Micro-Current Stimulation Can Modulate the Adipogenesis Process by Regulating the Insulin Signaling Pathway in 3T3-L1 Cells and ob/ob Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Micro-Current Stimulation
2.3. 3T3-L1 Cell Culture and Differentiation
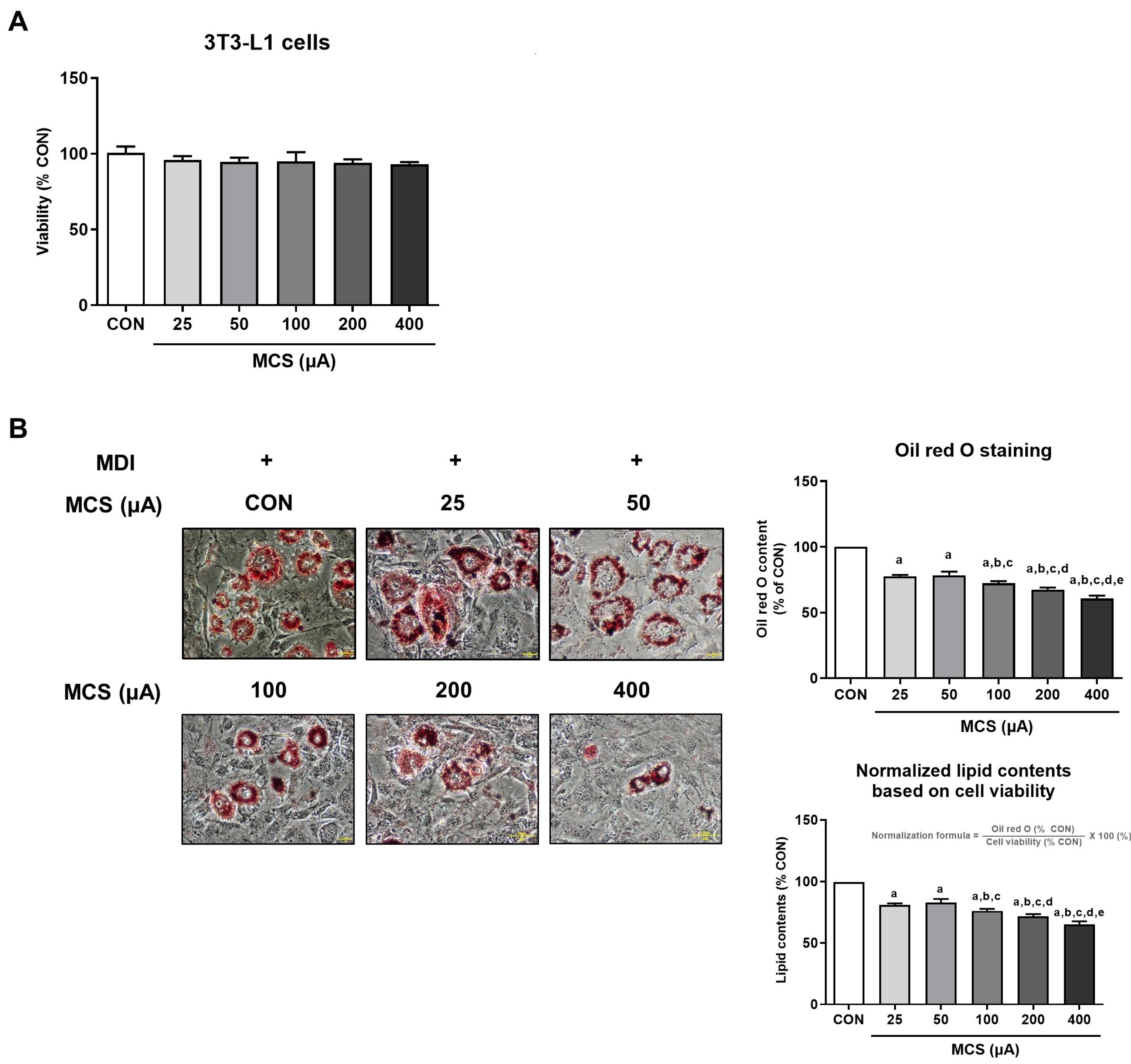
2.4. Cell Viability
2.5. Oil Red O Staining
2.6. Confocal Microscopy
2.7. Immunoblotting
2.8. Animals
2.9. In Vivo Micro-CT
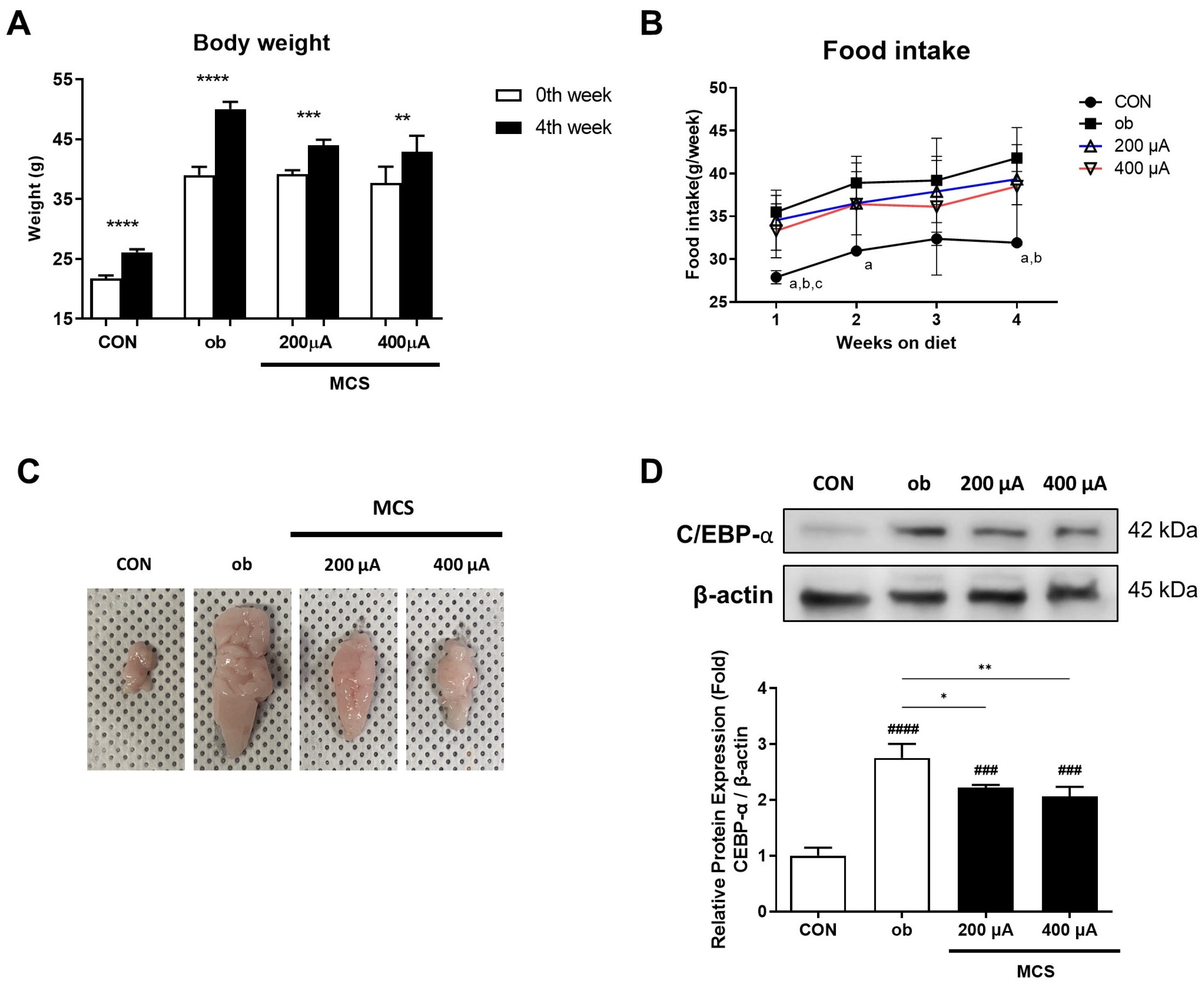
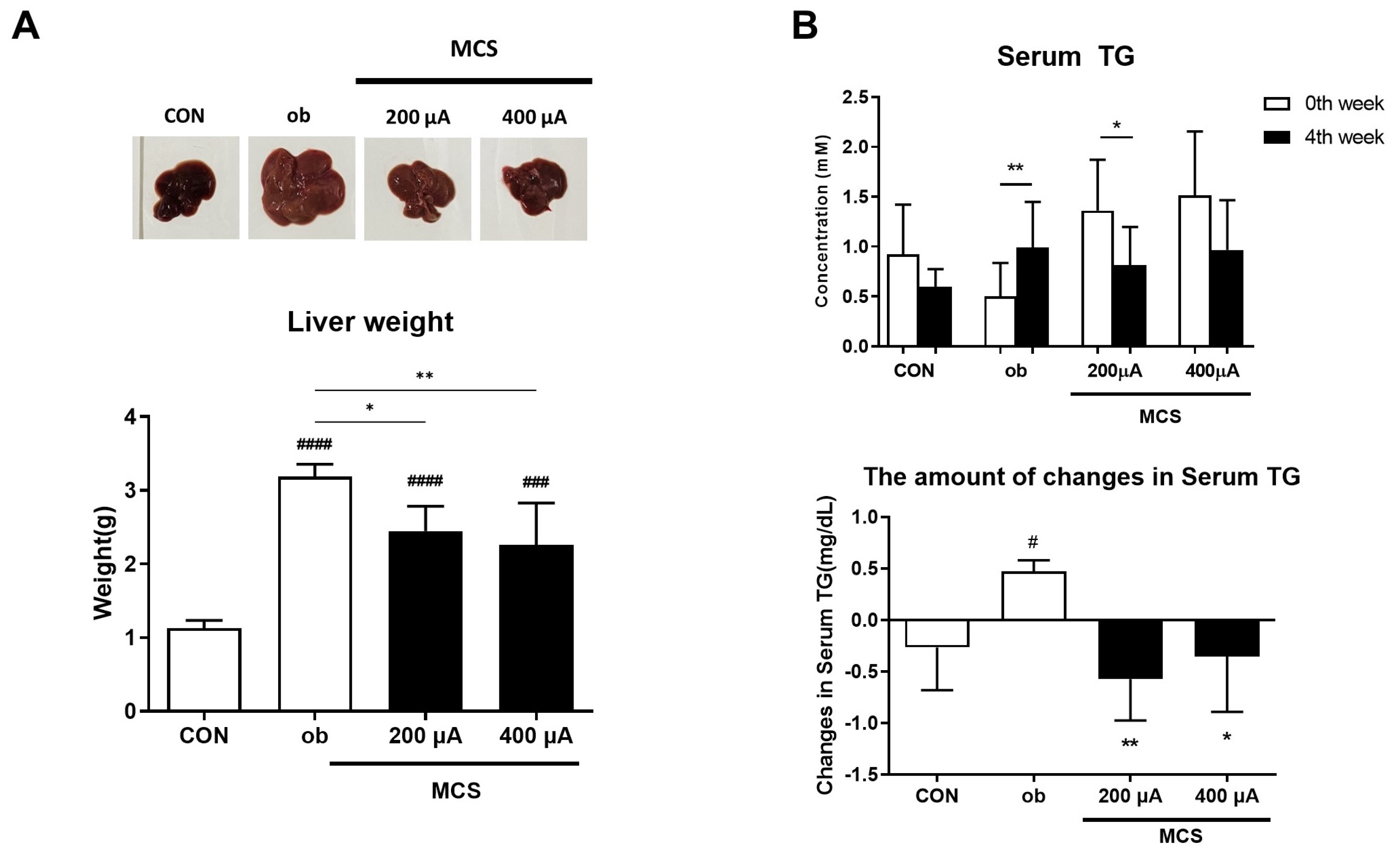
2.10. Body Weight and Liver Weight
2.11. Triglycerides in Serum
2.12. Statistical Analysis
3. Results
3.1. Analysis of Cell Viability and Intracellular Lipid Droplet Formation According to MCS Application and Its Intensity
3.2. Effect of MCS on Insulin Signaling Pathway
3.3. The Protein Expression of C/EBP-α and Nucleus Translocation of PPAR-γ in 3T3-L1 Adipocytes According to the Application of MCS
3.4. Changes in Body Weight and Food Intake and the Observation of the Appearance and the Protein Expression of C/EBP-α for the Epididymal Fat Pad
3.5. Observation of Abdominal Adipose Tissue Using Micro-CT Analysis
3.6. Observation of Liver and Changes in Serum Triglycerides (TG) Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, S.; Akram, Z.; Noreen, A.; Baig, M.T.; Sheikh, S.; Huma, A.; Jabeen, A.; Lodhi, M.; Khan, S.A.; Hudda, A. Overweight and obesity prevalence and predictors in people living in Karachi. J. Pharm. Res. Int. 2021, 33, 194–202. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight. Fact Sheet N 311. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/2012 (accessed on 31 December 2012).
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Lee, H.S.; Han, H.-K.; Choi, C.-I. Saikosaponin A and D inhibit adipogenesis via the AMPK and MAPK signaling pathways in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2021, 22, 11409. [Google Scholar] [CrossRef]
- Kong, L.; Xu, M.; Qiu, Y.; Liao, M.; Zhang, Q.; Yang, L.; Zheng, G. Chlorogenic acid and caffeine combination attenuates adipogenesis by regulating fat metabolism and inhibiting adipocyte differentiation in 3T3-L1 cells. J. Food Biochem. 2021, 45, e13795. [Google Scholar] [CrossRef]
- Haider, N.; Larose, L. Harnessing adipogenesis to prevent obesity. Adipocyte 2019, 8, 98–104. [Google Scholar] [CrossRef]
- Arner, E.; Westermark, P.O.; Spalding, K.L.; Britton, T.; Rydén, M.; Frisén, J.; Bernard, S.; Arner, P. Adipocyte turnover: Relevance to human adipose tissue morphology. Diabetes 2010, 59, 105–109. [Google Scholar] [CrossRef]
- MacDougald, O.A.; Lane, M.D. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 1995, 64, 345–373. [Google Scholar] [CrossRef]
- Lane, M.D.; Tang, Q.Q. From multipotent stem cell to adipocyte. Birth Defects Res. Part A Clin. Mol. Teratol. 2005, 73, 476–477. [Google Scholar] [CrossRef]
- Tang, Q.-Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Boulton, T.G.; Nye, S.H.; Robbins, D.J.; Ip, N.Y.; Radzlejewska, E.; Morgenbesser, S.D.; DePinho, R.A.; Panayotatos, N.; Cobb, M.H.; Yancopoulos, G.D. ERKs: A family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 1991, 65, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Miki, H.; Yamauchi, T.; Suzuki, R.; Komeda, K.; Tsuchida, A.; Kubota, N.; Terauchi, Y.; Kamon, J.; Kaburagi, Y.; Matsui, J. Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation. Mol. Cell. Biol. 2001, 21, 2521–2532. [Google Scholar] [CrossRef]
- Blüher, M.; Michael, M.D.; Peroni, O.D.; Ueki, K.; Carter, N.; Kahn, B.B.; Kahn, C.R. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell 2002, 3, 25–38. [Google Scholar] [CrossRef]
- Hwang, D.; Lee, H.; Lee, M.; Cho, S.; Kim, H.S. The Micro-Current Stimulation Inhibits Adipogenesis by Activating Wnt/β-Catenin Signaling. J. Biomed. Eng. Res. 2020, 41, 235–246. [Google Scholar]
- Pullar, C.E.; Isseroff, R.R. Cyclic AMP mediates keratinocyte directional migration in an electric field. J. Cell Sci. 2005, 118, 2023–2034. [Google Scholar] [CrossRef]
- Meng, X.; Arocena, M.; Penninger, J.; Gage, F.H.; Zhao, M.; Song, B. PI3K mediated electrotaxis of embryonic and adult neural progenitor cells in the presence of growth factors. Exp. Neurol. 2011, 227, 210–217. [Google Scholar] [CrossRef]
- Wang, E.; Zhao, M.; Forrester, J.V.; McCaig, C.D. Electric fields and MAP kinase signaling can regulate early wound healing in lens epithelium. Investig. Ophthalmol. Vis. Sci. 2003, 44, 244–249. [Google Scholar] [CrossRef]
- Sheikh, A.Q.; Taghian, T.; Hemingway, B.; Cho, H.; Kogan, A.B.; Narmoneva, D.A. Regulation of endothelial MAPK/ERK signalling and capillary morphogenesis by low-amplitude electric field. J. R. Soc. Interface 2013, 10, 20120548. [Google Scholar]
- Hernández-Bule, M.L.; Paíno, C.L.; Trillo, M.Á.; Úbeda, A. Electric stimulation at 448 kHz promotes proliferation of human mesenchymal stem cells. Cell. Physiol. Biochem. 2014, 34, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Rackauskas, G.; Saygili, E.; Rana, O.R.; Saygili, E.; Gemein, C.; Laucevicius, A.; Aidietis, A.; Marinskis, G.; Serpytis, P.; Plisiene, J. Subthreshold high-frequency electrical field stimulation induces VEGF expression in cardiomyocytes. Cell Transplant. 2015, 24, 1653–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; DeYoung, S.M.; Zhang, M.; Zhang, M.; Cheng, A.; Saltiel, A.R. Changes in integrin expression during adipocyte differentiation. Cell Metab. 2005, 2, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhang, Y.; Zhang, T.; Steckler, R.A.; Yang, X. Hop2 interacts with the transcription factor CEBPα and suppresses adipocyte differentiation. J. Biol. Chem. 2021, 297, 101264. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A complex interplay of multiple molecular determinants and pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-Y.; Chen, C.-W.; Chen, L.-K.; Chou, H.-Y.; Chang, C.-L.; Juan, C.-C. Curcumin attenuates adipogenesis by inducing preadipocyte apoptosis and inhibiting adipocyte differentiation. Nutrients 2019, 11, 2307. [Google Scholar] [CrossRef]
- Möckel, F.; Hoffmann, G.; Obermüller, R.; Drobnik, W.; Schmitz, G. Influence of water-filtered infrared-A (wIRA) on reduction of local fat and body weight by physical exercise. GMS Ger. Med. Sci. 2006, 4, Doc05. [Google Scholar]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef]
- Fleury, N.; Geldenhuys, S.; Gorman, S. Sun exposure and its effects on human health: Mechanisms through which sun exposure could reduce the risk of developing obesity and cardiometabolic dysfunction. Int. J. Environ. Res. Public Health 2016, 13, 999. [Google Scholar] [CrossRef]
- Hu, H.H.; Branca, R.T.; Hernando, D.; Karampinos, D.C.; Machann, J.; McKenzie, C.A.; Wu, H.H.; Yokoo, T.; Velan, S.S. Magnetic resonance imaging of obesity and metabolic disorders: Summary from the 2019 ISMRM Workshop. Magn. Reson. Med. 2020, 83, 1565–1576. [Google Scholar] [CrossRef]
- Marucci, A.; Antonucci, A.; De Bonis, C.; Mangiacotti, D.; Scarale, M.G.; Trischitta, V.; Di Paola, R. GALNT2 as a novel modulator of adipogenesis and adipocyte insulin signaling. Int. J. Obes. 2019, 43, 2448–2457. [Google Scholar] [CrossRef]
- Henriksbo, B.D.; Tamrakar, A.K.; Xu, J.; Duggan, B.M.; Cavallari, J.F.; Phulka, J.; Stampfli, M.R.; Ashkar, A.A.; Schertzer, J.D. Statins Promote Interleukin-1β–Dependent Adipocyte Insulin Resistance Through Lower Prenylation, Not Cholesterol. Diabetes 2019, 68, 1441–1448. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Cho, S.Y.; Yun, C.H.; Moon, Y.S.; Lee, T.R.; Kim, S.H. Transcriptional activation of Cidec by PPARγ2 in adipocyte. Biochem. Biophys. Res. Commun. 2008, 377, 297–302. [Google Scholar] [CrossRef]
- Brasaemle, D.L. Thematic review series: Adipocyte biology. The perilipin family of structural lipid droplet proteins: Stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 2007, 48, 2547–2559. [Google Scholar] [CrossRef]
- Wu, R.; Liu, Y.; Yao, Y.; Zhao, Y.; Bi, Z.; Jiang, Q.; Liu, Q.; Cai, M.; Wang, F.; Wang, Y. FTO regulates adipogenesis by controlling cell cycle progression via m6A-YTHDF2 dependent mechanism. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2018, 1863, 1323–1330. [Google Scholar] [CrossRef]
- Han, J.H.; Jang, K.W.; Park, M.H.; Myung, C.S. Garcinia cambogia suppresses adipogenesis in 3T3-L1 cells by inhibiting p90RSK and Stat3 activation during mitotic clonal expansion. J. Cell. Physiol. 2021, 236, 1822–1839. [Google Scholar] [CrossRef]
- Yu, H.-S.; Kim, W.-J.; Bae, W.-Y.; Lee, N.-K.; Paik, H.-D. Inula britannica inhibits adipogenesis of 3T3-L1 preadipocytes via modulation of mitotic clonal expansion involving ERK 1/2 and Akt signaling pathways. Nutrients 2020, 12, 3037. [Google Scholar] [CrossRef]
- Chang, E.; Kim, C.Y. Natural products and obesity: A focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef]
- Hwang, D.; Lee, H.; Lee, J.; Lee, M.; Cho, S.; Kim, T.; Kim, H. Micro-current stimulation has potential effects of hair growth-promotion on human hair follicle-derived papilla cells and animal model. Int. J. Mol. Sci. 2021, 22, 4361. [Google Scholar] [CrossRef]
- Zabielski, P.; Hady, H.R.; Chacinska, M.; Roszczyc, K.; Gorski, J.; Blachnio-Zabielska, A.U. The effect of high fat diet and metformin treatment on liver lipids accumulation and their impact on insulin action. Sci. Rep. 2018, 8, 7249. [Google Scholar] [CrossRef]
- Yokoyama, I.; Setoyama, O.; Urakawa, A.; Sugawara, M.; Jia, Y.; Komiya, Y.; Nagasao, J.; Arihara, K. Lysine-glucose Maillard reaction products promote longevity and stress tolerance in Caenorhabditis elegans via the insulin/IGF-1 signaling pathway. J. Funct. Foods 2021, 87, 104750. [Google Scholar] [CrossRef]
- Feng, J.; Wang, S.; Zuo, H.; Liu, X.; Li, G.; Yu, N. Relationship Research of PPAR-r Gene Polymorphisms with Adiponectin and Leptin in Type 2 Diabetes Mellitus. J. Clin. Nurs. Res. 2020, 4, 118–122. [Google Scholar] [CrossRef]
- Nagpal, R.; Mishra, S.P.; Yadav, H. Unique gut microbiome signatures depict diet-versus genetically induced obesity in mice. Int. J. Mol. Sci. 2020, 21, 3434. [Google Scholar] [CrossRef]
- Gutiérrez-Salmerón, M.; Lucena, S.R.; Chocarro-Calvo, A.; García-Martínez, J.M.; Orozco, R.M.M.; García-Jiménez, C. Metabolic and hormonal remodeling of colorectal cancer cell signalling by diabetes. Endocr. -Relat. Cancer 2021, 28, R191–R206. [Google Scholar] [CrossRef]
- Gebreyes, Y.F.; Goshu, D.Y.; Geletew, T.K.; Argefa, T.G.; Zemedu, T.G.; Lemu, K.A.; Waka, F.C.; Mengesha, A.B.; Degefu, F.S.; Deghebo, A.D. Prevalence of high bloodpressure, hyperglycemia, dyslipidemia, metabolic syndrome and their determinants in Ethiopia: Evidences from the National NCDs STEPS Survey, 2015. PLoS ONE 2018, 13, e0194819. [Google Scholar] [CrossRef]
- Selen, E.S.; Choi, J.; Wolfgang, M.J. Discordant hepatic fatty acid oxidation and triglyceride hydrolysis leads to liver disease. JCI Insight 2021, 6, e135626. [Google Scholar] [CrossRef]
- Singh, R.; Liu, H.; Shanklin, J.; Singh, V. Hydrothermal pretreatment for valorization of genetically engineered bioenergy crop for lipid and cellulosic sugar recovery. Bioresour. Technol. 2021, 341, 125817. [Google Scholar] [CrossRef]
- Rivera-Iñiguez, I.; Panduro, A.; Roman, S.; González-Aldaco, K. What do we know about nutrient-based strategies targeting molecular mechanisms associated with obesity-related fatty liver disease? Ann. Hepatol. 2023, 28, 100874. [Google Scholar] [CrossRef]
- Xue, H.; Zhao, H.; Wang, H.; Zhang, S. Indomethacin inhibits PGE2, regulates inflammatory response, participates in adipogenesis regulation, and improves success rate of fat transplantation in C57/B6 mice. Trop. J. Pharm. Res. 2019, 18, 2313–2318. [Google Scholar]
- Zhang, X. NAFLD related-HCC: The relationship with metabolic disorders. Obes. Fat. Liver Liver Cancer 2018, 1061, 55–62. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Lee, J.-H.; Kim, D.; Hwang, D.; Lee, M.; Chung, H.; Kim, T.-J.; Kim, H.S. Micro-Current Stimulation Can Modulate the Adipogenesis Process by Regulating the Insulin Signaling Pathway in 3T3-L1 Cells and ob/ob Mice. Life 2023, 13, 404. https://doi.org/10.3390/life13020404
Lee H, Lee J-H, Kim D, Hwang D, Lee M, Chung H, Kim T-J, Kim HS. Micro-Current Stimulation Can Modulate the Adipogenesis Process by Regulating the Insulin Signaling Pathway in 3T3-L1 Cells and ob/ob Mice. Life. 2023; 13(2):404. https://doi.org/10.3390/life13020404
Chicago/Turabian StyleLee, Hana, Jin-Ho Lee, Doyong Kim, Donghyun Hwang, Minjoo Lee, Halim Chung, Tack-Joong Kim, and Han Sung Kim. 2023. "Micro-Current Stimulation Can Modulate the Adipogenesis Process by Regulating the Insulin Signaling Pathway in 3T3-L1 Cells and ob/ob Mice" Life 13, no. 2: 404. https://doi.org/10.3390/life13020404
APA StyleLee, H., Lee, J.-H., Kim, D., Hwang, D., Lee, M., Chung, H., Kim, T.-J., & Kim, H. S. (2023). Micro-Current Stimulation Can Modulate the Adipogenesis Process by Regulating the Insulin Signaling Pathway in 3T3-L1 Cells and ob/ob Mice. Life, 13(2), 404. https://doi.org/10.3390/life13020404






