Ephedra alata Subsp. Alenda as a Novel Source of Bioactive Phytochemicals: Characterization Based on the Mass Spectrometry and Profiling of Antioxidant and Anti-Inflammatory Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Drugs
2.2. Plant Material, Collection, and Extraction
2.3. Phytochemistry (HPLC-DAD-QTOF-MS Analysis)
2.4. The Antioxidant Properties: In Vitro Study
2.4.1. Scavenging Ability toward DPPH
2.4.2. Superoxide Radical Scavenging Assay
2.4.3. Ferrous Ion Chelating Assay
2.5. In-Vitro Anti-Inflammatory Activity
2.5.1. COX-1 and COX-2 Inhibition Assay
2.5.2. Inhibition of Protein Denaturation
2.5.3. Ethical Clearance
2.5.4. Membrane Stabilization
2.6. Statistical Methods
3. Results and Discussion
3.1. HPLC-ESI-QTOF-MS Analysis
3.2. Antioxidants Activity
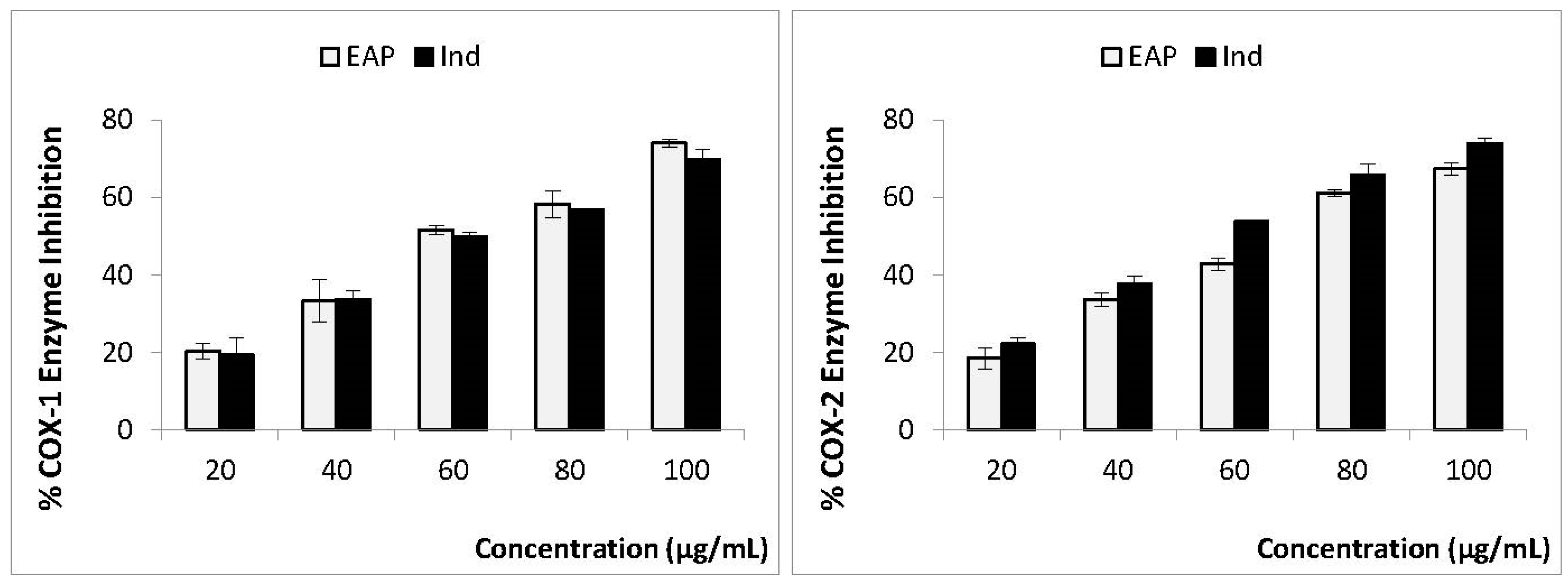
3.3. In Vitro Anti-Inflammatory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahnashi, M.H.; Alyami, B.A.; Alqahtani, Y.S.; Jan, M.S.; Rashid, U.; Sadiq, A.; Alqarni, A.O. Phytochemical Profiling of Bioactive Compounds, Anti-Inflammatory and Analgesic Potentials of Habenaria digitata Lindl.: Molecular Docking Based Synergistic Effect of the Identified Compounds. J. Ethnopharmacol. 2021, 273, 113976. [Google Scholar] [CrossRef] [PubMed]
- Tir, M.; Feriani, A.; Labidi, A.; Mufti, A.; Saadaoui, E.; Nasri, N.; Khaldi, A.; El Cafsi, M.; Tlili, N. Protective effects of phytochemicals of Capparis spinosa seeds with cisplatin and CCl4 toxicity in mice. Food Biosci. 2019, 28, 42–48. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Ghanadian, M.; Palizaban, A.; Mahnam, K.; Eshaghi, H.; Gheisari, B.; Sadeghi-Aliabadi, H. Cycloarta-23-Ene-3beta,25-Diol a Pentacyclic Steroid from Euphorbia Spinidens, as COX Inhibitor with Molecular Docking, and In Vivo Study of Its Analgesic and Anti-Inflammatory Activities in Male Swiss Mice and Wistar Rats. Prostaglandins Other Lipid Mediat. 2020, 150, 106473. [Google Scholar] [CrossRef]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular Pharmacology of Inflammation: Medicinal Plants as Anti-Inflammatory Agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Karim, S.S.; Mohamed, H.S.; Abdelhameed, M.F.; Amr AE, G.E.; Almehizia, A.A.; Nossier, E.S. Design, Synthesis and Molecular Docking of New Pyrazole-Thiazolidinones as Potent Anti-Inflammatory and Analgesic Agents with TNF-α Inhibitory Activity. Bioorganic Chem. 2021, 111, 104827. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dayma, V.; Dwivedi, A.; Baroliya, P.K.; Tripathi, I.P.; Vanangamudi, M.; Goswami, A.K. Synthesis of Sulpha Drug Based Hydroxytriazene Derivatives: Anti-Diabetic, Antioxidant, Anti-Inflammatory Activity and Their Molecular Docking Studies. Bioorganic Chem. 2020, 96, 103642. [Google Scholar] [CrossRef]
- Chandan, G.; Kumar, C.; Chibber, P.; Kumar, A.; Singh, G.; Satti, N.K.; Saini, R.V. Evaluation of Analgesic and Anti-Inflammatory Activities and Molecular Docking Analysis of Steroidal Lactones from Datura stramonium L. Phytomedicine 2021, 89, 153621. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Paul, A.; Majumder, M.; Sultan, R.A.; Emran, T.B. Pharmacological Evidence for the Use of Cissus assamica as a Medicinal Plant in the Management of Pain and Pyrexia. Biochem. Biophys. Rep. 2020, 21, 100715. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. A Review on Ethnopharmacological Applications, Pharmacological Activities, and Bioactive Compounds of Mangifera indica (Mango). Evid.-Based Complement. Altern. Med. 2017, 2017, 6949835. [Google Scholar] [CrossRef]
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N., Jr. The Essentiality of Arachidonic Acid in Infant Development. Nutr. Apr. 2016, 8, 216. [Google Scholar] [CrossRef]
- Munir, A.; Khushal, A.; Saeed, K.; Sadiq, A.; Ullah, R.; Ali, G.; Mumtaz, A. Synthesis, in-Vitro, in-Vivo Anti-Inflammatory Activities and Molecular Docking Studies of Acyl and Salicylic Acid Hydrazide Derivatives. Bioorganic Chem. 2020, 104, 104168. [Google Scholar] [CrossRef] [PubMed]
- Zafar, R.; Ullah, H.; Zahoor, M.; Sadiq, A. Isolation of Bioactive Compounds from Bergenia Ciliata (Haw.) Sternb Rhizome and Their Antioxidant and Anticholinesterase Activities. BMC Complement. Altern. Med. 2019, 19, 296. [Google Scholar] [CrossRef] [PubMed]
- Guenaou, I.; Nait Irahal, I.; Errami, A.; Lahlou, F.A.; Hmimid, F.; Bourhim, N. Bioactive Compounds from Ephedra Fragilis: Extraction Optimization, Chemical Characterization, Antioxidant and AntiGlycation Activities. Molecules 2021, 26, 5998. [Google Scholar] [CrossRef] [PubMed]
- Ibragic, S.; Barbini, S.; Oberlerchner, J.T.; Potthast, A.; Rosenau, T.; Böhmdorfer, S. Antioxidant Properties and Qualitative Analysis of Phenolic Constituents in Ephedra spp. by HPTLC Together with Injection Port Derivatization GC–MS. J. Chromatogr. B 2021, 1180, 122877. [Google Scholar] [CrossRef]
- Jaradat, N.; Hussen, F.; Al Ali, A. Preliminary Phytochemical Screening, Quantitative Estimation of Total Flavonoids, Total Phenols and Antioxidant Activity of Ephedra alata. J. Mater. Environ. Sci. 2015, 6, 1771–1778. [Google Scholar]
- Nawwar, M.A.M.; Barakat, H.H.; Buddrust, J.; Linscheidt, M. Alkaloidal, Lignan and Phenolic Constituents of Ephedra alata. Phytochemistry 1985, 24, 878–879. [Google Scholar] [CrossRef]
- Kittana, N.; Abu-Rass, H.; Sabra, R.; Manasra, L.; Hanany, H.; Jaradat, N.; Zaid, A.N. Topical Aqueous Extract of Ephedra alata Can Improve Wound Healing in an Animal Model. Chin. J. Traumatol. 2017, 20, 108–113. [Google Scholar] [CrossRef]
- Bourgou, S.; Ezzine, Y.; Ben Mansour, R.; Dakhlaoui, S.; Selmi, S.; Bouchkouel, S.; Msaada, K.; Aidi-Wannes, W.; Hiroko, I.; Megdiche-Ksouri, W. Preliminary Phytochemical Analysis, Antioxidant, Anti-Inflammatory and Anticancer Activities of Two Tunisian Ephedra Species: Ephedra alata and Ephedra fragilis. S. Afr. J. Bot. 2020, 135, 421–428. [Google Scholar]
- Dbeibia, A.; Taheur, F.B.; Altammar, K.A.; Haddaji, N.; Mahdhi, A.; Amri, Z.; Mzoughi, R.; Jabeur, C. Control of Staphylococcus Aureus Methicillin Resistant Isolated from Auricular Infections Using Aqueous and Methanolic Extracts of Ephedra alata. Saudi J. Biol. Sci. 2022, 29, 1021–1028. [Google Scholar] [CrossRef]
- Benabderrahim, M.A. Antioxidant Activity and Phenolic Profile of a Collection of Medicinal Plants from Tunisian Arid and Saharan Regions. Ind. Crops Prod. 2019, 138, 111427. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Heleno, S.A.; Bachari, K.; Dias, M.I.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds Characterization by LC-DAD- ESI/MSn and Bioactive Properties of Thymus algeriensis Boiss. Reut Ephedra Alata Decne Food Res. Int. 2019, 116, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Mufti, A.; Tir, M.; Zarei, A.; Mar Contreras, M.; Gómez-Cruz, I.; Feriani, A.; Ghazouani, L.; Saadaoui, E.; Allagui, M.S.; Harrath, A.H.; et al. Phytochemical Profiling of Ephedra alata Subsp. Alenda Seeds by High-Performance Liquid Chromatography—Electrospray Ionization—Quadrupole-Time-of-Flight-Mass Spectrometry (HPLC-ESI-QTOF-MS), Molecular Docking, and Antioxidant, Anti-Diabetic, and Acetylcholinesterase Inhibition. Anal. Lett. 2022, 55, 2450–2466. [Google Scholar] [CrossRef]
- Noui, A.; Boudiar, T.; Boulebd, H.; Gali, L.; Mar Contreras, M.; Segura-Carretero, A.; Nieto, G.; Akkal, S. HPLC–DAD–ESI/MS Profiles of Bioactive Compounds, Antioxidant and Anticholinesterase Activities of Ephedra alata subsp. alenda growing in Algeria. Nat. Prod. Res. 2022, 36, 5910–5915. [Google Scholar]
- Feriani, A.; Tir, M.; Aldahmash, W.; Mnafgui, K.; Hichem, A.; Gómez-Caravaca, A.M.; Del Mar Contreras, M.; Taamalli, A.; Alwasel, S.; Segura-Carretero, A.; et al. In Vivo Evaluation and Molecular Docking Studies of Schinus molle L. Fruit Extract Protective Effect against Isoproterenol-Induced Infarction in Rats. Environ. Sci. Pollut. Res. Int. 2022, 36, 5910–5915. [Google Scholar] [CrossRef]
- Contreras, M.M.; Gómez-Cruz, I.; Romero, I.; Castro, E. Olive Pomace-Derived Biomasses Fractionation through a Two-Step Extraction Based on the Use of Ultrasounds: Chemical Characteristics. Foods 2021, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Medfai, W.; Contreras MD, M.; Lama-Muñoz, A.; Mhamdi, R.; Oueslati, I.; Castro, E. How Cultivar and Extraction Conditions Affect Antioxidants Type and Extractability for Olive Leaves Valorization. ACS Sustain. Chem. Eng. 2020, 8, 5107–5118. [Google Scholar]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a Heated Histidine-Glucose Model System. I: Investigation of the Antioxidant Role of Histidine and Isolation of Antioxidants by High-Performance Liquid Chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]
- Yuan, Z.B.; Gao, R.M. Kinetics and mechanism of pyrogallol autoxidation. Chem. J. Chin. Univ. -Chin. 1997, 18, 1438–1441. [Google Scholar]
- Chew, Y.-L.; Goh, J.-K.; Lim, Y.-Y. Assessment of in-vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem. 2009, 116, 13–18. [Google Scholar] [CrossRef]
- Husseini, Y.; Sahraei, H.; Meftahi, G.H.; Dargahian, M.; Mohammadi, A.; Hatef, B.; Zardooz, H.; Ranjbaran, M.; Hosseini, S.B.; Alibeig, H.; et al. Analgesic and Anti-Inflammatory Activities of Hydro-Alcoholic Extract of Lavandula officinalis in Mice: Possible Involvement of the Cyclooxygenase Type 1 and 2 Enzymes. Rev. Bras. Farmacogn. 2016, 26, 102–108. [Google Scholar] [CrossRef]
- Ullah, H.A.; Zaman, S.; Juhara, F.; Akter, L.; Tareq, S.M.; Masum, E.H.; Bhat-tacharjee, R. Evaluation of Antinociceptive, In-Vivo and In-Vitro Anti-Inflammatory Activity of Ethanolic Extract of Curcuma zedoaria Rhizome. BMC Complement. Altern. Med. 2014, 14, 346. [Google Scholar] [CrossRef] [PubMed]
- Sadique, J.; Al-Rqobahs, W.A.; Bughaith, E.I.; Gindi, A.R. The Bio-Activity of Certain Medicinal Plants on the Stabilization of RBC Membranesystem. Fitoterapia 1989, 60, 525–532. [Google Scholar]
- Caveney, S.; Charlet, D.A.; Freitag, H.; Maier-Stolte, M.; Starratt, A.N. New Observations on the Secondary Chemistry of World Ephedra (Ephedraceae). Am. J. Bot. 2001, 88, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wang, S.; Liang, P.; Wang, Y.; Zhang, X.; Jia, Q.; He, L. Screening and Evaluation of Anti-SARS-CoV-2 Components from Ephedra sinica by ACE2/CMC-HPLC-IT-TOF-MS Approach. Anal. Bioanal. Chem. 2021, 413, 2995–3004. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Khalifeh, H.; Saad, F.; Serale, N.; Salis, A.; Damonte, G.; Lupidi, G.; Daher, A.; Vergani, L. Protective Effects of Extracts from Ephedra foeminea Forssk Fruits Against Oxidative Injury in Human Endothelial Cells. J. Ethnopharmacol. 2020, 260, 112976. [Google Scholar] [CrossRef]
- Calvano, C.D.; Glaciale, M.; Palmisano, F.; Cataldi, T.R.I. Glycosphingolipidomics of Donkey Milk by Hydrophilic Interaction Liquid Chromatography Coupled to ESI and Multistage MS. Electrophoresis 2018, 39, 1634–1644. [Google Scholar] [CrossRef]
- Naumoska, K.; Jug, U.; Metli_car, V.; Vovk, I. Oleamide, a Bioactive Compound, Unwittingly Introduced into the Human Body through Some Plastic Food/Beverages and Medicine Containers. Foods 2020, 9, 549. [Google Scholar] [CrossRef]
- Feriani, A.; Tir, M.; Mufti, A.; Caravaca, A.M.G.; Contreras, M.D.M.; Taamalli, A.; Carretero, A.S.; Aldawood, N.; Nahdi, S.; Alwasel, S.; et al. HPLC-ESI-QTOF-MS/MS Profiling and Therapeutic Effects of Schinus terebinthifolius and Schinus molle Fruits: Investigation of Their Antioxidant, Antidiabetic, Anti-Inflammatory and Antinociceptive Properties. Inflammopharmacology 2021, 29, 467–481. [Google Scholar] [CrossRef]
- Hamoudi, M.; Amroun, D.; Baghiani, A.; Khennouf, S.; Dahamna, S. Antioxidant, Anti-Inflammatory, and Analgesic Activities of Alcoholic Extracts of Ephedra Nebrodensis from Eastern Algeria. Turk. J. Pharm. Sci. 2021, 18, 574–580. [Google Scholar] [CrossRef]
- Ali, S.I.; El-Emary, G.A.E.; Mohamed, A.A. Effects of Gamma Irradiation on FT-IR Fingerprint, Phenolic Contents and Antioxidant Activity of Foeniculum vulgare and Carum carvi Seeds. Res. J. Pharm. Technol. 2018, 11, 3323–3329. [Google Scholar] [CrossRef]
- Tlili, N.; Yahia, Y.; Feriani, A.; Labidi, A.; Ghazouani, L.; Nasri, N.; Saadaoui, E.; Nasri, N. Schinus terebinthifolius vs. Schinus molle: A Comparative Study of the Effect of Species Andlocation on the Phytochemical Content of Fruits. Ind. Crops Prod. 2018, 122, 559–565. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A Review on Antioxidants, Prooxidants and Related Controversy: Natural and Synthetic Compounds, Screening and Analysis Methodologies and Future Perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Moraes, A.D.T.; de Miranda, M.D.S.; Jacob, Í.T.T.; da Cruz Amorim, C.A.; de Moura, R.O.; da Silva, S.Â.S.; Soares, M.B.P.; de Almeida, S.M.V.; de Lima Souza, T.R.C.; de Oliveira, J.F.; et al. Synthesis, in vitro and in vivo biological evaluation, COX-1/2 inhibition and molecular docking study of indole-N-acylhydrazone derivatives. Bioorg. Med. Chem. 2018, 26, 5388–5396. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Cisneros, M.Á.; Rios, M.Y.; Aguilar-Guadarrama, A.B.; Rao, P.P.; Aburto-Amar, R.; Rodríguez-López, V. In vitro COX-1 and COX-2 Enzyme Inhibitory Activities of Iridoids from Penstemon barbatus, Castilleja tenuiflora, Cresentia alata and Vitex mollis. Bioorganic Med. Chem. Lett. 2015, 25, 4505–4508. [Google Scholar] [CrossRef] [PubMed]
- Ketha, A.; Vedula, G.S.; Sastry, A.V.S. In vitro Antioxidant, Anti-Inflammatory, and Anticancer Activities of Methanolic Extract and Its Metabolites of Whole Plant Cardiospermum Canescens Wall. Future J. Pharm. Sci. 2020, 6, 11. [Google Scholar] [CrossRef]
- Rjeibi, I.; Saad, B.; Anouar, N.; Sana, S.; Sami, A.; Salah, M.; Hfaiedh, N. Brachychiton populneus as a Novel Source of Bioactive Ingredients with Therapeutic Effects: Antioxidant, Enzyme Inhibitory, Anti-Inflammatory Properties and LC–ESI-MS Profile. Inflammopharmacology 2019, 28, 563–574. [Google Scholar] [CrossRef]
- Osman, N.I.; Sidik, N.J.; Awal, A.; Adam, N.A.; Rezali, N.I. In vitro Xanthine Oxidase and Albumin Denaturation Inhibition Assay of Barringtonia racemosa L. and Total Phenolic Content Analysis for Potential Anti-Inflammatory Use in Gouty Arthritis. J. Intercult. Ethnopharmacol. 2016, 5, 343–349. [Google Scholar] [CrossRef]
- Dharmadeva, S.; Galgamuwa, L.S.; Prasadinie, C.; Kumarasinghe, N. In vitro Anti-Inflammatory Activity of Ficus racemosa L. Bark Using Albumin Denaturation Method. Ayu 2018, 39, 239. [Google Scholar] [CrossRef]
- Gunathilake, K.; Ranaweera, K.; Rupasinghe, H. In vitro Anti-Inflammatory Properties of Selected Green Leafy Vegetables. Biomedicines 2018, 6, 107. [Google Scholar] [CrossRef]
- Alamgeer, N.S.G.; Uttra, A.M.; Qaiser, M.N.; Ahsan, H. Appraisal of Anti-Arthritic and Nephroprotective Potential of Cuscuta reflexa. Pharm Biol. 2017, 55, 792–798. [Google Scholar] [CrossRef]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, Proanthocyanidins, Flavones and Flavonols in Some Plant Materials and Their Antioxidant Activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Ramadhan, U. In vitro Assessment of Anti-Inflammatory and COX-2 inhibitory action of some medicinal plants. J. Biol. Res. Boll. Della Soc. Ital. Biol. Sper. 2020, 93. [Google Scholar] [CrossRef]
- Morales León, A.J.; González Santisteban, A.; Peña Fuentes, D.; Guardia Puebla, Y.; Torres Rodríguez, E. In vitro Anti-Inflammatory Activity of Aqueous, Ethanolic and Ethereal Extracts of Rhizomes, Leaves and Stems of Anredera vesicaria. J. Anal. Pharm. Res. 2018, 7, 459–461. [Google Scholar] [CrossRef]
- Aitadafouri, M.; Mounnnieri, C.; Heyman, S.F.; Binistic, C.; Bon, C.; Godhold, J. 4-Alkoxybenzamides as New Potent Phospholipase A 2 Inhibitors. Biochem. Pharmacol. 1996, 51, 737–742. [Google Scholar]
- Gambhire, M.; Juvekar, A.; Wankhede, S. Evaluation of Anti-Inflammatory Activity of Methanol Extract of Barleria cristata Leaves by In Vivo and In vitro Methods. Int. J. Pharmacol. 2009, 7, 1–4. [Google Scholar]
- Kumar, V.; Bhat, Z.A.; Kumar, D.; Bohra, P.; Sheela, S. In–vitro Anti –Inflammatory Activity of Leaf Extracts of Basella alba Linn.Var Alba. Int. J. Drug Dev. Res. 2011, 3, 176–179. [Google Scholar]
- Rukshala, D.; de Silva, E.D.; Ranaweera, B.L.R.; Fernando, N.; Handunnetti, S.M. Anti-Inflammatory Effect of Leaves of Vernonia zeylanica in Lipopolysaccharide-Stimulated Raw 264.7 Macrophages and Carrageenan-Induced Rat Paw-Edema Model. J. Ethnopharmacol. 2021, 274, 114030. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, L.; Huang, G.; Zhou, T.; Zhang, X.; Leng, X.; Lin, J. Embelia Laeta Aqueous Extract Suppresses Acute Inflammation via Decreasing COX-2/INOS Expression and Inhibiting NF-ΚB Pathway. J. Ethnopharmacol. 2021, 281, 114575. [Google Scholar] [CrossRef]
- Wu, Z.; Kong, X.; Zhang, T.; Ye, J.; Fang, Z.; Yang, X. Pseudoephedrine/Ephedrine Shows Potent Anti-Inflammatory Activity against TNF-α-Mediated Acute Liver Failure Induced by Lipopolysaccharide/d-Galactosamine. Eur. J. Pharmacol. 2014, 724, 112–121. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Qi, F.; Sun, J.H.; Yan, J.Q.; Li, C.M.; Lv, X.C. Anti-Inflammatory Effects of Isorhamnetin on LPS-Stimulated Human Gingival Fibroblasts by Activating Nrf2 Signaling Pathway. Microb. Pathog. 2018, 120, 37–41. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Kim, H.-W.; Lee, M.-K.; Kim, H.-J.; Kim, J.-B.; Choe, J.-S.; Lee, Y.-M.; Jang, H.-H. Antioxidant and Anti-Inflammatory Activities in Relation to the Flavonoids Composition of Pepper (Capsicum annuum L.). Antioxidants 2020, 9, 986. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-M.; Lee, S.A.; Hong, J.H.; Kim, J.-S.; Kim, D.K.; Kim, C.S. Oleamide Suppresses Inflammatory Responses in LPS-Induced RAW264.7 Murine Macrophages and Alleviates Paw Edema in a Carrageenan-Induced Inflammatory Rat Model. Int. Immunopharmacol. 2018, 56, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Nixon, G.F. Sphingolipids in Inflammation: Pathological Implications and Potential Therapeutic Targets. Br. J. Pharmacol. 2009, 158, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2018, 8, 1957. [Google Scholar] [CrossRef]
- Gad, M.Z.; Azab, S.S.; Khattab, A.R.; Farag, M.A. Over a Century since Ephedrine Discovery: An Updated Revisit to Its Pharmacological Aspects, Functionality and Toxicity in Comparison to Its Herbal Extracts. Food Amp. Funct. 2021, 12, 9563–9582. [Google Scholar] [CrossRef]
- Limongelli, V.; Bonomi, M.; Marinelli, L.; Gervasio, F.L.; Cavalli, A.; Novellino, E.; Parrinello, M. Molecular Basis of Cyclooxygenase Enzymes (COXs) Selective Inhibition. Proc. Natl. Acad. Sci. USA 2010, 107, 5411–5416. [Google Scholar] [CrossRef]
- Statler, A.K.; Maani, C.; Kohli, A. Ephedrine; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547661/ (accessed on 1 January 2022).
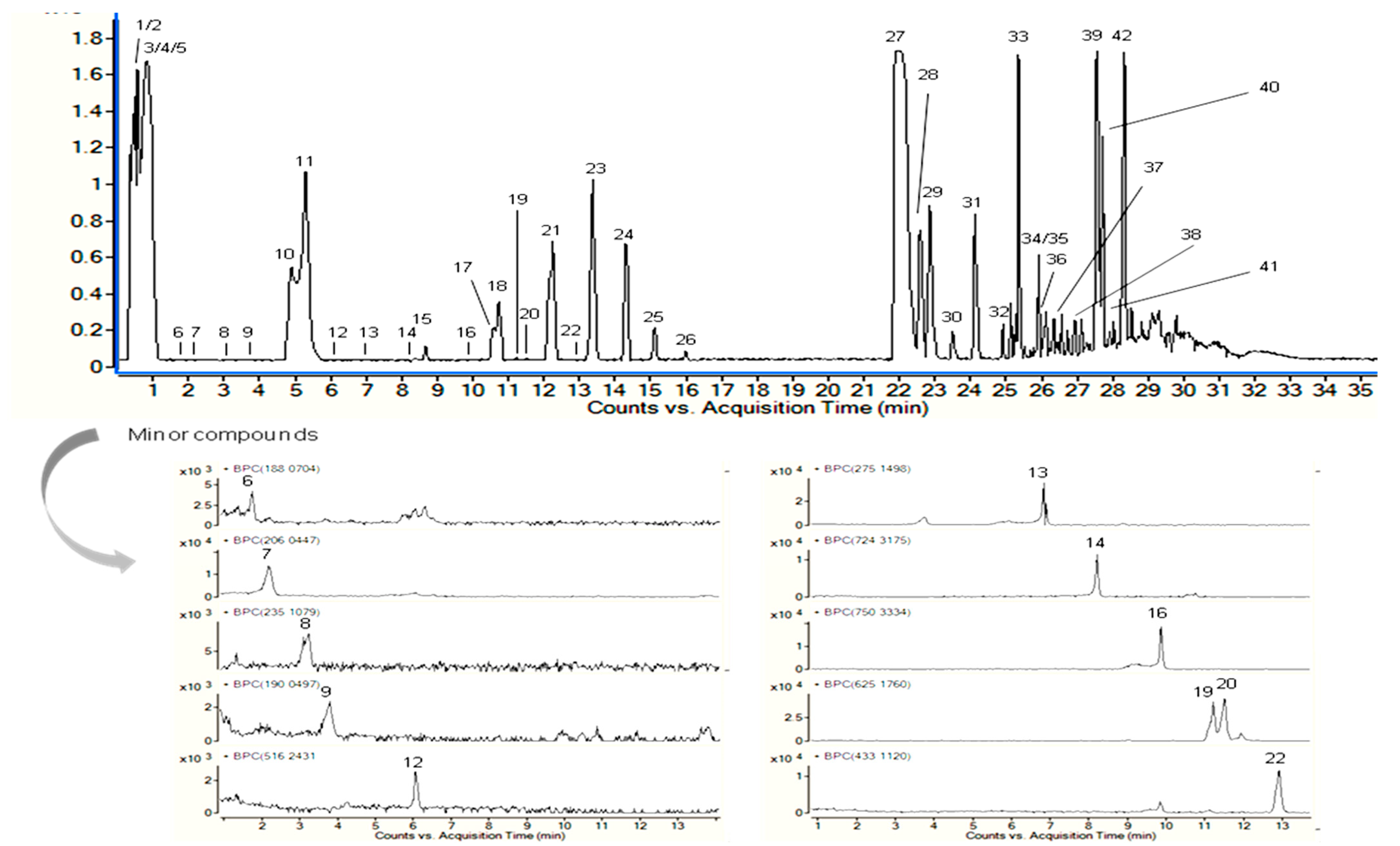

| No. | Proposed Compound | Molecular Formula | RT (min) | Mean Peak Area | Relative % a |
|---|---|---|---|---|---|
| 1 | Unknown | C5H13NO | 0.4 | 6,941,206 | 3.567 ± 0.013 |
| 2 | Methanoproline | C6H9NO2 | 0.5 | 327,766 | 0.168 ± 0.004 |
| 3 | Leucine/Isoleucine hexoside | C12H23NO7 | 0.6 | 9,895,290 | 5.085 ± 0.158 |
| 4 | Phenylalanine hexoside | C15H21NO7 | 0.8 | 32,493,414 | 16.699 ± 0.309 |
| 5 | Ephedrine derivative 1 (+ hexosyl + deoxyhexosyl) | C22H35NO10 | 1.1 | 47,146 | 0.024 ± 0.004 |
| 6 | Indoleacrylic acid | C11H9NO2 | 1.7 | 18,548 | 0.010 ± 0.001 |
| 7 | Hydroxykynurenic acid | C10H7NO4 | 2.2 | 110,717 | 0.057 ± 0.001 |
| 8 | Unknown | C12H14N2O3 | 3.2 | 58,993 | 0.030 ± 0.001 |
| 9 | Kynurenic acid | C10H7NO3 | 3.7 | 26,694 | 0.014 ± 0.003 |
| 10 | Unknown | C10H13NO2 | 4.9 | 6,082,634 | 3.126 ± 0.216 |
| 11 | Unknown | C10H13NO2 | 5.3 | 12,797,772 | 6.577 ± 0.119 |
| 12 | Ephedrine derivative 2 | C24H37NO11 | 6.1 | 7645 | 0.004 ± 0.000 |
| 13 | Unknown | C14H18N4O2 | 6.9 | 160,758 | 0.083 ± 0.003 |
| 14 | Ephedrine derivative 3 | C35H49NO15 | 8.2 | 39,773 | 0.020 ± 0.004 |
| 15 | Isoschaftoside | C26H28O14 | 8.7 | 558,228 | 0.287 ± 0.001 |
| 16 | Ephedrine derivative 4 | C37H51NO15 | 9.9 | 44,981 | 0.023 ± 0.002 |
| 17 | Unknown | C29H59NO9 | 10.7 | 1,133,485 | 0.583 ± 0.012 |
| 18 | Unknown | C29H59NO9 | 10.8 | 1,919,159 | 0.986 ± 0.106 |
| 19 | Isorhamnetin O-hexoside-O-deoxyhexoside 1 | C28H32O16 | 11.2 | 312,278 | 0.160 ± 0.010 |
| 20 | Isorhamnetin O-hexoside-O-deoxyhexoside 2 | C28H32O16 | 11.5 | 375,236 | 0.193 ± 0.000 |
| 21 | Unknown (compound 18 + C6H11NO) | C35H70N2O10 | 12.3 | 5,044,989 | 2.593 ± 0.091 |
| 22 | Kaempferol 3-O-rhamnoside | C21H20O10 | 12.9 | 89,651 | 0.046 ± 0.000 |
| 23 | Unknown (compound 19 + C6H11NO) | C41H81N3O11 | 13.4 | 2,472,859 | 1.271 ± 0.047 |
| 24 | Unknown (compound 20 + C6H11NO) | C47H92N4O12 | 14.3 | 565,680 | 0.291 ± 0.006 |
| 25 | Unknown (compound 21 + C6H11NO) | C53H103N5O13 | 15.1 | 74,822 | 0.038±0.000 |
| 26 | Tetradecasphinganine | C14H31NO2 | 16 | 675,136 | 0.347 ± 0.010 |
| 27 | Hexadecasphinganine | C16H35NO2 | 22 | 47,041,954 | 24.176 ± 1.961 |
| 28 | Phytosphingosine | C18H39NO3 | 22.7 | 58,02,618 | 2.982 ± 0.226 |
| 29 | Sphingolipid derivative 1 | C16H35NO3 | 22.8 | 6,817,998 | 3.504 ± 0.513 |
| 30 | Sphingolipid derivative 2 | C16H33NO3 | 23.5 | 627,400 | 0.322 ± 0.011 |
| 31 | Unknown | C14H31NO | 24.1 | 8,684,421 | 4.463 ± 0.566 |
| 32 | Sphingolipid derivative 3 | C18H39NO3 | 25 | 2,066,875 | 1.062 ± 0.186 |
| 33 | Unknown | C16H36NO | 25.4 | 4,771,780 | 2.452 ± 0.105 |
| 34 | 9,10-Dihydroxystearic acid | C18H36O4 | 25.8 | 32,234 | 0.017 ± 0.000 |
| 35 | Deoxysphinganine | C18H39NO | 25.9 | 403,861 | 0.208 ± 0.017 |
| 36 | Hydroxyoctadecatrienoic acid | C18H30O3 | 26.3 | 1,173,301 | 0.603 ± 0.025 |
| 37 | Unknown (choline derivative) | C28H47N3O6 | 26.5 | 80,220 | 0.041 ± 0.002 |
| 38 | Unknown | C20H37NO3 | 27 | 148,727 | 0.076 ± 0.004 |
| 39 | Unknown | C17H32N6O2 | 27.5 | 10,892,423 | 5.598 ± 0.659 |
| 40 | Oleamide | C18H35NO | 27.8 | 13,817,387 | 7.101 ± 0.705 |
| 41 | N-Palmitoylsphingosine | C34H67NO3 | 28 | 1,601,879 | 0.823 ± 0.440 |
| 42 | Unknown | C19H36N6O2 | 28.4 | 8,344,368 | 4.288 ± 0.626 |
| Sample | DPPH Radical Scavenging | Superoxide Radical Scavenging | Fe2+ Chelating |
|---|---|---|---|
| Ephedra alata pulp | 0.57 ± 0.05 ns | 0.55 ± 0.01 * | 0.51 ± 0.02 * |
| Ascorbic acid | 0.54 ± 0.01 | 0.63 ± 0.02 | 0.46 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mufti, A.; Contreras, M.d.M.; Gómez-Cruz, I.; Alshamrani, A.; Nahdi, S.; Mansour, L.; Alwasel, S.; Harrath, A.H.; Tlili, N. Ephedra alata Subsp. Alenda as a Novel Source of Bioactive Phytochemicals: Characterization Based on the Mass Spectrometry and Profiling of Antioxidant and Anti-Inflammatory Properties. Life 2023, 13, 323. https://doi.org/10.3390/life13020323
Mufti A, Contreras MdM, Gómez-Cruz I, Alshamrani A, Nahdi S, Mansour L, Alwasel S, Harrath AH, Tlili N. Ephedra alata Subsp. Alenda as a Novel Source of Bioactive Phytochemicals: Characterization Based on the Mass Spectrometry and Profiling of Antioxidant and Anti-Inflammatory Properties. Life. 2023; 13(2):323. https://doi.org/10.3390/life13020323
Chicago/Turabian StyleMufti, Afoua, María del Mar Contreras, Irene Gómez-Cruz, Abdullah Alshamrani, Saber Nahdi, Lamjed Mansour, Salah Alwasel, Abdel Halim Harrath, and Nizar Tlili. 2023. "Ephedra alata Subsp. Alenda as a Novel Source of Bioactive Phytochemicals: Characterization Based on the Mass Spectrometry and Profiling of Antioxidant and Anti-Inflammatory Properties" Life 13, no. 2: 323. https://doi.org/10.3390/life13020323
APA StyleMufti, A., Contreras, M. d. M., Gómez-Cruz, I., Alshamrani, A., Nahdi, S., Mansour, L., Alwasel, S., Harrath, A. H., & Tlili, N. (2023). Ephedra alata Subsp. Alenda as a Novel Source of Bioactive Phytochemicals: Characterization Based on the Mass Spectrometry and Profiling of Antioxidant and Anti-Inflammatory Properties. Life, 13(2), 323. https://doi.org/10.3390/life13020323








