Arachidonic Acid Added during the Differentiation Phase of 3T3-L1 Cells Exerts Anti-Adipogenic Effect by Reducing the Effects of Pro-Adipogenic Prostaglandins
Abstract
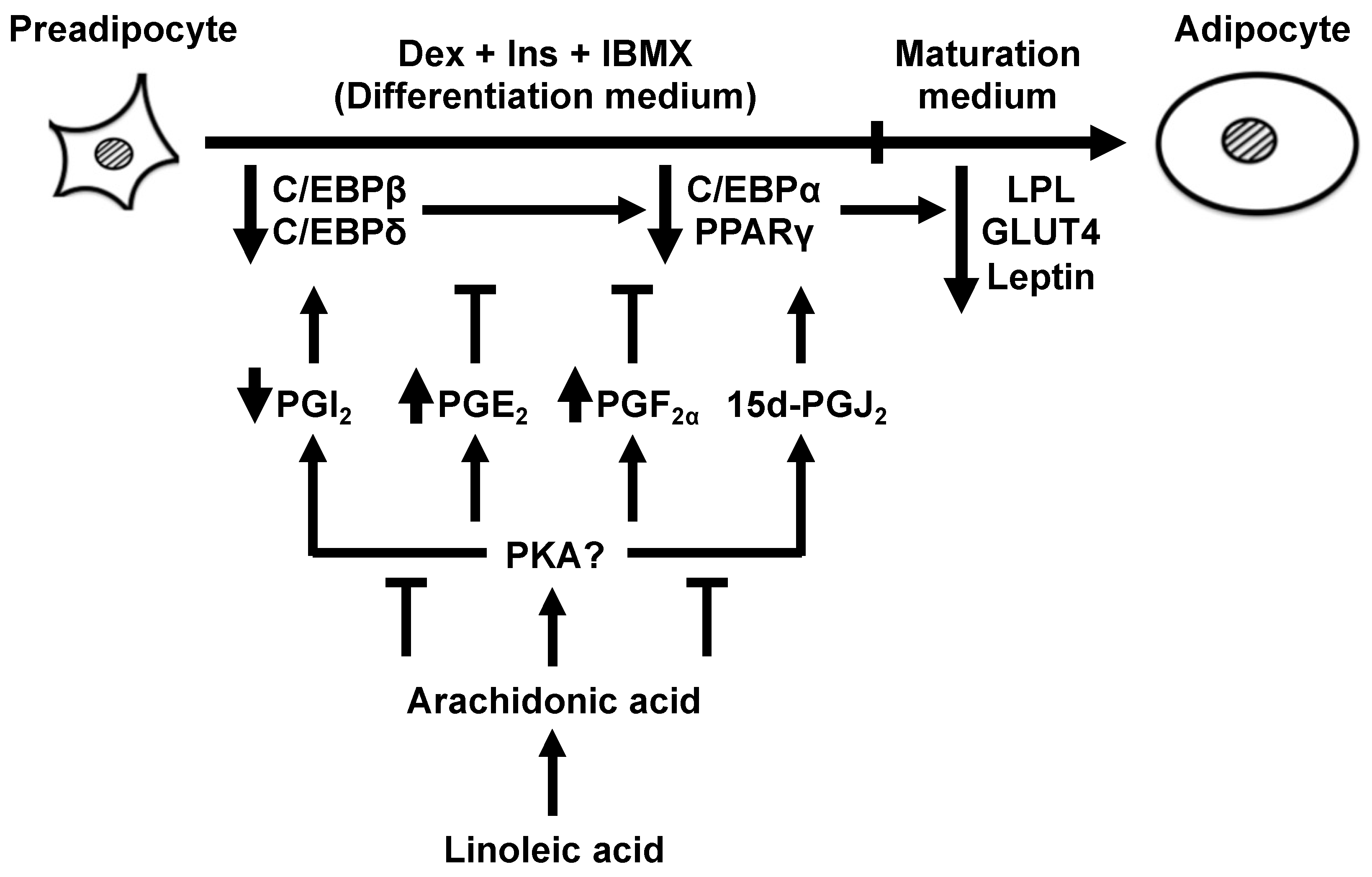
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Culture of 3T3-L1 Cells and Induction of Adipogenesis
2.3. Quantification of Intracellular TAGs and Cellular Proteins
2.4. Quantification of PGs by ELISA
2.5. Quantification of Gene Expression
2.6. Statistical Analyses
3. Results
3.1. Effects of AA or LA Added during Only the Differentiation Phase of 3T3-L1 Cells on MDI-Induced Adipogenesis
3.2. Types of PGs Biosynthesized by Adding AA during Only the Differentiation Phase of 3T3-L1 Cells and Effects of PGs Added during Only the Differentiation Phase on MDI-Induced Adipogenesis
3.3. Adipogenesis-Related Gene Expression in Response to AA during the Differentiation Phase
3.4. Effects of AA Coexistent with Pro-Adipogenic PGs during the Differentiation Phase of 3T3-L1 Cells on MDI-Induced Adipogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, C.N.; Ross, S.E.; Longo, K.A.; Bajnok, L.; Hemati, N.; Johnson, K.W.; Harrison, S.D.; MacDougald, O.A. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002, 277, 30998–31004. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Kehinde, O. Sublines of mouse 3T3 cells that accumulate lipid. Cell 1974, 1, 113–116. [Google Scholar] [CrossRef]
- Green, H.; Kehinde, O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 1975, 5, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Umek, R.M.; McKnight, S.L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xie, Y.; Bucher, N.L.; Farmer, S.R. Conditional ectopic expression of C/EBPβ in NIH-3T3 cells induces PPARγ and stimulates adipogenesis. Genes Dev. 1995, 9, 2350–2363. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.C.; Cao, Z.; Classon, M.; McKnight, S.L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995, 9, 168–181. [Google Scholar] [CrossRef]
- Wu, Z.; Bucher, N.L.; Farmer, S.R. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Mol. Cell. Biol. 1996, 16, 4128–4136. [Google Scholar] [CrossRef]
- Tzameli, I.; Fang, H.; Ollero, M.; Shi, H.; Hamm, J.K.; Kievit, P.; Hollenberg, A.N.; Flier, J.S. Regulated production of a peroxisome proliferator-activated receptor-γ ligand during an early phase of adipocyte differentiation in 3T3-L1 adipocytes. J. Biol. Chem. 2004, 279, 36093–36102. [Google Scholar] [CrossRef]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef]
- MacDougald, O.A.; Lane, M.D. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 1995, 64, 345–373. [Google Scholar] [CrossRef]
- Lowell, B.B. PPARγ: An essential regulator of adipogenesis and modulator of fat cell function. Cell 1999, 99, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 2000, 16, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Massiera, F.; Saint-Marc, P.; Seydoux, J.; Murata, T.; Kobayashi, T.; Narumiya, S.; Guesnet, P.; Amri, E.Z.; Negrel, R.; Ailhaud, G. Arachidonic acid and prostacyclin signaling promote adipose tissue development: A human health concern? J. Lipid Res. 2003, 44, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S. Prostacyclin: A major prostaglandin in the regulation of adipose tissue development. J. Cell Physiol. 2019, 234, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Forman, B.M.; Tontonoz, P.; Chen, J.; Brun, R.P.; Spiegelman, B.M.; Evans, R.M. 15-Deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 1995, 83, 803–812. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Lenhard, J.M.; Willson, T.M.; Patel, I.; Morris, D.C.; Lehmann, J.M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell 1995, 83, 813–819. [Google Scholar] [CrossRef]
- Mazid, M.A.; Chowdhury, A.A.; Nagao, K.; Nishimura, K.; Jisaka, M.; Nagaya, T.; Yokota, K. Endogenous 15-deoxy-Δ12,14-prostaglandin J2 synthesized by adipocytes during maturation phase contributes to upregulation of fat storage. FEBS Lett. 2006, 580, 6885–6890. [Google Scholar] [CrossRef]
- Hossain, M.S.; Chowdhury, A.A.; Rahman, M.S.; Nishimura, K.; Jisaka, M.; Nagaya, T.; Shono, F.; Yokota, K. Development of enzyme-linked immunosorbent assay for Δ12-prostaglandin J2 and its application to the measurement of endogenous product generated by cultured adipocytes during the maturation phase. Prostaglandins Other Lipid Mediat. 2011, 94, 73–80. [Google Scholar] [CrossRef]
- Aubert, J.; Saint-Marc, P.; Belmonte, N.; Dani, C.; Négrel, R.; Ailhaud, G. Prostacyclin IP receptor up-regulates the early expression of C/EBPβ and C/EBPδ in preadipose cells. Mol. Cell. Endocrinol. 2000, 160, 149–156. [Google Scholar] [CrossRef]
- Falcetti, E.; Flavell, D.M.; Staels, B.; Tinker, A.; Haworth, S.G.; Clapp, L.H. IP receptor-dependent activation of PPARγ by stable prostacyclin analogues. Biochem. Biophys. Res. Commun. 2007, 360, 821–827. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Tsuboi, H.; Okuno, Y.; Tamba, S.; Tsuchiya, S.; Tsujimoto, G.; Ichikawa, A. Microarray evaluation of EP4 receptor-mediated prostaglandin E2 suppression of 3T3-L1 adipocyte differentiation. Biochem. Biophys. Res. Commun. 2004, 322, 911–917. [Google Scholar] [CrossRef]
- Tsuboi, H.; Sugimoto, Y.; Kainoh, T.; Ichikawa, A. Prostanoid EP4 receptor is involved in suppression of 3T3-L1 adipocyte differentiation. Biochem. Biophys. Res. Commun. 2004, 322, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Inazumi, T.; Shirata, N.; Morimoto, K.; Takano, H.; Segi-Nishida, E.; Sugimoto, Y. Prostaglandin E2-EP4 signaling suppresses adipocyte differentiation in mouse embryonic fibroblasts via an autocrine mechanism. J. Lipid Res. 2011, 52, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.W.; Casimir, D.A.; Ntambi, J.M. The mechanism of inhibition of 3T3-L1 preadipocyte differentiation by prostaglandin F2α. Endocrinology 1996, 137, 5641–5650. [Google Scholar] [CrossRef]
- Fujimori, K.; Ueno, T.; Nagata, N.; Kashiwagi, K.; Aritake, K.; Amano, F.; Urade, Y. Suppression of adipocyte differentiation by aldo-keto reductase 1B3 acting as prostaglandin F2α synthase. J. Biol. Chem. 2010, 285, 8880–8886. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.; Pedersen, L.M.; Liaset, B.; Ma, T.; Petersen, R.K.; van den Berg, S.; Pan, J.; Müller-Decker, K.; Dülsner, E.D.; Kleemann, R.; et al. cAMP-dependent signaling regulates the adipogenic effect of n-6 polyunsaturated fatty acids. J. Biol. Chem. 2008, 283, 7196–7205. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.K.; Jørgensen, C.; Rustan, A.C.; Frøyland, L.; Muller-Decker, K.; Furstenberger, G.; Berge, R.K.; Kristiansen, K.; Madsen, L. Arachidonic acid-dependent inhibition of adipocyte differentiation requires PKA activity and is associated with sustained expression of cyclooxygenases. J. Lipid Res. 2003, 44, 2320–2330. [Google Scholar] [CrossRef]
- Khan, F.; Syeda, P.K.; Nartey, M.N.; Rahman, M.S.; Islam, M.S.; Nishimura, K.; Jisaka, M.; Shono, F.; Yokota, K. Pretreatment of cultured preadipocytes with arachidonic acid during the differentiation phase without a cAMP-elevating agent enhances fat storage after the maturation phase. Prostaglandins Other Lipid Mediat. 2016, 123, 16–27. [Google Scholar] [CrossRef]
- Nikolopoulou, E.; Papacleovoulou, G.; Jean-Alphonse, F.; Grimaldi, G.; Parker, M.G.; Hanyaloglu, A.C.; Christian, M. Arachidonic acid-dependent gene regulation during preadipocyte differentiation controls adipocyte potential. J. Lipid Res. 2014, 55, 2479–2490. [Google Scholar] [CrossRef] [PubMed]
- Syeda, P.K.; Hossain, M.S.; Chowdhury, A.A.; Rahman, M.S.; Nishimura, K.; Jisaka, M.; Nagaya, T.; Shono, F.; Yokota, K. Generation of monoclonal antibody for 15-deoxy-Δ12,14-prostaglandin J2 and development of enzyme-linked immunosorbent assay for its quantification in culture medium of adipocytes. Appl. Biochem. Biotechnol. 2012, 167, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Morishima, T.; Nagaya, T.; Jisaka, M.; Takinami, K. Modification of cultured Madin-Darby canine kidney cells with dietary unsaturated fatty acids and regulation of arachidonate cascade reaction. Biosci. Biotechnol. Biochem. 1996, 60, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Khan, F.; Syeda, P.K.; Nishimura, K.; Jisaka, M.; Nagaya, T.; Shono, F.; Yokota, K. Endogenous synthesis of prostacyclin was positively regulated during the maturation phase of cultured adipocytes. Cytotechnology 2014, 66, 635–646. [Google Scholar] [CrossRef]
- Kelton, J.G.; Blajchman, M.A. Prostaglandin I2 (prostacyclin). Can. Med. Assoc. J. 1980, 122, 175–179. [Google Scholar] [PubMed]
- Ji, Z.; Mei, F.C.; Cheng, X. Epac, not PKA catalytic subunit, is required for 3T3-L1 preadipocyte differentiation. Front. Biosci. (Elite Ed.) 2010, 2, 392–398. [Google Scholar] [CrossRef]
- Gabrielli, M.; Martini, C.N.; Brandani, J.N.; Iustman, L.J.; Romero, D.G.; del, C. Vila, M. Exchange protein activated by cyclic AMP is involved in the regulation of adipogenic genes during 3T3-L1 fibroblasts differentiation. Dev. Growth Differ. 2014, 56, 143–151. [Google Scholar] [CrossRef]
- Li, F.; Wang, D.; Zhou, Y.; Zhou, B.; Yang, Y.; Chen, H.; Song, J. Protein kinase A suppresses the differentiation of 3T3-L1 preadipocytes. Cell Res. 2008, 18, 311–323. [Google Scholar] [CrossRef]
- Reginato, M.J.; Krakow, S.L.; Bailey, S.T.; Lazar, M.A. Prostaglandins promote and block adipogenesis through opposing effects on peroxisome proliferator-activated receptor γ. J. Biol. Chem. 1998, 273, 1855–1858. [Google Scholar] [CrossRef]
- Fujimori, K.; Yano, M.; Ueno, T. Synergistic suppression of early phase of adipogenesis by microsomal PGE synthase-1 (PTGES1)-produced PGE2 and aldo-keto reductase 1B3-produced PGF2α. PLoS ONE 2012, 7, e44698. [Google Scholar] [CrossRef]
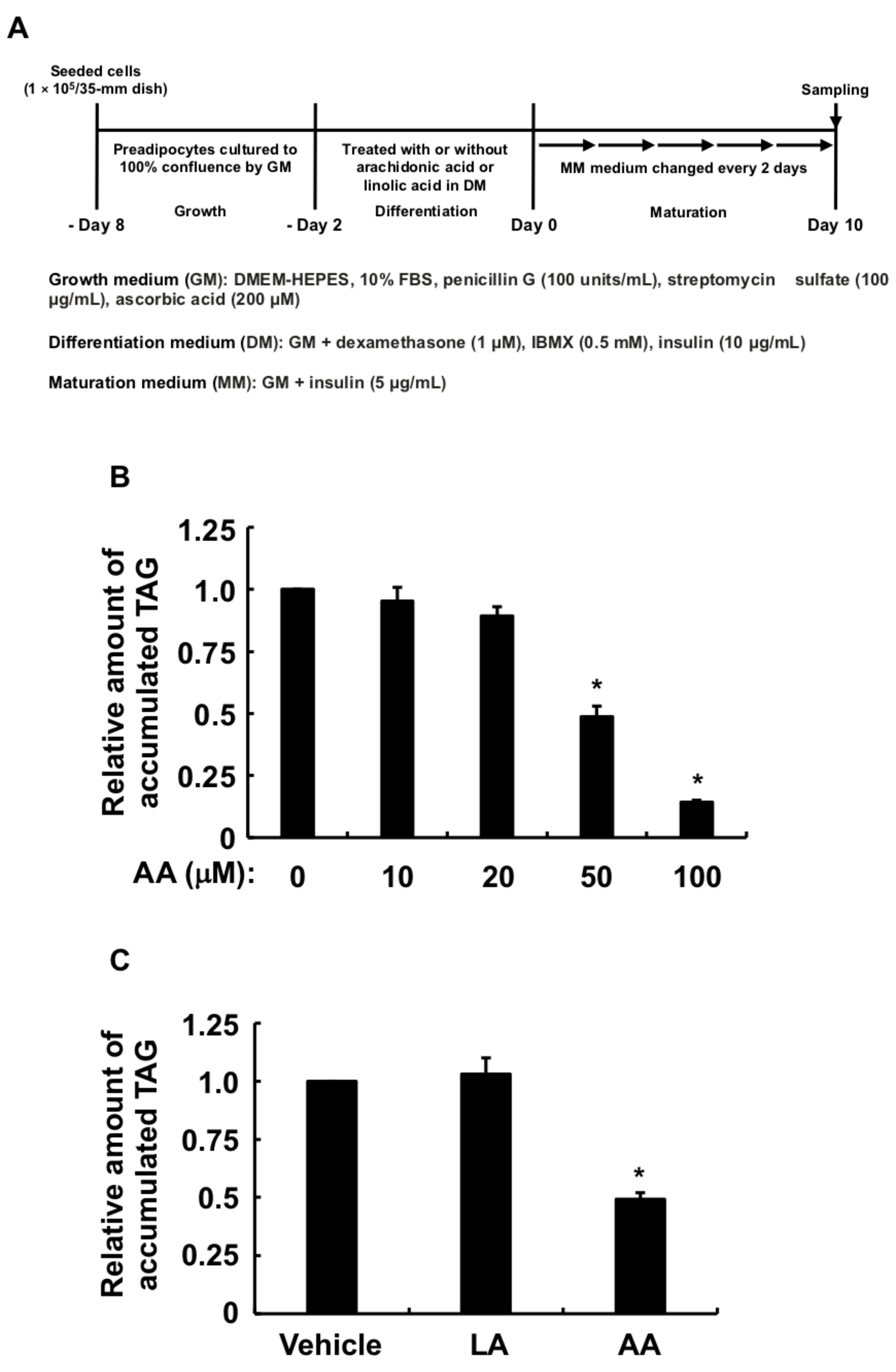

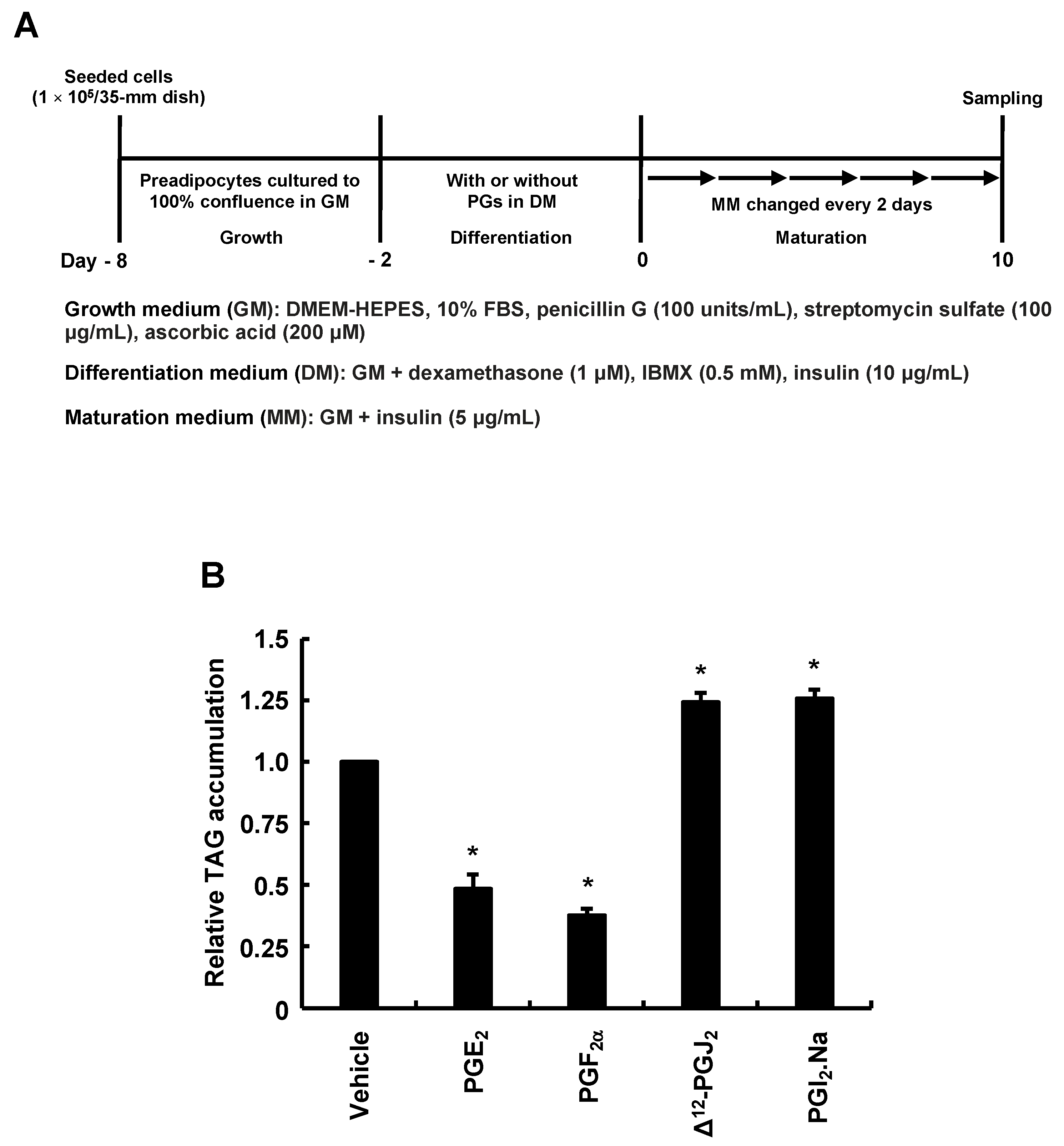
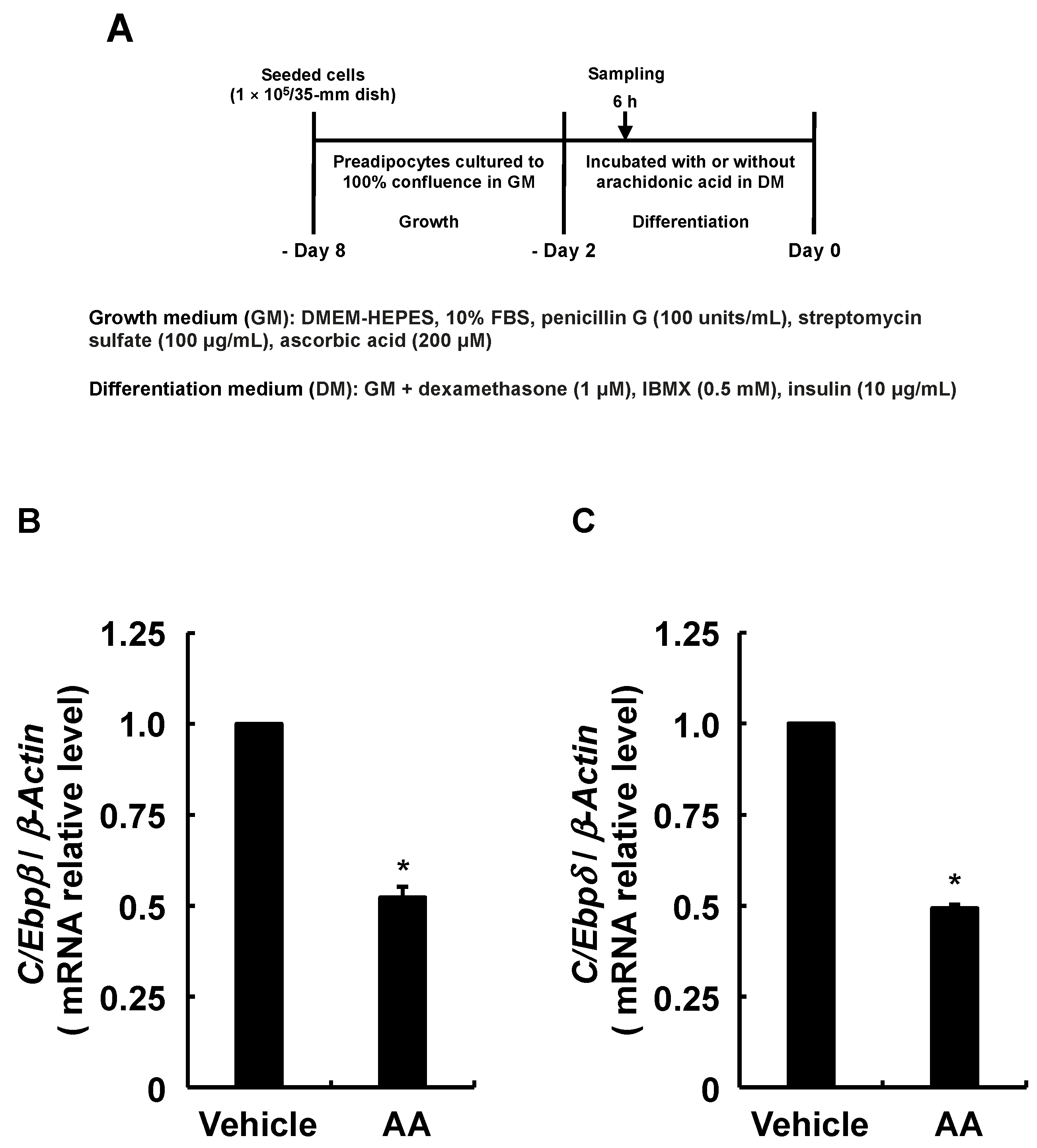
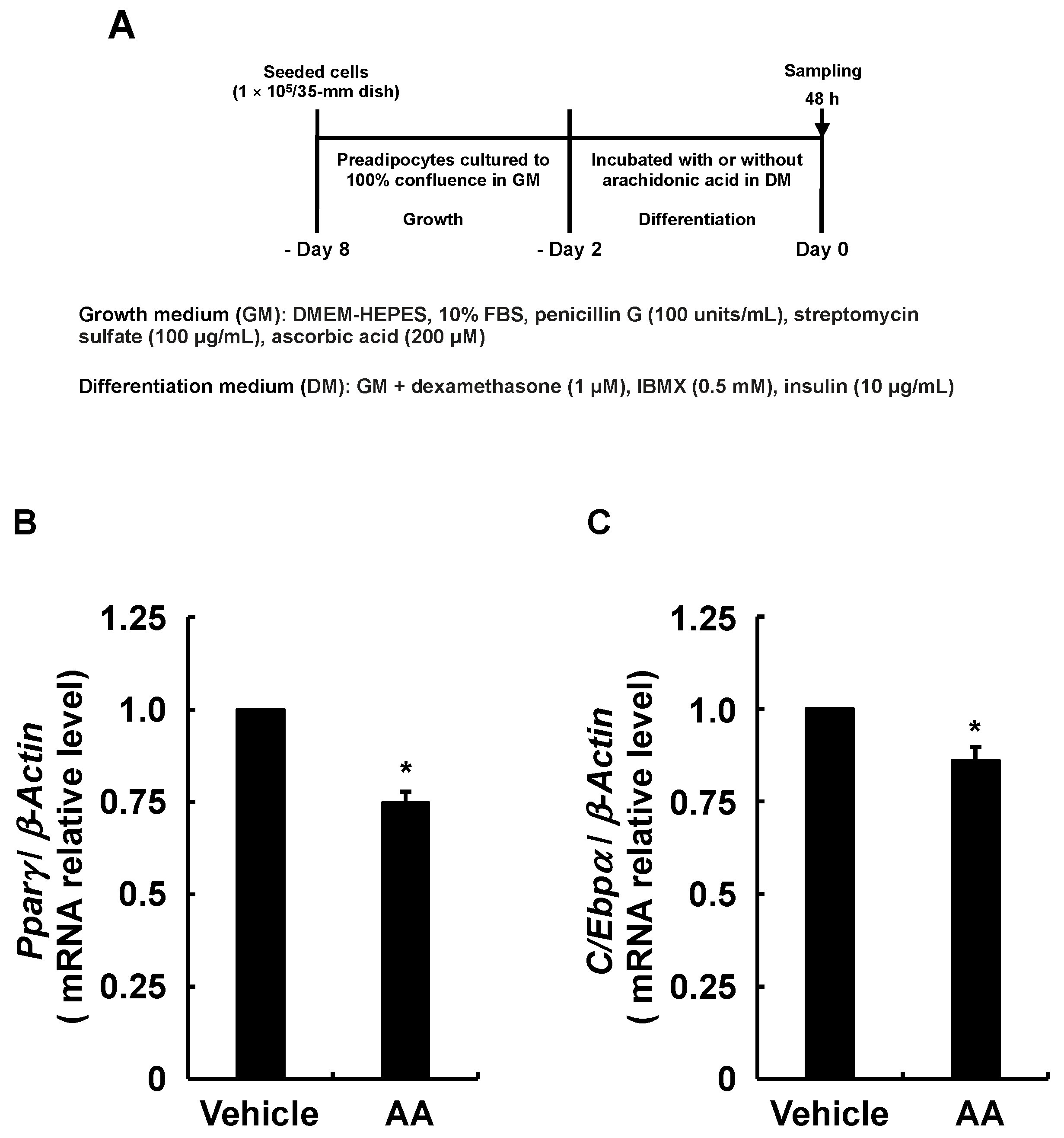


| Target Genes | Primers (5→3′) | Length (bp) | Tm (°C) | Product Length (bp) |
|---|---|---|---|---|
| C/Ebpβ | Fw: CGGGTTTCGGGACTTGAT | 18 | 56.97 | 93 |
| Rv: GCCCGGCTGACAGTTACAC | 19 | 61.03 | ||
| C/Ebpδ | Fw: GACTCCTGCCATGTACGACG | 20 | 60.53 | 118 |
| Rv: GTTGAAGAGGTCGGCGAAGA | 20 | 60.04 | ||
| Pparγ | Fw: CTTCGCTGATGCACTGCCTAT | 21 | 60.81 | 216 |
| Rv: GGGTCAGCTCTTGTGAATGGA | 21 | 60.00 | ||
| C/Ebpα | Fw: GCCAAGAAGTCGGTGGACA | 19 | 59.93 | 110 |
| Rv: GTCTCCACGTTGCGTTGTTT | 20 | 59.62 | ||
| Lpl | Fw: TTGCAGAGAGAGGACTCGGA | 20 | 59.96 | 125 |
| Rv: GGAGTTGCACCTGTATGCCT | 20 | 60.04 | ||
| Glut4 | Fw: GGATTCCATCCCACAAGGCA | 20 | 60.03 | 158 |
| Rv: CCAACACGGCCAAGACATTG | 20 | 60.04 | ||
| Leptin | Fw: TTTCACACACGCAGTCGGTA | 20 | 59.90 | 149 |
| Rv: CACATTTTGGGAAGGCAGGC | 20 | 60.04 | ||
| β-Actin | Fw: GCGGGCGACGATGCT | 15 | 59.84 | 197 |
| Rv: TGCCAGATCTTCTCCATGTCG | 21 | 59.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nartey, M.N.N.; Jisaka, M.; Syeda, P.K.; Nishimura, K.; Shimizu, H.; Yokota, K. Arachidonic Acid Added during the Differentiation Phase of 3T3-L1 Cells Exerts Anti-Adipogenic Effect by Reducing the Effects of Pro-Adipogenic Prostaglandins. Life 2023, 13, 367. https://doi.org/10.3390/life13020367
Nartey MNN, Jisaka M, Syeda PK, Nishimura K, Shimizu H, Yokota K. Arachidonic Acid Added during the Differentiation Phase of 3T3-L1 Cells Exerts Anti-Adipogenic Effect by Reducing the Effects of Pro-Adipogenic Prostaglandins. Life. 2023; 13(2):367. https://doi.org/10.3390/life13020367
Chicago/Turabian StyleNartey, Michael N. N., Mitsuo Jisaka, Pinky Karim Syeda, Kohji Nishimura, Hidehisa Shimizu, and Kazushige Yokota. 2023. "Arachidonic Acid Added during the Differentiation Phase of 3T3-L1 Cells Exerts Anti-Adipogenic Effect by Reducing the Effects of Pro-Adipogenic Prostaglandins" Life 13, no. 2: 367. https://doi.org/10.3390/life13020367
APA StyleNartey, M. N. N., Jisaka, M., Syeda, P. K., Nishimura, K., Shimizu, H., & Yokota, K. (2023). Arachidonic Acid Added during the Differentiation Phase of 3T3-L1 Cells Exerts Anti-Adipogenic Effect by Reducing the Effects of Pro-Adipogenic Prostaglandins. Life, 13(2), 367. https://doi.org/10.3390/life13020367







