Evaluation of the Antiparasitic, Antihepatotoxicity, and Antioxidant Efficacy of Quercetin and Chitosan, Either Alone or in Combination, against Infection Induced by Giardia lamblia in Male Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of G. lamblia Cysts Used for the Inoculum of Infection
2.2. Ethical Statement
2.3. Determination of the Sample Size
2.4. Compounds Used
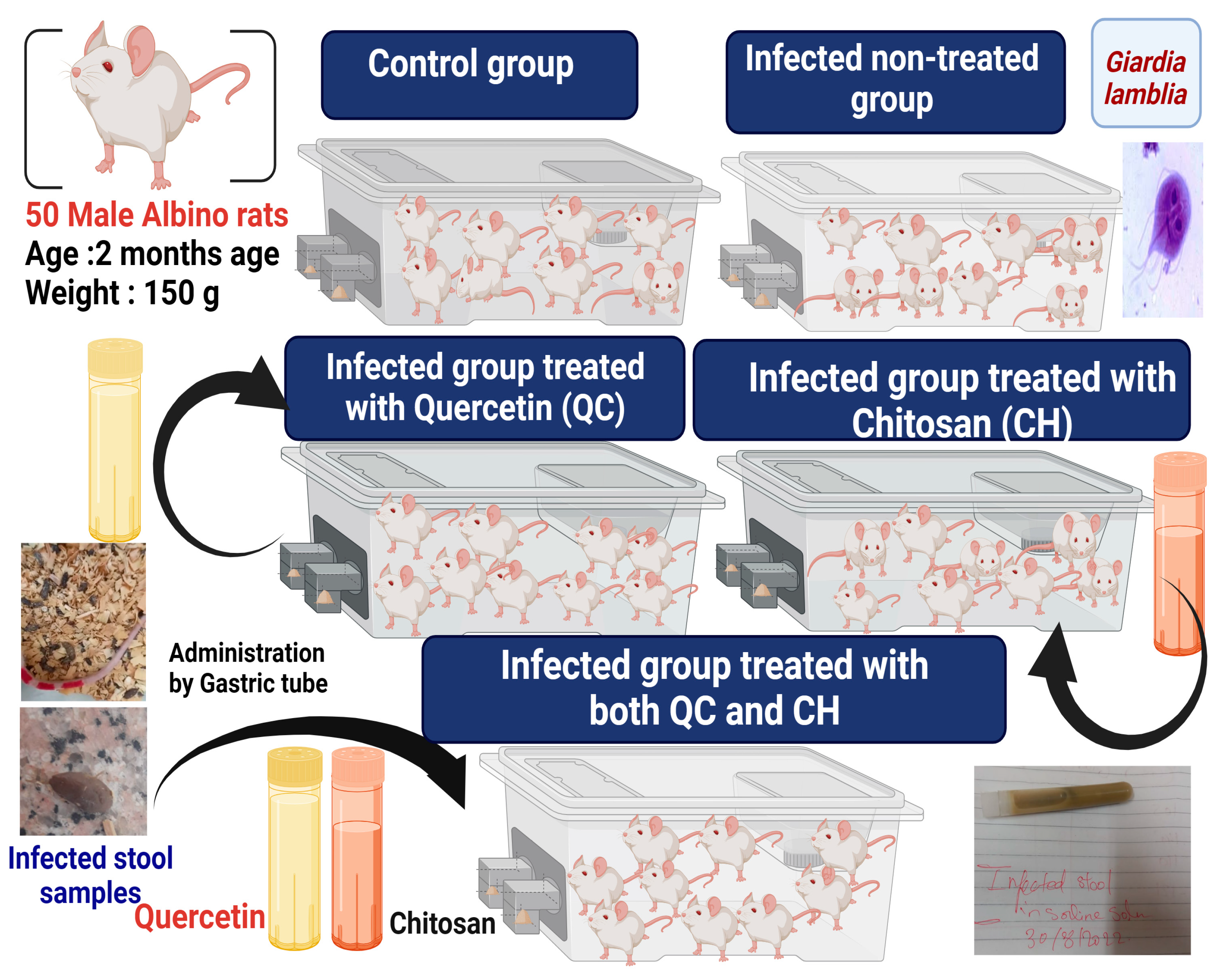
2.5. Experimental Animals
2.6. Assessment of the Efficacy of the Treatment
2.6.1. Parasitological Evaluation
2.6.2. Histopathological Evaluation
2.6.3. Transmission Electron Microscope Examination (TEM)
2.6.4. Assessment of Hepatorenal Functions
2.6.5. Evaluation of Antioxidant Enzymes in Liver Tissues
2.7. Statistical Analysis
3. Results
3.1. Parasitological Assessment of Infected Non-Treated and Treated Groups
G. lamblia Cyst Count in the Stools
3.2. Histopathological Examination Results
3.3. Transmission Electron Microscope (TEM) Examinations
3.4. Biochemical Evaluation of Hepatorenal Functions
3.5. Changes in Oxidative Stress in the Infected Non-Treated Group and Different Infected Treated Groups
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baz, M.G.; Elmarhoumy, S.M.; Saied, E.M.; Zoghroban, H.S. Evaluation of the efficacy of gold nanoparticles on Giardia lamblia infection in experimental animals. Exp. Parasitol. 2022, 238, 108277. [Google Scholar] [CrossRef] [PubMed]
- Elkholy, W.A.; Elkholy, M.A.; Elsokary, A.N.; Alshehri, E.; Al-Quraishy, S.; Abdel-Gaber, R.; Shaheen, H.A.A. Therapeutic and prophylactic effects of Punica granatum peel extract versus metronidazole in murine Giardiasis intestinalis. J. King Saud Univ. Sci. 2022, 34, 102321. [Google Scholar] [CrossRef]
- Ankarklev, J.; Jerlström-Hultqvist, J.; Ringqvist, E.; Troell, K.; Svärd, S.G. Behind the smile: Cell biology and disease mechanisms of Giardia species. Nat. Rev. Microbiol. 2010, 8, 413–422. [Google Scholar] [CrossRef]
- Pecková, R.; Doležal, K.; Sak, B.; Květoňová, D.; Kváč, M.; Nurcahyo, W.; Foitová, I. Effect of Piper betle on Giardia intestinalis infection in vivo. Exp. Parasitol. 2018, 184, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.N.; Fèvre, E.M.; Sripa, B.; et al. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001920. [Google Scholar] [CrossRef] [PubMed]
- Savioli, L.; Smith, H.; Thompson, A. Giardia and Cryptosporidium join the neglected diseases initiative. Parasitol. Today 2022, 22, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.H.; Weng, S.C.; Wu, J.H.; Tung, S.Y.; Su, L.H.; Lin, M.H.; Lee, G.A. DNA topoisomerase IIIβ promotes cyst generation by inducing cyst wall protein gene expression in Giardia lamblia. Open Biol. 2020, 10, 190228. [Google Scholar] [CrossRef]
- El-Gendy, A.M.L.; Hammed, M.A.A.M.; Ghallab, M.M.I.; Abdel Aziz, M.O.; Ibrahim, S.M. Therapeutic Effect of Chitosan Nanoparticles and Metronidazole in Treatment of Experimentally Giardiasis Infected Hamsters. Iran. J. Parasitol. 2021, 16, 32–42. [Google Scholar] [CrossRef]
- Petri, J.R. Therapy of intestinal protozoa. Trends Parasitol. 2003, 19, 523–526. [Google Scholar] [CrossRef]
- Long, K.Z.; Rosado, J.L.; Montoya, Y.; de Lourdes Solano, M.; Hertzmark, E.; DuPont, H.L.; Santos, J.I. Effect of vitamin A and zinc supplementation on gastrointestinal parasitic infections among Mexican children. Pediatrics 2007, 120, e846–e855. [Google Scholar] [CrossRef]
- Abu El-Ezz, N.M. Effect of Nigella sativa and Alliumcepa oils on Trichinella spiralis in experimentally infected rats. J. Egypt. Soc. Parasitol. 2005, 35, 511–523. [Google Scholar] [PubMed]
- Hamza, R.Z.; El-Shenawy, N.; Ismail, H. Protective effects of blackberry and quercetin on sodium fluoride-induced oxidative stress and histological changes in the hepatic, renal, testis and brain tissue of male rat. J. Basic Clin. Physiol. Pharmacol. (JBCPP) 2015, 26, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Refat, M.S.; Hamza, R.Z.; Adam, A.M.A.; Saad, H.A.; Gobouri, A.A.; Al-Harbi, F.S.; Al-Salmi, F.A.; Al-talhi, T.; El-Megharbel, S.M. Quercetin/Zinc complex and stem cells: A new drug therapy to ameliorate glycometabolic control and pulmonary dysfunction in diabetes mellitus: Structural characterization and genetic studies. PLoS ONE 2021, 16, e0246265. [Google Scholar] [CrossRef]
- Zamin, L.L.; Filippi-Chiela, E.C.; Dillenburg-Pilla, P.; Horn, F.; Salbego, C.; Lenz, G. Resveratrol and quercetin cooperate to induce senescence-like growth arrest in C6 rat glioma cells. Cancer Sci. 2009, 100, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Vessal, M.; Hemmati, M.; Vasei, M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 135, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Al-Baqami, N.M.; Hamza, R.Z. Synergistic antioxidant capacities of vanillin and chitosan nanoparticles against reactive oxygen species, hepatotoxicity, and genotoxicity induced by aging in male Wistar rats. Hum. Exp. Toxicol. 2021, 40, 183–202. [Google Scholar] [CrossRef]
- Vo, T.S.; Kim, J.A.; Ngo, D.H.; Chang-Suk, K. Protective effect of chitosan oligosaccharides against FceRI-mediated RBL-2H3 mast cell activation. Process Biochem. 2012, 47, 327–330. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Salmi, F.A.; El-Shenawy, N.S. Chitosan and Lecithin Ameliorate Osteoarthritis Symptoms Induced by Monoiodoacetate in a Rat Model. Molecules 2020, 25, 5738. [Google Scholar] [CrossRef]
- Dyab, A.K.; Yones, D.A.; Ibraheim, Z.Z.; Hassan, T.M. Anti-giardial therapeutic potential of dichloromethane extracts of Zingiber officinale and Curcuma longa in vitro and in vivo. Parasitol. Res. 2016, 115, 2637–2645. [Google Scholar] [CrossRef]
- Venkatesh, B.M.S.; Rao, R.; Vivekanand, N. A comparative study of concentration techniques for detection of intestinal parasitic infections—To evaluate the prevalence and to identify a better method of concentration technique at a tribal tertiary care hospital. IOSR J. Dent. Med. Sci. 2016, 15, 42–46. [Google Scholar] [CrossRef]
- Ammar, A.I.A.; Mahmoud, S.S.M.; El Hefnawy, N.N. Effect of ginger on hamsters infected by Giardia lamblia. J. Environ. Stud. 2014, 1, 45–56. [Google Scholar]
- Hayat, M.A. (Ed.) Basic Techniques for Transmission Electron Microscopy, 1st ed.; Macmillan Press: New York, NY, USA, 1986; ISBN 978012333926322. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1970, 95, 351–358. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Couri, D.; Abdel-Rahman, M.S. Effect of chlorine dioxide and metabolites on glutathione-dependent system in rat, mouse and chicken blood. J. Environ. Pathol. Toxicol. 1979, 3, 451–460. [Google Scholar] [PubMed]
- Chan, Y.H. Biostatistics 102: Quantitative data—Parametric & non-parametric tests. Singap. Med. J. 2003, 44, 391–396. [Google Scholar]
- Halliez, M.C.M.; Buret, A.G. Extra-intestinal and long term consequences of Giardia duodenalis infections. World J. Gastroenterol. 2013, 19, 8974–8985. [Google Scholar] [CrossRef]
- Luther, A.B.; Platts-Mills, J.A. Giardia: A pathogen or commensal for children in high prevalence settings? Curr. Opin. Infect. Dis. 2016, 29, 502–507. [Google Scholar]
- Müller, J.; Hemphill, A.; Müller, N. Physiological aspects of nitro drug resistance in Giardia lamblia. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Oshiba, S.F.; Yaseein, R.I.; El-Shennawy, A.M.; Aiad, H.A.; El-Wakil, E.A. In vivo effect of pomegranate (Punica granatum) extracts versus Nitazoxanide drug on the ileum of experimentally infected mice with Cryptosporidium parvum oocysts. J. Am. Sci. 2018, 14, 27–39. [Google Scholar]
- Hezarjaribi, H.Z.; Elmi, T.; Dayer, M.S.; Gholami, S.; Fakhar, M.; Akbariqomi, M.; Ghaffarifar, F. A systematic review of the effects of Iranian pharmaceutical plant extracts on Giardia lamblia. Asian Pac. J. Trop. Dis. 2015, 5, 925–929. [Google Scholar] [CrossRef]
- Hanif, H.; Abdollahi, V.; Jouni, F.J.; Nikoukar, M.; Esboei, B.R.; Shams, E.; Vazini, H. Quercetin nano phytosome: As a novel Anti-leishmania and Anti-malarial natural product. J. Parasit. Dis. 2023, 47, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan nanoparticles: A promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Goy, R.C.; De-Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Chabra, A.; Esboei, B.R.; Habibi, E.; Monadi, T.; Azadbakht, M.; Elmi, T.; Valian, H.K.; Akhtari, J.; Fakhar, M.; Naghshvar, F. Effects of some natural products from fungal and herbal sources on Giardia lamblia in vivo. Parasitology 2019, 146, 1188–1198. [Google Scholar] [CrossRef]
- Amaral, F.M.M.; Ribeiro, M.N.; Barbosa-Filho, J.M.; Reis, A.S.; Nascimento, F.R.F.; Macedo, R.O. Plants and chemical constituents with giardicidal activity. Braz. J. Pharmacogn. 2006, 16, 696–720. [Google Scholar] [CrossRef]
- Johns, T.; Faubert, G.M.; Kokwaro, J.O.; Mahunnah, R.L.A.; Kimanani, E.K. Anti-giardial activity of gastrointestinal remedies of the Luo of East Africa. J. Ethnopharmacol. 1995, 46, 17–23. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Abdel-Misih, S.R.Z.; Bloomston, M. Liver Anatomy. Surg. Clin. N. Am. 2010, 90, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Guo, X.; Zhou, F.; Xiao, L.; Liu, J.; Jiang, C.; Xing, M.; Yao, P. Quercetin alleviates ethanol-induced liver steatosis associated with improvement of lipophagy. Food Chem. Toxicol. 2019, 125, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ranelletti, F.O.; Maggiano, N.; Serra, F.G.; Ricci, R.; Larocca, L.M.; Lanza, P.; Scambia, G.; Fattorossi, A.; Capelli, A.; Piantelli, M. Quercetin inhibits p21-RAS expression in human colon cancer cell lines and in primary colorectal tumors. Int. J. Cancer 2000, 85, 438–445. [Google Scholar] [CrossRef]
- Ismail, A.; Abdel-Magied, A.A.; Elhenawy, A.A.; El-Nahas, H.A. Association Between Giardia Genotype and Oxidative Stress Biomarkers Among Giardia-Infected Children: A Case–Control Study. Acta Parasitol. 2022, 67, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Abd Al-Wahab, S.A.; Mahdi, J.K.; Mahdi, N.K. Oxidative stress among patients with some different parasitic infections. Med. J. Basrah Univ. 2009, 27, 66–70. [Google Scholar]
- El-Kady, N.A.M.; Abdel-Rahman, I.A.M.; Fouad, S.S.; Allemailem, K.S.; Istivan, T.; Ahmed, S.F.M.; Hasan, A.; Osman, H.A.; Elshabrawy, H.A. Pomegranate Peel Extract Is a Potential Alternative Therapeutic for Giardiasis. Antibiotics 2021, 10, 705. [Google Scholar] [CrossRef]
- Abdel-Fattah, N.S.; Nada, O.H. Effect of propolis versus metronidazole and their combined use in treatment of acute experimental giardiasis. J. Egypt Soc. Parasitol. 2007, 37, 691–710. [Google Scholar]
- Scott, K.G.; Logan, M.R.; Klammer, G.M.; Teoh, D.A.; Buret, A.G. Jejunal brush border microvillous alterations in Giardia muris-infected mice: Role of T lymphocytes and interleukin-6. Infect. Immun. 2000, 68, 3412–3418. [Google Scholar] [CrossRef]
- Rezvani, M.; Mohammadnejad, J.; Narmani, A.; Bidaki, K. Synthesis and in vitro study of modified chitosan-polycaprolactam nanocomplex as delivery system. Int. J. Biol. Macromol. 2018, 113, 1287–1293. [Google Scholar] [CrossRef]
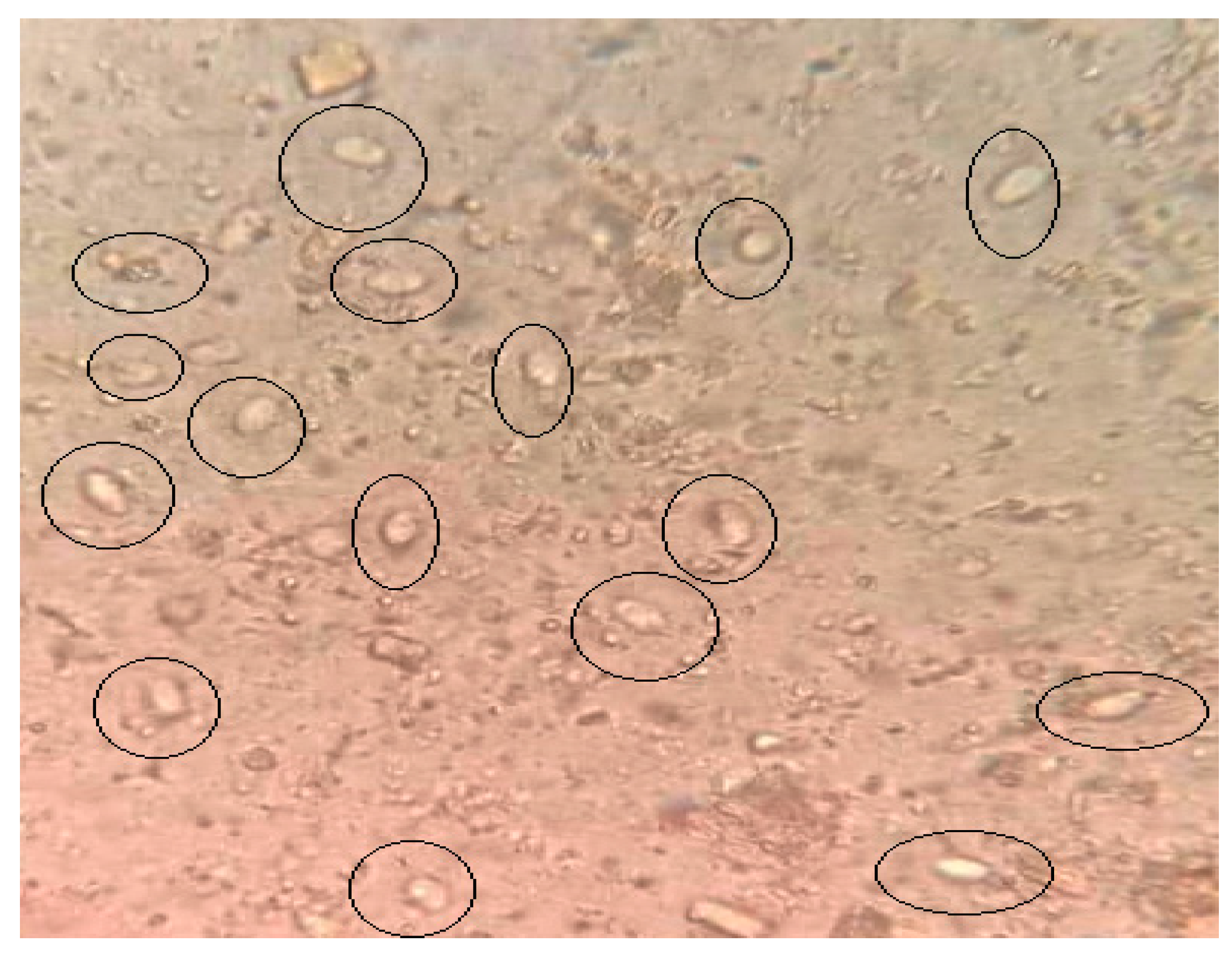


| Fecal Cyst Count | Infected Non-Treated Group | Infected Group Plus QC | Infected Group Plus CH | Infected Group Plus QC and CH |
|---|---|---|---|---|
| Range of cysts | 9.9–14.65 | 1.05–1.6 ** | 1.4–2.8 ** | 0.1–0.7 *** |
| % of reduction | 0% | 90% | 85% | 99% |
| Control Group | Infected Non-Treated Group | Infected Group + QC | Infected Group + CH | Infected Group + QC + CH | |
|---|---|---|---|---|---|
| ALT (U/L) | 15.25 ± 2.05 e | 65.25 ± 3.25 a | 40.02 ± 4.25 bc | 47.09 ± 3.25 b | 24.69 ± 2.69 d |
| AST (U/L) | 18.25 ± 2.87 e | 77.58 ± 3.25 a | 35.69 ± 2.98 c | 38.98 ± 4.56 bc | 22.68 ± 1.68 d |
| Creatinine (mg/dL) | 0.63 ± 0.12 e | 1.08 ± 0.41 a | 0.70 ± 0.32 c | 0.79 ± 0.14 bc | 0.66 ± 0.41 de |
| Urea (mg/dL) | 24.02 ± 2.67 e | 32.02 ± 5.02 a | 27.05 ± 3.02 c | 28.05 ± 2.54 bc | 24.69 ± 3.65 de |
| Control Group | Infected Non-Treated Group | Infected Group + QC | Infected Group + CH | Infected Group + QC + CH | |
|---|---|---|---|---|---|
| SOD (U/g) | 18.68 ± 2.02 ab | 10.03 ± 1.65 e | 13.02 ± 1.87 cd | 12.87 ± 1.98 d | 16.25 ± 2.32 b |
| CAT (U/g) | 8.65 ± 1.87 ab | 1.65 ± 0.98 e | 6.35 ± 1.25 cd | 5.98 ± 1.68 d | 8.05 ± 2.69 b |
| GSH (U/g) | 14.25 ± 2.65 a | 5.25 ± 1.65 d | 10.96 ± 2.98 c | 10.09 ± 1.87 c | 12.98 ± 3.65 b |
| MDA (U/g) | 10.09 ± 1.69 e | 18.69 ± 1.65 a | 13.75 ± 2.36 c | 14.52 ± 1.65 bc | 12.02 ± 1.65 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albogami, B. Evaluation of the Antiparasitic, Antihepatotoxicity, and Antioxidant Efficacy of Quercetin and Chitosan, Either Alone or in Combination, against Infection Induced by Giardia lamblia in Male Rats. Life 2023, 13, 2316. https://doi.org/10.3390/life13122316
Albogami B. Evaluation of the Antiparasitic, Antihepatotoxicity, and Antioxidant Efficacy of Quercetin and Chitosan, Either Alone or in Combination, against Infection Induced by Giardia lamblia in Male Rats. Life. 2023; 13(12):2316. https://doi.org/10.3390/life13122316
Chicago/Turabian StyleAlbogami, Bander. 2023. "Evaluation of the Antiparasitic, Antihepatotoxicity, and Antioxidant Efficacy of Quercetin and Chitosan, Either Alone or in Combination, against Infection Induced by Giardia lamblia in Male Rats" Life 13, no. 12: 2316. https://doi.org/10.3390/life13122316
APA StyleAlbogami, B. (2023). Evaluation of the Antiparasitic, Antihepatotoxicity, and Antioxidant Efficacy of Quercetin and Chitosan, Either Alone or in Combination, against Infection Induced by Giardia lamblia in Male Rats. Life, 13(12), 2316. https://doi.org/10.3390/life13122316







