Detection of Three Sarcocystis Species (Apicomplexa) in Blood Samples of the Bank Vole and Yellow-Necked Mouse from Lithuania
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Sample Collection
2.2. Molecular Analysis of A. flavicollis and C. glareolus Blood Samples
2.3. Statistical Analysis
3. Results
3.1. Detection Rates of Sarcocystis spp. in the Blood Samples of A. flavicollis and C. glareolus
3.2. Molecular Characterisation of Sarcocystis spp. in A. flavicollis and C. glareolus
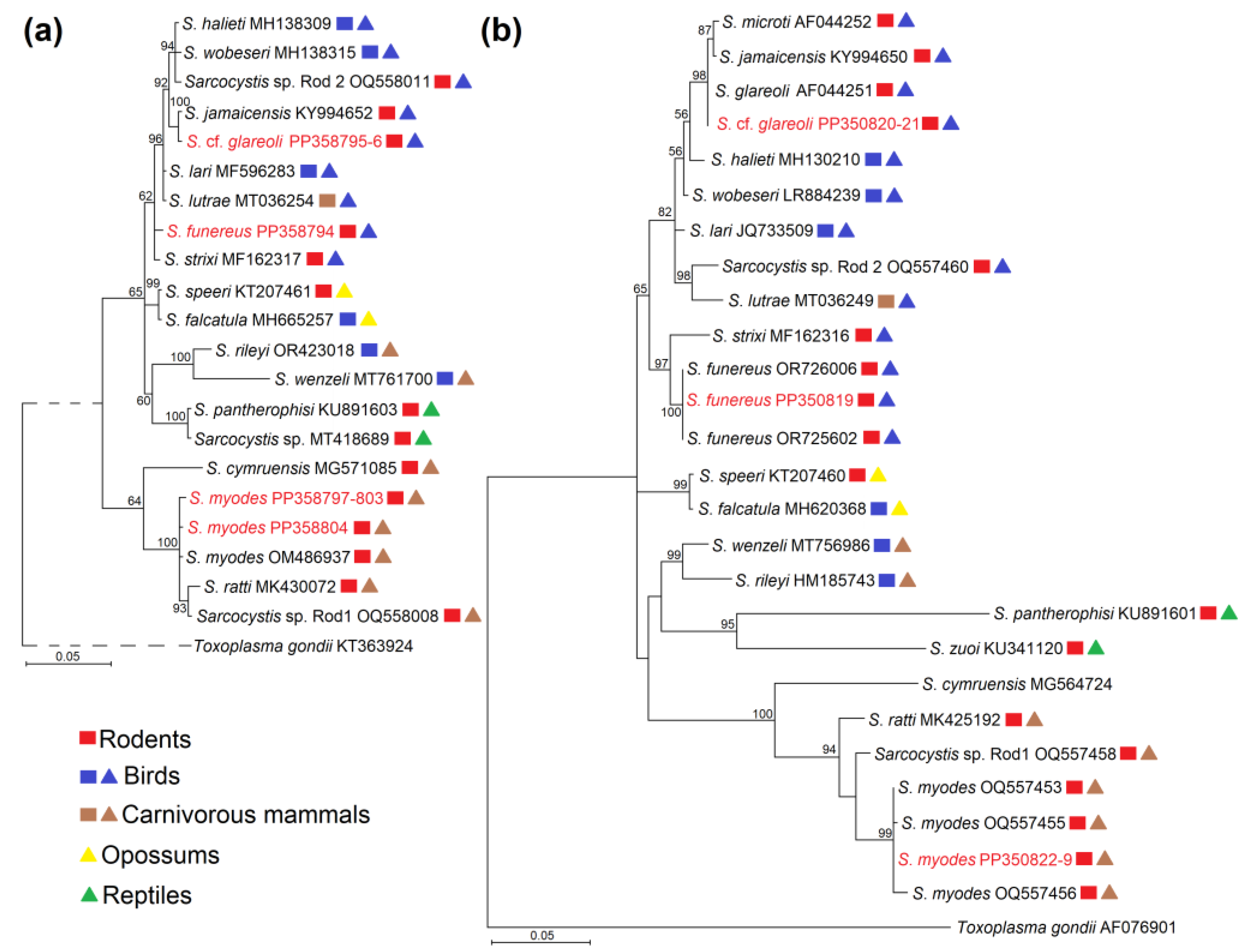
3.3. Phylogenetic Analysis of Identified Sarcocystis Species
4. Discussion
4.1. Prevalence of Sarcocystis spp. in Rodents
4.2. Detection of Sarcocystis spp. in Blood Samples of Intermediate Hosts
4.3. Characteristics of Sarcocystis spp. in A. flavicollis and C. glareolus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Fayer, R. Sarcocystis spp. in Human Infections. Clin. Microbiol. Rev. 2004, 17, 894–902. [Google Scholar] [CrossRef]
- Decker Franco, C.; Schnittger, L.; Florin-Christensen, M. Sarcocystis. In Parasitic Protozoa of Farm Animals and Pets; Springer International Publishing: Cham, Switzerland, 2018; pp. 103–124. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lindsay, D.S.; Saville, W.J.A.; Reed, S.M.; Granstrom, D.E.; Speer, C.A. A Review of Sarcocystis neurona and Equine Protozoal Myeloencephalitis (EPM). Vet. Parasitol. 2001, 95, 89–131. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Barr, B.C.; Nordhausen, R.; James, E.R.; Magargal, S.L.; Murray, M.; Conrad, P.A.; Toy-Choutka, S.; Jessup, D.A.; Grigg, M.E. Ultrastructural and Molecular Confirmation of the Development of Sarcocystis neurona Tissue Cysts in the Central Nervous System of Southern Sea Otters (Enhydra lutris nereis). Int. J. Parasitol. 2009, 39, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Levine, N.D. The Taxonomy of Sarcocystis (Protozoa, Apicomplexa) Species. J. Parasitol. 1986, 72, 372. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Navalón, B.; Anastasio-Giner, B.; Cano-Fructuoso, M.; Sanchez-Martínez, P.; Llopis-Morant, A.; Perez-Castarlenas, B.; Goyena, E.; Berriatua Fernández De Larrea, E. Short Communication. Sarcocystis Infection: A Major Cause of Carcass Condemnation in Adult Sheep in Spain. Span. J. Agric. Res. 2012, 10, 388. [Google Scholar] [CrossRef]
- Dahmana, H.; Granjon, L.; Diagne, C.; Davoust, B.; Fenollar, F.; Mediannikov, O. Rodents as Hosts of Pathogens and Related Zoonotic Disease Risk. Pathogens 2020, 9, 202. [Google Scholar] [CrossRef]
- Votýpka, J.; Hypša, V.; Jirku, M.; Flegr, J.; Vavra, J.; Lukes, J. Molecular Phylogenetic Relatedness of Frenkelia spp. (Protozoa, Apicomplexa) to Sarcocystis falcatula Stiles 1893: Is the Genus Sarcocystis Paraphyletic? J. Eukaryot. Microbiol. 1998, 45, 137–141. [Google Scholar] [CrossRef]
- Mugridge, N.B.; Morrison, D.A.; Johnson, A.M.; Luton, K.; Dubey, J.P. Phylogenetic Relationships of the Genus Frenkelia: A Review of Its History and New Knowledge Gained from Comparison of Large Subunit Ribosomal Ribonucleic Acid Gene Sequences. Int. J. Parasitol. 1999, 29, 957–972. [Google Scholar] [CrossRef]
- Jäkel, T.; Khoprasert, Y.; Endepols, S.; Archer-Baumann, C.; Suasa-ard, K.; Promkerd, P.; Kliemt, D.; Boonsong, P.; Hongnark, S. Biological Control of Rodents Using Sarcocystis singaporensis. Int. J. Parasitol. 1999, 29, 1321–1330. [Google Scholar] [CrossRef]
- Bentancourt Rossoli, J.V.; Moré, G.; Soto-Cabrera, A.; Moore, D.P.; Morrell, E.L.; Pedrana, J.; Scioli, M.V.; Campero, L.M.; Basso, W.; Hecker, Y.P.; et al. Identification of Sarcocystis spp. in Synanthropic (Muridae) and Wild (Cricetidae) Rodents from Argentina. Parasitol. Res. 2024, 123, 31. [Google Scholar] [CrossRef]
- Canova, V.; Helman, E.; Robles, M.D.R.; Abba, A.M.; Moré, G. First Report of Sarcocystis spp. (Apicomplexa, Sarcocystidae) in Lagostomus maximus (Desmarest, 1917) (Rodentia, Chinchillidae) in Argentina. Int. J. Parasitol. Parasites Wildl. 2023, 20, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-J.; Liu, Q.; Yang, Y.-F.; Esch, G.W.; Guo, Y.-M.; Zou, F.-C. Sarcocystis eothenomysi n. sp. (Apicomplexa: Sarcocystidae) from the Large Oriental Vole Eothenomys miletus (Thomas) (Cricetidae: Microtinae) from Anning, China. Syst. Parasitol. 2014, 89, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-J.; Liu, T.-T.; Liu, Q.; Esch, G.W.; Chen, J.-Q. Sarcocystis clethrionomyelaphis Matuschka, 1986 (Apicomplexa: Sarcocystidae) Infecting the Large Oriental Vole Eothenomys miletus (Thomas) (Cricetidae: Microtinae) and Its Phylogenetic Relationships with Other Species of Sarcocystis Lankester, 1882. Syst. Parasitol. 2015, 91, 273–279. [Google Scholar] [CrossRef]
- Rudaitytė-Lukošienė, E.; Jasiulionis, M.; Balčiauskas, L.; Prakas, P.; Stirkė, V.; Butkauskas, D. Morphological and Molecular Description of Sarcocystis myodes n. sp. from the Bank Vole (Clethrionomys glareolus) in Lithuania. Biology 2022, 11, 512. [Google Scholar] [CrossRef]
- El-Morsey, A.; Abdo, W.; Sultan, K.; Elhawary, N.M.; AbouZaid, A.A. Ultrastructural and Molecular Identification of the Sarcocysts of Sarcocystis tenella and Sarcocystis arieticanis Infecting Domestic Sheep (Ovis aries) from Egypt. Acta Parasit. 2019, 64, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, B.; De La Fuente, C.; Alunda, J.M.; Luzón, M. Molecular Characterisation of Five Sarcocystis Species in Domestic Sheep (Ovis aries) from Spain. Parasitol. Res. 2020, 119, 215–231. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, R.; Sang, X.; Zhang, X.; Li, M.; Li, X.; Yang, N.; Jiang, T. A Systematic Meta-Analysis of Global Sarcocystis Infection in Sheep and Goats. Pathogens 2023, 12, 902. [Google Scholar] [CrossRef]
- Hu, J.-J.; Huang, S.; Wen, T.; Esch, G.W.; Liang, Y.; Li, H.-L. Sarcocystis spp. in Domestic Sheep in Kunming City, China: Prevalence, Morphology, and Molecular Characteristics. Parasite 2017, 24, 8. [Google Scholar] [CrossRef]
- Jäkel, T.; Raisch, L.; Richter, S.; Wirth, M.; Birenbaum, D.; Ginting, S.; Khoprasert, Y.; Mackenstedt, U.; Wassermann, M. Morphological and Molecular Phylogenetic Characterization of Sarcocystis kani sp. nov. and Other Novel, Closely Related Sarcocystis spp. Infecting Small Mammals and Colubrid Snakes in Asia. Int. J. Parasitol. Parasites Wildl. 2023, 22, 184–198. [Google Scholar] [CrossRef]
- Prakas, P.; Stirkė, V.; Šneideris, D.; Rakauskaitė, P.; Butkauskas, D.; Balčiauskas, L. Protozoan Parasites of Sarcocystis spp. in Rodents from Commercial Orchards. Animals 2023, 13, 2087. [Google Scholar] [CrossRef]
- Baranauskaitė, A.; Strazdaitė-Žielienė, Ž.; Servienė, E.; Butkauskas, D.; Prakas, P. Molecular Identification of Protozoan Sarcocystis in Different Types of Water Bodies in Lithuania. Life 2022, 13, 51. [Google Scholar] [CrossRef]
- Moré, G.; Bacigalupe, D.; Basso, W.; Rambeaud, M.; Venturini, M.C.; Venturini, L. Serologic Profiles for Sarcocystis sp. and Neospora caninum and Productive Performance in Naturally Infected Beef Calves. Parasitol. Res. 2010, 106, 689–693. [Google Scholar] [CrossRef]
- Decker Franco, C.; Romero, S.; Ferrari, A.; Schnittger, L.; Florin-Christensen, M. Detection of Sarcocystis aucheniae in Blood of Llama Using a Duplex Semi-Nested PCR Assay and Its Association with Cyst Infestation. Heliyon 2018, 4, e00928. [Google Scholar] [CrossRef]
- Martin, M.; Decker Franco, C.; Romero, S.; Carletti, T.; Schnittger, L.; Florin-Christensen, M. Molecular Detection of Sarcocystis aucheniae in the Blood of Llamas from Argentina. Rev. Argent. Microbiol. 2016, 48, 200–205. [Google Scholar] [CrossRef]
- Moustafa, M.A.M.; Shimozuru, M.; Mohamed, W.; Taylor, K.R.; Nakao, R.; Sashika, M.; Tsubota, T. First Molecular Detection and Characterization of Hepatozoon and Sarcocystis spp. in Field Mice and Voles from Japan. Parasitol. Res. 2017, 116, 2321–2325. [Google Scholar] [CrossRef] [PubMed]
- Kamani, J.; Harrus, S.; Nachum-Biala, Y.; Gutiérrez, R.; Mumcuoglu, K.Y.; Baneth, G. Prevalence of Hepatozoon and Sarcocystis spp. in Rodents and Their Ectoparasites in Nigeria. Acta Trop. 2018, 187, 124–128. [Google Scholar] [CrossRef]
- Usluca, S.; Celebi, B.; Karasartova, D.; Gureser, A.S.; Matur, F.; Oktem, M.A.; Sozen, M.; Karatas, A.; Babur, C.; Mumcuoglu, K.Y.; et al. Molecular Survey of Babesia microti (Aconoidasida: Piroplasmida) in Wild Rodents in Turkey. J. Med. Entomol. 2019, 56, 1605–1609. [Google Scholar] [CrossRef]
- Gjerde, B.; Hilali, M.; Mawgood, S.A. Molecular Characterisation of Three Regions of the Nuclear Ribosomal DNA Unit and the Mitochondrial cox1 Gene of Sarcocystis fusiformis from Water Buffaloes (Bubalus bubalis) in Egypt. Parasitol. Res. 2015, 114, 3401–3413. [Google Scholar] [CrossRef] [PubMed]
- Sudan, V.; Shanker, D.; Paliwal, S.; Kumar, R.; Singh, A. Phylogenetics of Sarcocystis fusiformis Isolates Based on 18S rRNA and cox1 Genes. Microb. Pathog. 2021, 159, 105144. [Google Scholar] [CrossRef]
- Baltrūnaitė, L.; Kitrytė, N.; Križanauskienė, A. Blood Parasites (Babesia, Hepatozoon and Trypanosoma) of Rodents, Lithuania: Part I. Molecular and Traditional Microscopy Approach. Parasitol. Res. 2020, 119, 687–694. [Google Scholar] [CrossRef]
- Prūsaitė, J. Fauna of Lithuania: Mammals; Mokslas: Vilnius, Lithuania, 1988; p. 295. [Google Scholar]
- Hellgren, O.; Waldenström, J.; Bensch, S. A New PCR Assay for Simultaneous Studies of Leucocytozoon, Plasmodium, and Haemoproteus from Avian Blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Terman, R.C. Weights of Selected Organs of Deer Mice (Peromyscus maniculatus bairdii) from Asymp-Totic Laboratory Populations. J. Mammal. 1969, 50, 311–320. [Google Scholar] [CrossRef]
- Myllymäki, A. Demographic Mechanisms in the Fluctuating Populations of the Field Vole Microtus agrestis. Oikos 1977, 29, 468–493. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L.; Janonytė, A. Reproduction of the Root Vole (Microtus oeconomus) at the Edge of Its Distribution Range. Turk. J. Zool. 2012, 36, 668–675. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Milne, I.; Wright, F.; Rowe, G.; Marshall, D.F.; Husmeier, D.; McGuire, G. TOPALi: Software for Automatic Identification of Recombinant Sequences within DNA Multiple Alignments. Bioinformatics 2004, 20, 1806–1807. [Google Scholar] [CrossRef] [PubMed]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for Parasitologists—A Primer to Quantitative Parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Q.; Wu, Y.; Yang, H.; Bergelson, J.; Kreitman, M.; Tian, D. Variation in the Ratio of Nucleotide Substitution and Indel Rates across Genomes in Mammals and Bacteria. Mol. Biol. Evol. 2009, 26, 1523–1531. [Google Scholar] [CrossRef]
- Máca, O.; Kouba, M.; Langrová, I.; Panská, L.; Korpimäki, E.; González-Solís, D. The Tengmalm’s Owl Aegolius funereus (Aves, Strigidae) as the Definitive Host of Sarcocystis funereus sp. nov. (Apicomplexa). Front. Vet. Sci. 2024, 11, 1356549. [Google Scholar] [CrossRef]
- Arnastauskiené, T.; Grikieniené, J. Infection of small mammals with sarcosporidians in the South-Eastern Baltic region. Ecology 1993, 2, 47–56. [Google Scholar]
- Grikienienė, J.; Malakauskas, M.; Mažeikytė, R.; Balčiauskas, L.; Senutaitė, J. Muscle Parasites (Sarcocystis, Trichinella, Alaria) of Wild Mammals in Lithuania. Theriol. Litu. 2001, 1, 29–46. [Google Scholar]
- Prakas, P.; Butkauskas, D. Protozoan Parasites from Genus Sarcocystis and Their Investigations in Lithuania. Ekologija 2012, 58, 45–58. [Google Scholar] [CrossRef]
- Prakas, P.; Kirillova, V.; Gavarāne, I.; Grāvele, E.; Butkauskas, D.; Rudaitytė-Lukošienė, E.; Kirjušina, M. Morphological and Molecular Description of Sarcocystis ratti n. sp. from the Black Rat (Rattus rattus) in Latvia. Parasitol. Res. 2019, 118, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Grikienienė, J. Investigations into Endoparasites of Small Mammals in the Environs of Lake Drūkšiai. Acta Zool. Litu. 2005, 15, 109–114. [Google Scholar] [CrossRef]
- Grikienienė, J.; Mažeikytė, R. Investigation of Sarcosporidians (Sarcocystis) of Small Mammals in Kamasta Landscape Reserve and Its Surroundings. Acta Zool. Litu. 2000, 10, 55–68. [Google Scholar] [CrossRef]
- Soveri, T.; Henttonen, H.; Tanskanen, R.; Husu-Kallio, J.; Haukisalmi, V.; Sukura, A.; Laakkonen, J. Disease Patterns in Field and Bank Vole Populations during a Cyclic Decline in Central Finland. Comp. Immunol. Microbiol. Infect. Dis. 2000, 23, 73–89. [Google Scholar] [CrossRef]
- Svobodová, M.; Vorisek, P.; Votýpka, J.; Weidinger, K. Heteroxenous Coccidia (Apicomplexa: Sarcocystidae) in the Populations of Their Final and Intermediate Hosts: European Buzzard and Small Mammals. Acta Protozool. 2004, 43, 251–260. [Google Scholar]
- Inoue, I.; Yamada, M.; Yoshimi, Y.; Imai, Y.; Utsugi, I.; Suzuki, R.; Nogami, S.; Fujita, E.; Takahashi, K.; Tsuchiya, K.; et al. Prevalence of Sarcocystis (Protozoa, Apicomplexa) in Voles in Japan. Jpn. J. Parasitol. 1990, 39, 415–417. [Google Scholar]
- Hoogenboom, I.; Dijkstra, C. Sarcocystis cernae: A Parasite Increasing the Risk of Predation of Its Intermediate Host, Microtus arvalis. Oecologia 1987, 74, 86–92. [Google Scholar] [CrossRef]
- Tillmann, T.; Kamino, K.; Mohr, U. Sarcocystis muris—A Rare Case in Laboratory Mice. Lab. Anim. 1999, 33, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Meingassner, J.G.; Burtscher, H. Doppelinfektion Des Gehirns Mit Frenkelia Species Und Toxoplasma gondii Bei Chinchilla laniger [Double Infection of the Brain with Frenkelia Species and Toxoplasma gondii in Chinchilla laniger—Author’s Trans.]. Vet. Pathol. 1977, 14, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Modrý, D.; Votýpka, J.; Svobodová, M. Note on the Taxonomy of Frenkelia microti (Findlay & Middleton, 1934) (Apicomplexa: Sarcocystidae). Syst. Parasitol. 2004, 58, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Grikienienė, J.; Mažeikytė, R.; Balčiauskas, L. The First Data on Brain Parasites of the Genus Frenkelia (Protista: Coccidia) in Some Small Rodent Species in Lithuania. Acta Zool. Litu. 2003, 13, 21–27. [Google Scholar] [CrossRef]
- Krücken, J.; Blümke, J.; Maaz, D.; Demeler, J.; Ramünke, S.; Antolová, D.; Schaper, R.; Von Samson-Himmelstjerna, G. Small Rodents as Paratenic or Intermediate Hosts of Carnivore Parasites in Berlin, Germany. PLoS ONE 2017, 12, e0172829. [Google Scholar] [CrossRef] [PubMed]
- Waindok, P.; Özbakış-Beceriklisoy, G.; Janecek-Erfurth, E.; Springer, A.; Pfeffer, M.; Leschnik, M.; Strube, C. Parasites in Brains of Wild Rodents (Arvicolinae and Murinae) in the City of Leipzig, Germany. Int. J. Parasitol. Parasites Wildl. 2019, 10, 211–217. [Google Scholar] [CrossRef]
- Fichet-Calvet, E.; Kia, E.B.; Giraudoux, P.; Quéré, J.P.; Delattre, P.; Ashford, R.W. Frenkelia Parasites in a Small Mammal Community. Dynamics of Infection and Effect on the Host. Parasite 2004, 11, 301–310. [Google Scholar] [CrossRef][Green Version]
- Deter, J.; Bryja, J.; Chaval, Y.; Galan, M.; Henttonen, H.; Laakkonen, J.; Voutilainen, L.; Vapalahti, O.; Vaheri, A.; Salvador, A.R.; et al. Association between the DQA MHC Class II Gene and Puumala Virus Infection in Myodes glareolus, the Bank Vole. Infect. Genet. Evol. 2008, 8, 450–458. [Google Scholar] [CrossRef]
- Fernández-Escobar, M.; Millán, J.; Chirife, A.D.; Ortega-Mora, L.M.; Calero-Bernal, R. Molecular Survey for Cyst-Forming Coccidia (Toxoplasma gondii, Neospora caninum, Sarcocystis spp.) in Mediterranean Periurban Micromammals. Parasitol. Res. 2020, 119, 2679–2686. [Google Scholar] [CrossRef]
- Huaman, J.L.; Pacioni, C.; Forsyth, D.M.; Pople, A.; Hampton, J.O.; Helbig, K.J.; Carvalho, T.G. Evaluation of Haemoparasite and Sarcocystis Infections in Australian Wild Deer. Int. J. Parasitol. Parasites Wildl. 2021, 15, 262–269. [Google Scholar] [CrossRef]
- Basso, W.; Alvarez Rojas, C.A.; Buob, D.; Ruetten, M.; Deplazes, P. Sarcocystis Infection in Red Deer (Cervus elaphus) with Eosinophilic Myositis/Fasciitis in Switzerland and Involvement of Red Foxes (Vulpes vulpes) and Hunting Dogs in the Transmission. Int. J. Parasitol. Parasites Wildl. 2020, 13, 130–141. [Google Scholar] [CrossRef]
- Upton, S.J.; McKown, R.D. The Red-Tailed Hawk, Buteo jamaicensis, a Native Definitive Host of Frankelia microti (Apicomplexa) in North America. J. Wildl. Dis. 1992, 28, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Guo, Y.; Ma, C.; Deng, S.; Hu, J.; Zhang, Y. Redescription and Molecular Characterization of Sarcocysts of Sarcocystis cymruensis from Norway Rats (Rattus norvegicus) and Sarcocystis ratti from Black Rats (R. rattus) in China. Parasitol. Res. 2020, 119, 3785–3791. [Google Scholar] [CrossRef] [PubMed]
- Černá, Z.; Loučková, M. Microtus arvalis as the Intermediate Host of a Coccidian from the Kestrel (Falco tinnunculus). Folia Parasitol. 1976, 23, 110. [Google Scholar]
- Dubey, J. Sarcocystis montanaenis and S. microti sp. n. from the Meadow Vole (Microtus pennsylvanicus). Proc. Helminthol. Soc. Wash. 1983, 50, 318–324. [Google Scholar]
- Lindsay, D.S.; Upton, S.J.; Blagburn, B.L.; Toivio-Kinnucan, M.; McAllister, C.T.; Trauth, S.E. Sporocysts Isolated from the Southern Copperhead (Agkistrodon contortrix contortrix) Produce Sarcocystis montanaensis-like Sarcocysts in Prairie Voles (Microtus ochrogastei). J. Wildl. Dis. 1991, 27, 148–152. [Google Scholar] [CrossRef][Green Version]
- Antunes Murata, F.H.; Cerqueira-Cézar, C.K.; Thompson, P.C.; Tiwari, K.; Mowery, J.D.; Verma, S.K.; Rosenthal, B.M.; Sharma, R.N.; Dubey, J.P. Sarcocystis cymruensis: Discovery in Western Hemisphere in the Brown Rat (Rattus norvegicus) from Grenada, West Indies: Redescription, Molecular Characterization, and Transmission to IFN-γ Gene Knockout Mice via Sporocysts from Experimentally Infected Domestic Cat (Felis catus). Parasitol. Res. 2018, 117, 1195–1204. [Google Scholar] [CrossRef]
- Pérez, P.O.; Wibbelt, G.; Brinkmann, A.; Puentes, J.A.G.; Tuh, F.Y.Y.; Lakim, M.B.; Nitsche, A.; Wells, K.; Jäkel, T. Description of Sarcocystis scandentiborneensis sp. nov. from Treeshrews (Tupaia minor, T. tana) in Northern Borneo with Annotations on the Utility of COI and 18S rDNA Sequences for Species Delineation. Int. J. Parasitol. Parasites Wildl. 2020, 12, 220–231. [Google Scholar] [CrossRef]
- Watthanakaiwan, V.; Sukmak, M.; Hamarit, K.; Kaolim, N.; Wajjwalku, W.; Muangkram, Y. Molecular Characterization of the Ribosomal DNA Unit of Sarcocystis singaporensis, Sarcocystis zamani and Sarcocystis zuoi from Rodents in Thailand. J. Vet. Med. Sci. 2017, 79, 1412–1418. [Google Scholar] [CrossRef]
- Hu, J.; Sun, J.; Guo, Y.; Zeng, H.; Zhang, Y.; Tao, J. Infection of the Asian Gray Shrew Crocidura attenuata (Insectivora: Soricidae) with Sarcocystis attenuati n. sp. (Apicomplexa: Sarcocystidae) in China. Parasit. Vectors 2022, 15, 13. [Google Scholar] [CrossRef]
- Máca, O.; Kouba, M.; Korpimäki, E.; González-Solís, D. Molecular Identification of Sarcocystis sp. (Apicomplexa, Sarcocystidae) in Offspring of Tengmalm’s Owls, Aegolius funereus (Aves, Strigidae). Front. Vet. Sci. 2021, 8, 804096. [Google Scholar] [CrossRef] [PubMed]
- Máca, O. Molecular Identification of Sarcocystis lutrae in the European Otter (Lutra lutra) and the European Badger (Meles meles) from the Czech Republic. Parasitol. Res. 2018, 117, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Juozaitytė-Ngugu, E.; Švažas, S.; Šneideris, D.; Rudaitytė-Lukošienė, E.; Butkauskas, D.; Prakas, P. The Role of Birds of the Family Corvidae in Transmitting Sarcocystis Protozoan Parasites. Animals 2021, 11, 3258. [Google Scholar] [CrossRef]
- Wiesner, J. A New Sarcosporidian Species [Unnamed] of Clethrionomys glareolus Inhabiting the Owl Aegolius funereus as Definitive Host. J. Protozool. 1981, 27, 72A. [Google Scholar]
- Khalil, H.; Ecke, F.; Evander, M.; Hörnfeldt, B. Selective Predation on Hantavirus-Infected Voles by Owls and Confounding Effects from Landscape Properties. Oecologia 2016, 181, 597–606. [Google Scholar] [CrossRef] [PubMed]
| Habitat | Trapped | Infected (%) | Sarcocystis Species |
|---|---|---|---|
| Mature deciduous forest | 18 | 2 (11.11%) | S. myodes |
| Mature mixed forest | 33 | 0 | - |
| Planted young forest | 10 | 0 | - |
| Bog | 8 | 1 (12.50%) | S. myodes |
| Natural meadow | 34 | 4 (11.76%) | S. myodes and S. funereus * |
| Shrubby meadow | 22 | 0 | - |
| Arable land | 18 | 2 (11.11%) | S. cf. glareoli |
| Total | 143 | 9 (6.29%) |
| Habitat | Trapped | Infected |
|---|---|---|
| Mature deciduous forest | 33 | 0 |
| Mature mixed forest | 45 | 0 |
| Planted young forest | 5 | 0 |
| Bog | 8 | 0 |
| Natural meadow | 31 | 0 |
| Shrubby meadow | 29 | 0 |
| Arable land | 63 | 2 (3.17%) * |
| Total | 214 | 2 (0.93%) |
| Genetic Similarity with the Most Closely Related Species by Different Genes | |||
|---|---|---|---|
| Sarcocystis species | cox1 | Sarcocystis species | 28S rRNA |
| S. myodes (619 bp) | S. myodes (99.84–100%), Sarcocystis sp. Rod1 (99.52–99.68%), S. ratti (99.03–99.19%) | S. myodes (735 bp) | S. myodes (99.46–100%), Sarcocystis sp. Rod1 (97.82%), S. ratti (96.46%) |
| S. cf. glareoli (619 bp) | S. jamaicensis (100%), Sarcocystis sp. SCMW1 (99.68%), S. lutrae, S. corvusi, S. columbae, S. halieti, S. lari (99.52%), S. wobeseri, S. cornixi, Sarcocystis sp. ex Accipiter cooperi, Sarcocystis sp. Rod2 (99.35%), S. turdusi (99.19%), S. caninum, S. arctica, S. strixi, S. cf. strixi (99.03%) | S. cf. glareoli (726 bp) | S. glareolid (99.86%), S. jamaicensis (99.72%), S. microti (98.62%) |
| S. funereus (619 bp) | S. strixi (99.52%), S. lutrae, S. lari (99.35%), Sarcocystis sp. Ex Accipiter cooperi, Sarcocystis sp. SCMW1 (99.19%), S. corvusi, S. columbae, S. halieti (99.03%) | S. funereus (721 bp) | S. funereus (99.72–100%), S. lari (95.60%), S. strixi (95.25%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakas, P.; Gudiškis, N.; Kitrytė, N.; Bagdonaitė, D.L.; Baltrūnaitė, L. Detection of Three Sarcocystis Species (Apicomplexa) in Blood Samples of the Bank Vole and Yellow-Necked Mouse from Lithuania. Life 2024, 14, 365. https://doi.org/10.3390/life14030365
Prakas P, Gudiškis N, Kitrytė N, Bagdonaitė DL, Baltrūnaitė L. Detection of Three Sarcocystis Species (Apicomplexa) in Blood Samples of the Bank Vole and Yellow-Necked Mouse from Lithuania. Life. 2024; 14(3):365. https://doi.org/10.3390/life14030365
Chicago/Turabian StylePrakas, Petras, Naglis Gudiškis, Neringa Kitrytė, Dovilė Laisvūnė Bagdonaitė, and Laima Baltrūnaitė. 2024. "Detection of Three Sarcocystis Species (Apicomplexa) in Blood Samples of the Bank Vole and Yellow-Necked Mouse from Lithuania" Life 14, no. 3: 365. https://doi.org/10.3390/life14030365
APA StylePrakas, P., Gudiškis, N., Kitrytė, N., Bagdonaitė, D. L., & Baltrūnaitė, L. (2024). Detection of Three Sarcocystis Species (Apicomplexa) in Blood Samples of the Bank Vole and Yellow-Necked Mouse from Lithuania. Life, 14(3), 365. https://doi.org/10.3390/life14030365








