]
- Culbertson, K.A.; Garland, M.S.; Walton, R.K.; Zemaitis, L.; Pocius, V.M. Long-term monitoring indicates shifting fall migration timing in monarch butterflies (Danaus plexippus). Glob. Chang. Biol. 2022, 28, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Holloway, J.D.; Hill, J.K.; Thomas, C.D.; Chen, I.; Ho, C.K. Reduced body sizes in climate-impacted Borneo moth assemblages are primarily explained by range shifts. Nat. Commun. 2019, 10, 4612. [Google Scholar] [CrossRef]
- Gérard, M.; Martinet, B.; Maebe, K.; Marshall, L.; Smagghe, G.; Vereecken, N.J.; Vray, S.; Rasmont, P.; Michez, D. Shift in size of bumblebee queens over the last century. Glob. Chang. Biol. 2020, 26, 1185–1195. [Google Scholar] [CrossRef]
- Tscholl, T.; Nachman, G.; Spangl, B.; Walzer, A. Heat waves affect prey and predators differently via developmental plasticity: Who may benefit most from global warming? Pest Manag. Sci. 2022, 78, 1099–1108. [Google Scholar] [CrossRef]
- Gérard, M.; Vanderplanck, M.; Wood, T.; Michez, D. Global warming and plant–pollinator mismatches. Emerg. Top. Life Sci. 2020, 4, 77–86. [Google Scholar] [CrossRef]
- Hamann, E.; Blevins, C.; Franks, S.J.; Jameel, M.I.; Anderson, J.T. Climate change alters plant–herbivore interactions. New Phytol. 2021, 229, 1894–1910. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.E.; Lehmann, P.; Gotthard, K. Longer and warmer prewinter periods reduce post-winter fitness in a diapausing insect. Funct. Ecol. 2022, 36, 1151–1162. [Google Scholar] [CrossRef]
- Le Lann, C.; Van Baaren, J.; Visser, B. Dealing with predictable and unpredictable temperatures in a climate change context: The case of parasitoids and their hosts. J. Exp. Biol. 2021, 224, jeb238626. [Google Scholar] [CrossRef]
- Hance, T.; van Baaren, J.; Vernon, P.; Boivin, G. Impact of Extreme Temperatures on Parasitoids in a Climate Change Perspective. Annu. Rev. Entomol. 2006, 52, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Machekano, H.; Mvumi, B.M.; Nyamukondiwa, C. Loss of coevolved basal and plastic responses to temperature may underlie trophic level host-parasitoid interactions under global change. Biol. Control 2018, 118, 44–54. [Google Scholar] [CrossRef]
- Engel, E.; Lau, D.; Godoy, W.A.; Pasini, M.P.; Malaquias, J.B.; Santos, C.D.; Pivato, J.; Pereira, P.R.d.S. Oscillation, synchrony, and multi-factor patterns between cereal aphids and parasitoid populations in southern Brazil. Bull. Entomol. Res. 2022, 112, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Biale, H.; Geden, C.J.; Chiel, E. Heat adaptation of the house fly (Diptera: Muscidae) and its associated parasitoids in Israel. J. Med. Entomol. 2020, 57, 113–121. [Google Scholar] [CrossRef]
- Marshall, K.E.; Gotthard, K.; Williams, C.M. Evolutionary impacts of winter climate change on insects. Curr. Opin. Insect Sci. 2020, 41, 54–62. [Google Scholar] [CrossRef]
- Morse, D.H. Rapid phenological change differs across four trophic levels over 15 years. Oecologia 2021, 196, 577–587. [Google Scholar] [CrossRef]
- Alford, L.; Louâpre, P.; Mougel, F.; van Baaren, J. Measuring the evolutionary potential of a winter-active parasitic wasp to climate change. Oecologia 2020, 194, 41–50. [Google Scholar] [CrossRef]
- Schneider, L.; Comte, V.; Rebetez, M. Increasingly favourable winter temperature conditions for major crop and forest insect pest species in Switzerland. Agric. For. Meteorol. 2021, 298–299, 108315. [Google Scholar] [CrossRef]
- Biella, P.; Ćetković, A.; Gogala, A.; Neumayer, J.; Sárospataki, M.; Šima, P.; Smetana, V. Northwestward range expansion of the bumblebee Bombus haematurus into Central Europe is associated with warmer winters and niche conservatism. Insect Sci. 2021, 28, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Lindestad, O.; von Schmalensee, L.; Lehmann, P.; Gotthard, K. Variation in butterfly diapause duration in relation to voltinism suggests adaptation to autumn warmth, not winter cold. Funct. Ecol. 2020, 34, 1029–1040. [Google Scholar] [CrossRef]
- Andrade, T.O.; Krespi, L.; Bonnardot, V.; van Baaren, J.; Outreman, Y. Impact of change in winter strategy of one parasitoid species on the diversity and function of a guild of parasitoids. Oecologia 2016, 180, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Tapia, A.; Alvarez-Baca, J.K.; Tougeron, K.; van Baaren, J.; Lavandero, B.; Le Lann, C. Composition and structure of winter aphid–parasitoid food webs along a latitudinal gradient in Chile. Oecologia 2022, 200, 425–440. [Google Scholar] [CrossRef]
- Mehrnejad, M.R.; Copland, M.J.W. Diapause strategy in the parasitoid Psyllaephagus pistaciae. Entomol. Exp. Appl. 2005, 116, 109–114. [Google Scholar] [CrossRef]
- Tougeron, K.; Le Lann, C.; Brodeur, J.; van Baaren, J. Are aphid parasitoids from mild winter climates losing their winter diapause? Oecologia 2017, 183, 619–629. [Google Scholar] [CrossRef]
- Tougeron, K.; Damien, M.; Le Lann, C.; Brodeur, J.; van Baaren, J. Rapid Responses of Winter Aphid-Parasitoid Communities to Climate Warming. Front. Ecol. Evol. 2018, 6, 173. [Google Scholar] [CrossRef]
- Marasco, V. Diapausing insects don’t like it hot. J. Exp. Biol. 2022, 225, jeb243491. [Google Scholar] [CrossRef]
- Polgár, L.A.; Darvas, B.; Völkl, W. Induction of dormancy in aphid parasitoids: Implications for enhancing their field effectiveness. Agric. Ecosyst. Environ. 1995, 52, 19–23. [Google Scholar] [CrossRef]
- Brodeur, J.; McNeil, J.N. Biotic and abiotic factors involved in diapause induction of the parasitoid, Aphidius nigripes (Hymenoptera: Aphidiidae). J. Insect Physiol. 1989, 35, 969–974. [Google Scholar] [CrossRef]
- Polgár, L.A.; Hardie, J. Diapause induction in aphid parasitoids. Entomol. Exp. Appl. 2000, 97, 21–27. [Google Scholar] [CrossRef]
- Larson, E.L.; Tinghitella, R.M.; Taylor, S.C.A. Insect hybridization and climate change. Front. Ecol. Evol. 2019, 7, 348. [Google Scholar] [CrossRef]
- Toxopeus, J.; Gadey, L.; Andaloori, L.; Sanaei, M.; Ragland, G.J. Costs of averting or prematurely terminating diapause associated with slow decline of metabolic rates at low temperature. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 255, 110920. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Chen, J.; Chen, L.; Chen, C.; Wu, S. Exposure to mild temperatures decreases overwintering larval survival and post-diapause reproductive potential in the rice stem borer Chilo suppressalis. J. Pest Sci. 2017, 90, 117–125. [Google Scholar] [CrossRef]
- Forister, M.L.; Halsch, C.A.; Nice, C.C.; Fordyce, J.A.; Dilts, T.E.; Oliver, J.C.; Prudic, K.L.; Shapiro, A.M.; Wilson, J.K.; Glassberg, J. Fewer butterflies seen by community scientists across the warming and drying landscapes of the American West. Science 2021, 371, 1042–1045. [Google Scholar] [CrossRef]
- Dahlhoff, E.P.; Dahlhoff, V.C.; Grainger, C.A.; Zavala, N.A.; Otepola-Bello, D.; Sargent, B.A.; Roberts, K.T.; Heidl, S.J.; Smiley, J.T.; Rank, N.E. Getting chased up the mountain: High elevation may limit performance and fitness characters in a montane insect. Funct. Ecol. 2019, 33, 809–818. [Google Scholar] [CrossRef]
- Stefanescu, C.; Asís, J.D.; Baños-Picón, L.; Cerdà, X.; Marcos García, M.A.; Micó, E.; Ricarte, A.; Tormos, J. Diversidad de insectos polinizadores en la península ibérica. Ecosistemas 2018, 27, 9–22. [Google Scholar] [CrossRef]
- Soroye, P.; Newbold, T.; Kerr, J. Climate change contributes to widespread declines among bumble bees across continents. Science 2020, 367, 685–688. [Google Scholar] [CrossRef]
- Warren, M.S.; Maes, D.; van Swaay, C.A.M.; Goffart, P.; Van Dyck, H.; Bourn, N.A.D.; Wynhoff, I.; Hoare, D.; Ellis, S. The decline of butterflies in Europe: Problems, significance, and possible solutions. Proc. Natl. Acad. Sci. USA 2021, 118, e2002551117. [Google Scholar] [CrossRef]
- Engelhardt, E.K.; Biber, M.F.; Dolek, M.; Fartmann, T.; Hochkirch, A.; Leidinger, J.; Löffler, F.; Pinkert, S.; Poniatowski, D.; Voith, J.; et al. Consistent signals of a warming climate in occupancy changes of three insect taxa over 40 years in central Europe. Glob. Chang. Biol. 2022, 28, 3998–4012. [Google Scholar] [CrossRef] [PubMed]
- Forister, M.L.; Pelton, E.M.; Black, S.H. Declines in insect abundance and diversity: We know enough to act now. Conserv. Sci. Pract. 2019, 1, e80. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect Declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef] [PubMed]
- Halsch, C.A.; Shapiro, A.M.; Fordyce, J.A.; Nice, C.C.; Thorne, J.H.; Waetjen, D.P.; Forister, M.L. Insects and recent climate change. Proc. Natl. Acad. Sci. USA 2021, 118, e2002543117. [Google Scholar] [CrossRef] [PubMed]
- Lister, B.C.; Garcia, A. Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc. Natl. Acad. Sci. USA 2018, 115, E10397–E10406. [Google Scholar] [CrossRef] [PubMed]
- Raven, P.H.; Wagner, D.L. Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. USA 2021, 118, e2002548117. [Google Scholar] [CrossRef]
- Janzen, D.H.; Hallwachs, W. To us insectometers, it is clear that insect decline in our Costa Rican tropics is real, so let’s be kind to the survivors. Proc. Natl. Acad. Sci. USA 2021, 118, e2002546117. [Google Scholar] [CrossRef] [PubMed]
- Van Klink, R.; Bowler, D.E.; Gongalsky, K.B.; Swengel, A.B.; Gentile, A.; Chase, J.M. Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 2020, 368, 417–420. [Google Scholar] [CrossRef]
- Warren, R.; Price, J.; Graham, E.; Forstenhaeusler, N.; VanDerWal, J. The projected effect on insects, vertebrates, and plants of limiting global warming to 1.5 C rather than 2 C. Science 2018, 360, 791–795. [Google Scholar] [CrossRef]
- Outhwaite, C.L.; McCann, P.; Newbold, T. Agriculture and climate change are reshaping insect biodiversity worldwide. Nature 2022, 605, 97–102. [Google Scholar] [CrossRef]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. 2020, 95, 802–821. [Google Scholar] [CrossRef] [PubMed]
- Thierry, M.; Hrček, J.; Lewis, O.T. Mechanisms structuring host–parasitoid networks in a global warming context: A review. Ecol. Entomol. 2019, 44, 581–592. [Google Scholar] [CrossRef]
- Khelifa, R.; Blanckenhorn, W.U.; Roy, J.; Rohner, P.T.; Mahdjoub, H. Usefulness and limitations of thermal performance curves in predicting ectotherm development under climatic variability. J. Anim. Ecol. 2019, 88, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Ramos Aguila, L.C.; Akutse, K.S.; Bamisile, B.S.; Sánchez Moreano, J.P.; Ashraf, H.J.; Zhou, C.; Li, X.; Wang, L. Endophytically colonized Citrus limon seedlings by Beauveria bassiana hampered development, reproduction and progeny fitness of Diaphorina citri. J. Appl. Entomol. 2021, 146, 229–242. [Google Scholar] [CrossRef]
- Mutamiswa, R.; Chidawanyika, F.; Nyamukondiwa, C. Comparative assessment of the thermal tolerance of spotted stemborer, Chilo partellus (Lepidoptera: Crambidae) and its larval parasitoid, Cotesia sesamiae (Hymenoptera: Braconidae). Insect Sci. 2018, 25, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.E.; Hill, C.A.; Kingsolver, J.G. Differing thermal sensitivities in a host–parasitoid interaction: High, fluctuating developmental temperatures produce dead wasps and giant caterpillars. Funct. Ecol. 2021, 35, 675–685. [Google Scholar] [CrossRef]
- Andrade, G.S.; Pratissoli, D.; Dalvi, L.P.; Desneux, N.; dos Santos Junior, H.J.G. Performance of four Trichogramma species (Hymenoptera: Trichogrammatidae) as biocontrol agents of Heliothis virescens (Lepidoptera: Noctuidae) under various temperature regimes. J. Pest Sci. 2011, 84, 313–320. [Google Scholar] [CrossRef]
- Wu, L.-H.; Hoffmann, A.A.; Thomson, L.J. Potential impact of climate change on parasitism efficiency of egg parasitoids: A meta-analysis of Trichogramma under variable climate conditions. Agric. Ecosyst. Environ. 2016, 231, 143–155. [Google Scholar] [CrossRef]
- de Pedro, L.; Beitia, F.; Sabater-Muñoz, B.; Asís, J.D.; Tormos, J. Effect of temperature on the developmental time, survival of immatures and adult longevity of Aganaspis daci (Hymenoptera: Figitidae), a natural enemy of Ceratitis capitata (Diptera: Tephritidae). Crop Protect. 2016, 85, 17–22. [Google Scholar] [CrossRef]
- Qiu, B.; Zhou, Z.-S.; Luo, S.-P.; Xu, Z.-F. Effect of Temperature on Development, Survival, and Fecundity of Microplitis manilae (Hymenoptera: Braconidae). Environ. Entomol. 2012, 41, 657–664. [Google Scholar] [CrossRef]
- Pardikes, N.; Revilla, T.; Lue, C.H.; Thierry, M.; Souto-Vilarós, D.; Hrcek, J. Effects of phenological mismatch under warming are modified by community context. Glob. Chang. Biol. 2022, 28, 4013–4026. [Google Scholar] [CrossRef] [PubMed]
- Pardikes, N.; Revilla, T.; Lue, C.H.; Thierry, M.; Souto-Villaros, D.; Hrcek, J. Community context modifies response of host-parasitoid interactions to phenological mismatch under warming. Authorea Prepr. 2021. [Google Scholar] [CrossRef]
- Mołoń, M.; Dampc, J.; Kula-Maximenko, M.; Zebrowski, J.; Mołoń, A.; Dobler, R.; Durak, R.; Skoczowski, A. Effects of Temperature on Lifespan of Drosophila melanogaster from Different Genetic Backgrounds: Links between Metabolic Rate and Longevity. Insects 2020, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; You, M.; Atlihan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.-J.; Fu, J.-W.; Xu, Y.-Y. Age-stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Zhang, B.; Leonard, S.P.; Li, Y.; Moran, N.A. Obligate bacterial endosymbionts limit thermal tolerance of insect host species. Proc. Natl. Acad. Sci. USA 2019, 116, 24712–24718. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.B.; Blanford, S. Thermal biology in insect-parasite interactions. Trends Ecol. Evol. 2003, 18, 344–350. [Google Scholar] [CrossRef]
- Ashraf, H.J.; Ramos Aguila, L.C.; Akutse, K.S.; Ilyas, M.; Abbasi, A.; Li, X.; Wang, L. Comparative microbiome analysis of Diaphorina citri and its associated parasitoids Tamarixia radiata and Diaphorencyrtus aligarhensis reveals Wolbachia as a dominant endosymbiont. Environ. Microbiol. 2022, 24, 1638–1652. [Google Scholar] [CrossRef]
- Jason, M.M.; Marjorie, A.H. Molecular Survey of Endosymbionts in Florida Populations of Diaphorina citri (Hemiptera: Psyllidae) and Its Parasitoids Tamarixia radiata (Hymenoptera: Eulophidae) and Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae). Fla. Entomol. 2008, 91, 294–304. [Google Scholar] [CrossRef]
- Hague, M.T.J.; Shropshire, J.D.; Caldwell, C.N.; Statz, J.P.; Stanek, K.A.; Conner, W.R.; Cooper, B.S. Temperature effects on cellular host-microbe interactions explain continent-wide endosymbiont prevalence. Curr. Biol. 2022, 32, 878–888.e8. [Google Scholar] [CrossRef]
- Newton, I.L.G.; Rice, D.W. The Jekyll and Hyde symbiont: Could Wolbachia be a nutritional mutualist? J. Bacteriol. 2020, 202, e00589-19. [Google Scholar] [CrossRef]
- Ayyasamy, A.; Kempraj, V.; Pagadala Damodaram, K.J. Endosymbiotic Bacteria Aid to Overcome Temperature Induced Stress in the Oriental Fruit Fly, Bactrocera dorsalis. Microb. Ecol. 2021, 82, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Buxton, M.; Nyamukondiwa, C.; Dalu, T.; Cuthbert, R.N.; Wasserman, R.J. Implications of increasing temperature stress for predatory biocontrol of vector mosquitoes. Parasites Vectors 2020, 13, 604. [Google Scholar] [CrossRef] [PubMed]
- Pintanel, P.; Tejedo, M.; Salinas-Ivanenko, S.; Jervis, P.; Merino-Viteri, A. Predators like it hot: Thermal mismatch in a predator-prey system across an elevational tropical gradient. J. Anim. Ecol. 2021, 90, 1985–1995. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos Aguila, L.C.; Li, X.; Akutse, K.S.; Bamisile, B.S.; Sánchez Moreano, J.P.; Lie, Z.; Liu, J. Host–Parasitoid Phenology, Distribution, and Biological Control under Climate Change. Life 2023, 13, 2290. https://doi.org/10.3390/life13122290
Ramos Aguila LC, Li X, Akutse KS, Bamisile BS, Sánchez Moreano JP, Lie Z, Liu J. Host–Parasitoid Phenology, Distribution, and Biological Control under Climate Change. Life. 2023; 13(12):2290. https://doi.org/10.3390/life13122290
Chicago/Turabian StyleRamos Aguila, Luis Carlos, Xu Li, Komivi Senyo Akutse, Bamisope Steve Bamisile, Jessica Paola Sánchez Moreano, Zhiyang Lie, and Juxiu Liu. 2023. "Host–Parasitoid Phenology, Distribution, and Biological Control under Climate Change" Life 13, no. 12: 2290. https://doi.org/10.3390/life13122290
APA StyleRamos Aguila, L. C., Li, X., Akutse, K. S., Bamisile, B. S., Sánchez Moreano, J. P., Lie, Z., & Liu, J. (2023). Host–Parasitoid Phenology, Distribution, and Biological Control under Climate Change. Life, 13(12), 2290. https://doi.org/10.3390/life13122290






