Examination of Different Sporidium Numbers of Ustilago maydis Infection on Two Hungarian Sweet Corn Hybrids’ Characteristics at Vegetative and Generative Stages
Abstract
:1. Introduction
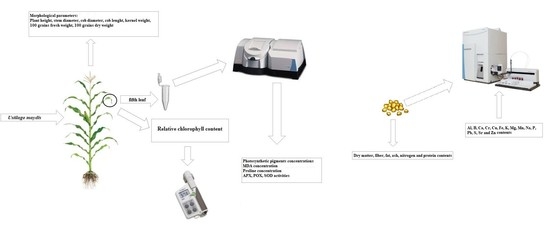
2. Materials and Methods
2.1. Experimental Conditions
2.2. Amount of Photosynthetic Pigments
2.3. MDA Content Determination
2.4. Activities of Antioxidant Enzymes
2.5. Determination of Proline
2.6. Measurement of Morphological Parameters
2.7. Measurement of the Quality Parameters of Grains
- S = amount of 0.1 M H2SO4 (mL) in the volumetric flask.
- L = amount of 0.2 M NaOH (mL) consumed for the back titration of sulphuric acid.
- B = Sample weight (g).
- 0.0028016 = nitrogen content which is corresponding to 1 mL of 0.1 M sulphuric acid quantity (g).
- 6.25 = conversion factor.
- m0 = the plant sample weight (g).
- m1 = the plant sample and ashing crucible weight (g).
- m2 = the ashing crucible weight (g)
- a: the fiber which contains ash weight (g), A–B.
- b: the ash weight (g), C–D.
- m: the sample weight (g).
- A: Dried porcelain dish weight (g) with plant samples.
- B: Dried porcelain dish weight (g) without plant samples.
- m0: the sample weight (g).
- m1: the flask, pumice stone, and dried sample weight (g).
- m2: the flask and pumice stone weight (g).
2.8. Measurement of Mineral Elements Concentration
2.9. Method of Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spiertz, J.H.J.; Ewert, F. Crop production and resource use to meet the growing demand for food, feed and fuel: Opportunities and constraints. NJAS—Wagening J. Life Sci. 2009, 56, 281–300. [Google Scholar] [CrossRef]
- Reinprecht, Y.; Schram, L.; Smith, T.H.; Pauls, K.P. Enhancing In-crop Diversity in Common Bean by Planting Cultivar Mixtures and Its Effect on Productivity. Front. Sustain. Food Syst. 2020, 4, 126. [Google Scholar] [CrossRef]
- Vesco, P.; Kovacic, M.; Mistry, M.; Croicu, M. Climate variability, crop and conflict: Exploring the impacts of spatial concentration in agricultural production. J. Peace Res. 2021, 58, 98–113. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Ali, G.; Gillespie, A.; Wagner-Riddle, C. Soil Organic Matter as Catalyst of Crop Resource Capture. Front. Environ. Sci. 2020, 8, 50. [Google Scholar] [CrossRef]
- D’Odorico, P.; Chiarelli, D.D.; Rosa, L.; Bini, A.; Zilberman, D.; Rulli, M.C. The global value of water in agriculture. Proc. Natl. Acad. Sci. 2020, 117, 21985–21993. [Google Scholar] [CrossRef]
- Anami, B.S.; Malvade, N.N.; Palaiah, S. Classification of yield affecting biotic and abiotic paddy crop stresses using field images. Inf. Process. Agric. 2019, 7, 272–285. [Google Scholar] [CrossRef]
- Ashkavand, P.; Zarafshar, M.; Tabari, M.; Mirzaie, J.; Nikpour, A.; Bordbar, S.K.; Struve, D.K.; Striker, G.G. Application of SiO2 nanopartic lesa spre treatment al leviates the impact of drought on the physiological performance of Prunusmahaleb (Rosaceae). Boletín De La Soc. Argent. De Botánica 2018, 53, 207. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and Crop Yield. Plant Biol. 2018, 21, 31–38. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Tan, D.K.Y. Impact of High Temperature and Drought Stresses on Chickpea Production. Agronomy 2018, 8, 145. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Das, S.; Bansal, S.; Singh, G.; Sardar, S.; Dhar, H.; Ram, H. Heavy metal stress in rice: Uptake, transport, signaling, and tolerance mechanisms. Physiol. Plant. 2021, 173, 430–448. [Google Scholar] [CrossRef]
- Kaur, H.; Kohli, S.K.; Khanna, K.; Bhardwaj, R. Scrutinizing the impact of water deficit in plants: Transcriptional regulation, signaling, photosynthetic efficacy, and management. Physiol. Plant. 2021, 172, 935–962. [Google Scholar] [CrossRef]
- Iqbal, Z.; Iqbal, M.S.; Hashem, A.; Abd-Allah, E.F.; Ansari, M.I. Plant Defense Responses to Biotic Stress and Its Interplay With Fluctuating Dark/Light Conditions. Front. Plant Sci 2021, 12, 631810. [Google Scholar] [CrossRef]
- Juroszek, P.; Von Tiedemann, A. Plant pathogens, insect pests and weeds in a changing global climate: A review of approaches, challenges, research gaps, key studies and concepts. J. Agric. Sci. 2013, 151, 163–188. [Google Scholar] [CrossRef]
- Hassan, M.A.; El Nemr, A. Pesticidespollution: Classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Walley, J.W.; Shen, Z.; McReynolds, M.R.; Schmelz, E.A.; Briggs, S.P. Fungal-induced protein hyper acetylation in maize identified by acetylome profiling. Proc. Natl. Acad. Sci. USA 2018, 115, 201717519. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plantcuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Delgado, P.; Fernández, V.; Graca, J.; Cabral, V.; Gil, L. The presence of cutan limits the interpretation of cuticular chemistry and structure: Ficus elastica leaf as an example. Physiol. Plant. 2016, 157, 205–220. [Google Scholar] [CrossRef]
- Chassot, C.; Nawrath, C.; Métraux, J.P. The cuticle: Not only a barrier for defence. Plant Signal. Behav. 2008, 3, 142–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef]
- Rosenthal, G.A.; Berenbaum, M.R. Herbivores: Their Interaction with Secondary Plant Metabolites, Vol II Ecological and Evolutionary Processes, 2nd ed.; Academic Press: San Diego, CA, USA, 1992. [Google Scholar]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Yourman, L.F.; Jeffers, S.M. Resistance to benzimidazole and dicarboximide fungicides in 406 greenhouse isolates of Botrytis cinerea. Plant. Dis. 1999, 83, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.C.; Stahman, M.A. Chemical nature of disease resistance in plants. Annu. Rev. Plant Physiol. 1995, 6, 351–366. [Google Scholar] [CrossRef]
- Lattanzio, V.; Cicco, V.D.; Venere, D.D.; Lima, G.; Salerno, M.I. Antifungal activity of phenolics against fungi commonly encountered during storage. Ital. J. Food Sci. 1994, 6, 23–30. [Google Scholar]
- Harborne, J.B. Recent advances in chemical ecology. Nat. Prod. Rep. 1989, 6, 85–109. [Google Scholar] [CrossRef]
- Ito, S.; Ihara, T.; Tamura, H.; Tanaka, S.; Ikeda, T.; Kajihara, H.; Dissanayake, C.; Abdel-Motaal, F.F.; El-Sayed, M.A. alpha-Tomatine, the major saponin in tomato, induces programmed cell death mediated by reactive oxygen species in the fungal pathogen Fusarium oxysporum. FEBS Lett. 2007, 581, 3217–3222. [Google Scholar] [CrossRef] [PubMed]
- Arcas, M.C.; Botía, J.M.; Ortuño, A.M.; Del Río, J.A. UV irradiation alters the levels of flavonoids involved in the defence mechanism of Citrus aurantium fruits against Penicillium digitatum. Eur. J. Plant Pathol. 2000, 106, 617–622. [Google Scholar] [CrossRef]
- Weidenbörner, M.; Jha, H.C. Optimization of the concentrations of flavone and flavanone with regard to their antifungal activities on four Deuteromycotina. J. Plant Dis. Prot. 1994, 101, 662–665. [Google Scholar]
- Del-Río, J.; Báidez, A.; Botía, J.; Ortuño, A. Enhancement of phenolic compounds in olive plants (Olea europaea L.) and their influence on resistance against Phytophthora sp. Food Chem. 2003, 83, 75–78. [Google Scholar] [CrossRef]
- Friend, J. Plant phenolics, lignification and plant disease. Suppression of Phytophthora Infection in Citrus Infected with Viroids. In Progressin Phytochemistry; Reinhold, L., Harborne, J.B., Swain, T., Eds.; Pergamon Press: Oxford, UK, 1981; pp. 197–261. Available online: https://www.researchgate.net/publication/262561984_Suppression_of_Phytophthora_Infection_in_Citrus_Infected_with_Viroids (accessed on 10 August 2022).
- Johnson, P.N. Effects of soil phosphate level and shade on plant growth and mycorrhizas. N. Z. J. Bot. 1976, 14, 333–340. [Google Scholar] [CrossRef]
- Overeem, J.C. Pre-existing antimicrobial substances in plants and their role in disease resistance. In Biochemical Aspects of Plant-Parasite Relationships; Friend, J., Threlfall, D.R., Eds.; Academic Press: London, UK, 1976; pp. 195–205. [Google Scholar]
- Camejo, D.; Guzmán-Cedeño, Á.; Moreno, A. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Dumanovic, J.; Nepovimova, E.; Natic, M.; Kuca, K.; Jacevic, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 2003, 119, 355–364. [Google Scholar] [CrossRef]
- Farmer, E.E.; Mueller, M.J. ROS-Mediated Lipid Peroxidation and RES-Activated Signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Romero-Puertas, M.C. Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks. Ann. Bot. 2015, 116, 475–485. [Google Scholar] [CrossRef]
- Huang, S.; Van-Aken, O.; Schwarzlander, M.; Belt, K.; Millar, A.H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef]
- Dietz, K.J.; Turkan, I.; Krieger-Liszkay, A. Redox- and reactive oxygen species-dependent signaling into and out of the photosynthesizing chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Ascorbate peroxidase from rice seedlings: Properties of enzyme isoforms, effects of stresses and protective roles of osmolytes. Plant Sci. 2004, 167, 541–550. [Google Scholar] [CrossRef]
- Boguszewska, D.; Grudkowska, M.; Zagdañska, B. Drought-responsive antioxidant enzymes in potato (Solanum tuberosum L.). Potato Res. 2010, 53, 373–382. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Aydogdu, M.; Boyraz, N.; Kaya, Y. Effect on yield losses on maize (Zea mays L.) caused by corn smut (Ustilago maydis (DC) Corda). J. Turk. Phytopath. 2015, 44, 23–30. [Google Scholar]
- Pataky, J.K.; Snetselaar, K.M. Common smut of corn. Plant Health Instr. 2006. [Google Scholar] [CrossRef]
- Koeck, M.; Adrienne, R.; Hardham, B.; Doddsa, P.N. The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell Microbiol. 2011, 13, 1849–1857. [Google Scholar] [CrossRef]
- Billett, E.E.; Burnett, J.H. The host-parasite physiology of the maize smut fungus, Ustilago maydis I. The effect of smut infection on maize growth. Phys. Plant Pathol. 1978, 12, 93–102. [Google Scholar] [CrossRef]
- Sierra-Campos, E.; Pardo, J.P. The relationship between the antioxidant system and the virulence in Ustilago maydis, a fungal pathogen. Microbiología 2009, 51, 7–17. [Google Scholar]
- Pathi, K.M.; Rink, P.; Budhagatapalli, N.; Betz, R.; Saado, I.; Hiekel, S.; Becker, M.; Djamei, A.; Kumlehn, J. Engineering smut resistance in maize by site-directed mutagenesis of LIPOXYGENASE 3. Front. Plant Sci. 2020, 11, 543895. [Google Scholar] [CrossRef] [PubMed]
- Szőke, L.; Moloi, M.J.; Kovács, G.E.; Biró, G.; Radócz, L.; Hájos, M.T.; Kovács, B.; Rácz, D.; Danter, M.; Tóth, B. The Application of Phytohormones as Biostimulants in Corn Smut Infected Hungarian Sweet and Fodder Corn Hybrids. Plants 2021, 10, 1822. [Google Scholar] [CrossRef]
- Clough, G.H.; Blatchford, S.; Hamm, P.B. Common smut reduces sweet corn yield and ear processing quality. HortScience 2011, 46, 1507–1511. [Google Scholar] [CrossRef]
- Pál-Fám, F.; Keszthelyi, S.; Pozsgai, J. Analysis of prolifications on hailstormed maize and the biotic stress caused by corn smut fungus. Növénytermelés 2012, 60, 149–152. [Google Scholar]
- Keszthelyi, S.; Kerepesi, I.; Pál-Fám, F.; Pozsgai, J. Study of germination ability and chemical composition of maize seeds affected by hailstorm and common smut (Ustilago maydis (DC.) Corda). Növényvédelem 2008, 44, 435–439. [Google Scholar]
- Lévai, L. The Effect of Smut Gall Tumour Infection on Iron and Zinc Uptake and Distribution in Maize Seedlings. Acta Agraria Debr. 2004, 15, 27–32. [Google Scholar] [CrossRef]
- Cao, J.; Cheng, C.; Yang, J.; Wang, Q. Pathogen infection drives patterns of nutrient resorption in citrus plants. Sci. Rep. 2015, 5, 14675. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Hwang, S.G.; Huang, Y.M.; Huang, C.H. Effects of Trichoderma asperellum on nutrient uptake and Fusarium wilt of tomato. Crop Prot. 2018, 110, 275–282. [Google Scholar] [CrossRef]
- Yu, X.; Luo, Q.; Huang, K.; Yang, G.; He, G. Prospecting for Microelement Function and Biosafety Assessment of Transgenic Cereal Plants. Front. Plant Sci. 2018, 9, 326. [Google Scholar] [CrossRef]
- Expert, D.; Enard, C.; Masclaux, C. The role of iron in plant host-pathogen interactions. Trends Microbiol. 1996, 4, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Szőke, L.; Tóth, B. Examination of the effect of corn smut infection on plant physiological parameters in a small scale field experiment. In Proceedings of the 44th Conference for Students of Agriculture and Veterinary Medicine with International Participation, Novi Sad, Serbia, 15 December 2020; pp. 37–43. [Google Scholar]
- Moran, R.; Porath, D. Chlorophyll determination in intact tissues using N, N-dimethylformamide. Plant Physiol. 1980, 65, 478–479. [Google Scholar] [CrossRef]
- Wellburn, R.A. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvent with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E. Production and scavenging of reactive oxygen species in Fagus sylvatica seeds during storage at varied temperature and humidity. J. Plant Physiol. 2005, 162, 873–885. [Google Scholar] [CrossRef]
- Mishra, N.P.; Mishra, R.K.; Singhal, G.S. Changes in the activities of antioxidant enzymes during exposure of intact wheat leaves to strong visible light at different temperatures in the presence of protein synthesis inhibitors. Plant Physiol. 1993, 102, 903–910. [Google Scholar] [CrossRef]
- Zeislin, N.; Ben-Zaken, R. Peroxidases, phenylalanine ammonia-lyase and lignification in peduncles of rose flowers. Plant Physiol. Biochem. 1991, 29, 147–151. [Google Scholar]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y. Extraction and Determination of Proline. PrometheusWiki. 2011. Available online: https://prometheuswiki.rsb.anu.edu.au/tikipagehistory.php?page=Extraction%20and%20determination%20of%20proline&preview=14 (accessed on 10 September 2022).
- Csapó, J.; Albert, C.; És Kiss, D. Analitikai kémia Élelmiszermérnököknek; Scientia Kiadó: Kolozsvár, Romania, 2020; ISBN 978-606-975-034-6. [Google Scholar]
- Tóth, B.; Bóka, K.; Rothmann, L.; Moloi, M. The Effect of Four Industrial By-Products on the Photosynthetic Pigments, Dry Weight and Ultrastructure of Zea mays L. Biol. Bull. 2021, 48, 296–305. [Google Scholar] [CrossRef]
- Hanusz, Z.; Tarasińska, J. Normalization of the Kolmogorov–Smirnov and Shapiro–Wilk tests of normality. Biometr. Lett. 2015, 52, 85–93. [Google Scholar] [CrossRef]
- Cavus, M. Comparison of one-way ANOVA tests under unequal variances in terms of median p-values. Commun. Stat. Simul. Comput. 2022, 1–14. [Google Scholar] [CrossRef]
- Midway, S.; Robertson, M.; Flinn, S.; Kaller, M. Comparing multiple comparisons: Practical guidance for choosing the best multiple cmparisons test. Peer J. 2020, 8, e10387. [Google Scholar] [CrossRef] [PubMed]
- Frommer, D.; Radócz, L.; Veres, S. Changes of relative chlorophyll content in sweet corn leaves of different ages infected by corn smut. Agric. Conspec. Sci. 2019, 84, 189–192. [Google Scholar]
- Szőke, L.; Kovács, G.E.; Radócz, L.; Takácsné Hájos, M.; Kovács, B.; Tóth, B. Impact of corn smut [Ustilago maydis (DC.) Corda] sporidium numbers on some physiological parameters of fodder corn in field experiment, NRI Book of Abstracts. In Proceedings of the Plant Protection Conference 61: Scientific Session of the Institute of Plant Protection, Poznan, Poland, 10–12 February 2021; p. 116. [Google Scholar]
- Szőke, L.; Kovács, G.E.; Radócz, L.; Hájos, M.T.; Kovács, B.; Tóth, B. Investigation of Ustilago maydis infection on some physiological parameters and phenotypic traits of maize. Int. J. Innov. Approaches Agric. Res. 2020, 4, 396–406. [Google Scholar] [CrossRef]
- Szőke, L.; Kovács, G.E.; Radócz, L.; Takácsné Hájos, M.; Kovács, B.; Tóth, B. The physiological changes of sweet corn infected by corn smut (Ustilago maydis (DC.) Corda) in greenhouse experiment, NRI Book of Abstracts. In Proceedings of the Plant Protection Conference 61: Scientific Session of the Institute of Plant Protection, Poznan, Poland, 10–12 February 2021; p. 117. [Google Scholar]
- Frommer, D.; Veres, S.; Radócz, L. Susceptibility of stem infected sweet corn hybrids to common smut disease. Acta Agrar. Debr. 2018, 74, 55–57. [Google Scholar] [CrossRef]
- Shimizu, N.; Hosogi, N.; Hyon, G.S.; Jiang, S.; Inoue, K.; Park, P. Reactive oxygen species (ROS) generation and ROS induced lipid peroxidation are associated with plant membrane modifications in host cells in response to AK-toxin from Alternaria alternata Japanese pear pathotype. J. Gen. Plant Pathol. 2006, 72, 6–15. [Google Scholar] [CrossRef]
- Meena, M.; Zehra, A.; Dubey, M.K.; Aamir, M.; Gupta, V.K.; Upadhyay, R.S. Comparative Evaluation of Biochemical Changes in Tomato (Lycopersicon esculentum Mill.) Infected by Alternaria alternata and Its Toxic Metabolites (TeA, AOH, and AME). Front. Plant Sci. 2016, 7, 1408. [Google Scholar] [CrossRef]
- Xi, D.; Li, X.; Gao, L.; Zhang, Z.; Zhu, Y.; Zhu, H. Application of exogenous salicylic acid reduces disease severity of Plasmodiophora brassicae in pakchoi (Brassica campestris ssp. chinensis Makino). PLoS ONE 2021, 16, e0248648. [Google Scholar] [CrossRef]
- Chen, Y.E.; Cui, J.M.; Su, Y.Q.; Yuan, S.; Yuan, M.; Zhang, H.Y. Influence of stripe rust infection on the photosynthetic characteristics and antioxidant system of susceptible and resistant wheat cultivars at the adult plant stage. Front. Plant Sci. 2015, 6, 779. [Google Scholar] [CrossRef]
- Radwan, D.E.M.; Ismail, K.S. The Impact of Hydrogen Peroxide Against Cucumber Green Mottle Mosaic Virus Infection in Watermelon Plants. Pol. J. Environ. Stud. 2020, 29, 3771–3782. [Google Scholar] [CrossRef]
- Kovács, G.E.; Szőke, L.; Tóth, B.; Kovács, B.; Bojtor, C.; Illés, Á.; Radócz, L., Jr.; Moloi, M.J.; Radócz, L. The Physiological and Biochemical Responses of European Chestnut (Castanea sativa L.) to Blight Fungus (Cryphonectria parasitica (Murill) Barr). Plants 2021, 10, 2136. [Google Scholar] [CrossRef] [PubMed]
- Vanacker, H.; Carver, T.L.; Foyer, C.H. Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 1998, 117, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Dey, N.; Roy, U.K.; Aditya, M.; Bhattacharjee, S. Defensive strategies of ROS in Programmed Cell Death associated with hypertensive response in plant pathogenesis. Ann. Syst. Biol. 2020, 3, 001–009. [Google Scholar] [CrossRef]
- Fortunato, A.A.; Debona, D.; Bernardeli, A.M.A.; Rodrigues, F.Á. Changes in the Antioxidant System in Soybean Leaves Infected by Corynespora cassiicola. Phytopathology 2015, 105, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Fulton, H.R. Recent Notes on the Chestnut Bark Disease. In Pennsylvania Chestnut Blight Conference Report; Penn State University Libaries: Harrisburg, PA, USA, 1912; pp. 48–56. Available online: https://digital.libraries.psu.edu/digital/collection/digitalbks2/id/13444 (accessed on 22 July 2022).
- Allen, D.J.; McKee, I.F.; Farage, P.K.; Baker, N.R. Analysis of limitations to CO2 assimilation on exposure of leaves of two Brassica napus cultivars to UV-B. Plant Cell Environ. 1997, 20, 633–640. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; van Staden, J. A regulatory role for proline metabolism in stimulating Arabidopsis thaliana seed germination. Plant Growth Regul. 2003, 39, 41–50. [Google Scholar] [CrossRef]
- Talanova, V.V.; Titov, A.F.; Boeva, N.P. Effect of increasing concentrations of lead and cadmium on cucumber seedlings. Biol. Plant. 2000, 43, 441–444. [Google Scholar] [CrossRef]
- Pazarlar, S.; Gümüs, M.; Öztekin, G.B. The Effects of Tobacco mosaic virus Infection on Growth and Physiological Parameters in Some Pepper Varieties (Capsicum annuum L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 427. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Kerepesi, I.; Pál-Fám, F.; Pozsgai, J. Germination, enzyme activity and nutrient contents of hail stormed corn in the case of corn smut fungus [Ustilago maydis (DC.) Corda]. Cereal Res. Commun. 2008, 36, 196–198. [Google Scholar]
- Shattuck, V.I.; Brolley, B.; Stobbs, L.W.; Lougheed, E.C. The effect of turnip mosaic virus infection on the mineral content and storability of field-grown rutabaga. Commun. Soil Sci. Plant Anal. 1989, 20, 581–595. [Google Scholar] [CrossRef]
- Cesco, S.; Tolotti, A.; Nadalini, S.; Rizzi, S.; Valentinuzzi, F.; Mimmo, T.; Porfido, C.; Allegretta, I.; Giovannini, O.; Perazzolli, M.; et al. Plasmopara viticola infection affects mineral elements allocation and distribution in Vitis vinifera leaves. Sci. Rep. 2020, 10, 18759. [Google Scholar] [CrossRef]
- Dordas, C. Role of Nutrients in Controlling Plant Diseases in Sustainable Agriculture: A Review. Agron. Sustain. Dev. 2009, 28, 33–46. [Google Scholar] [CrossRef] [Green Version]
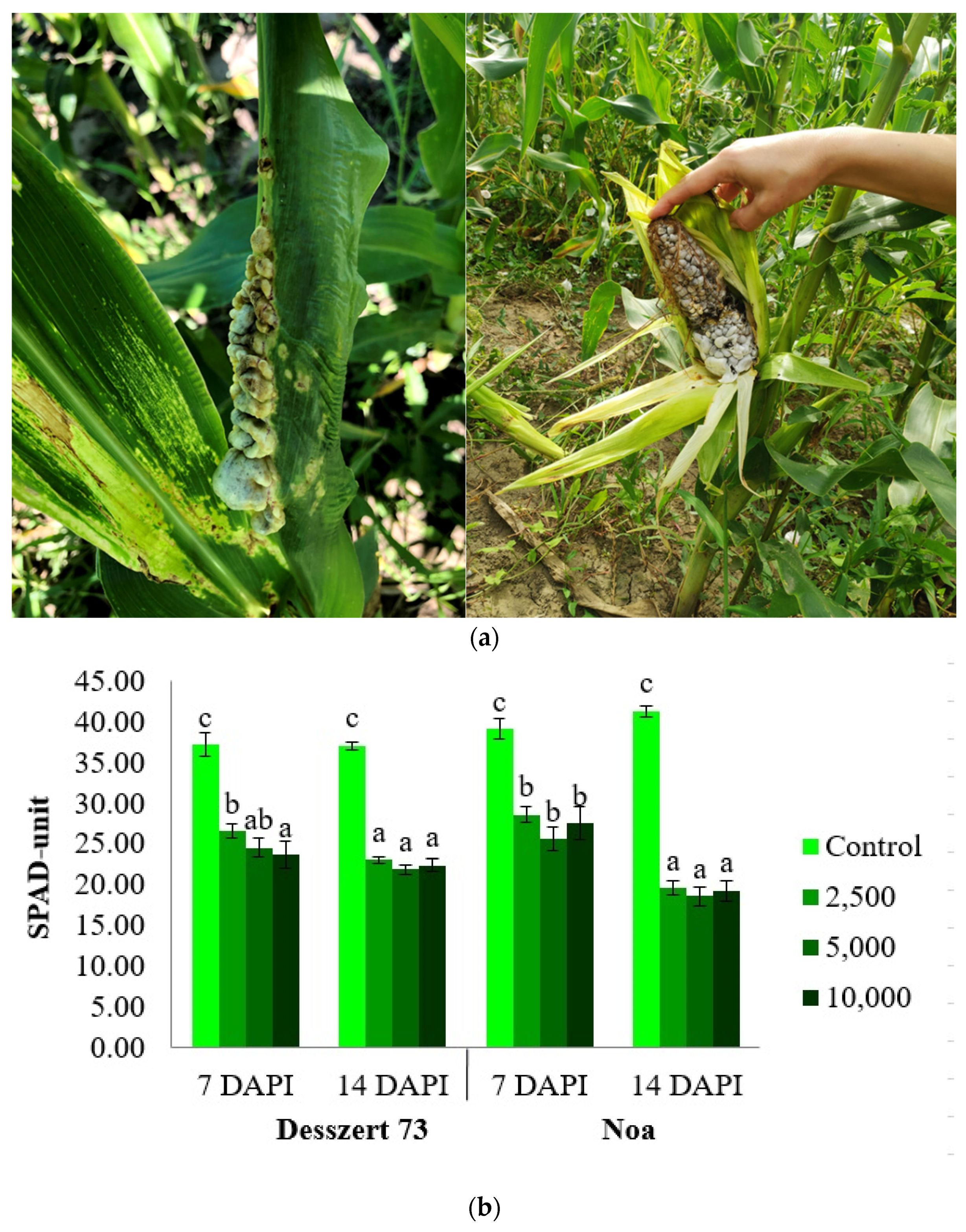



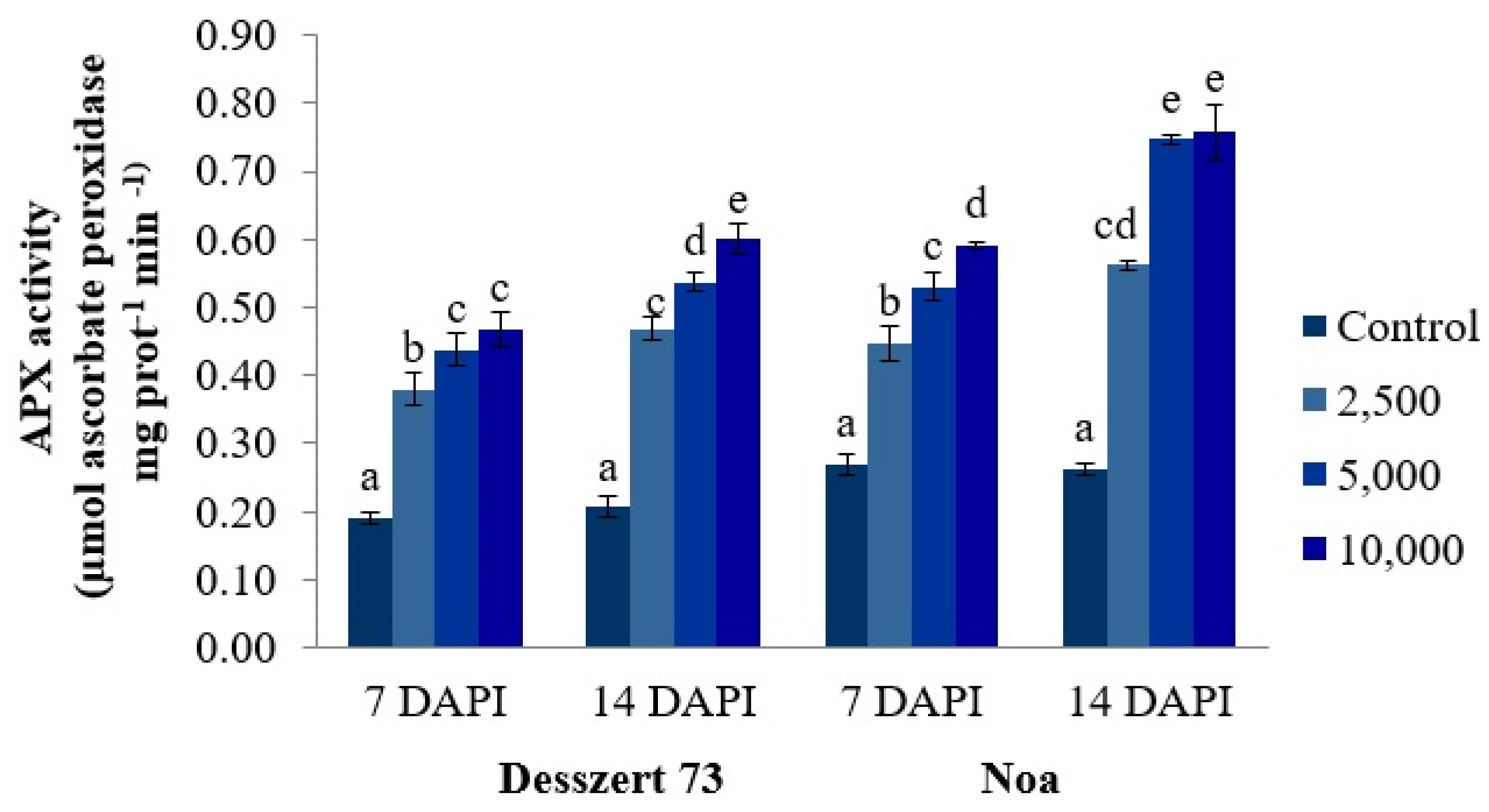

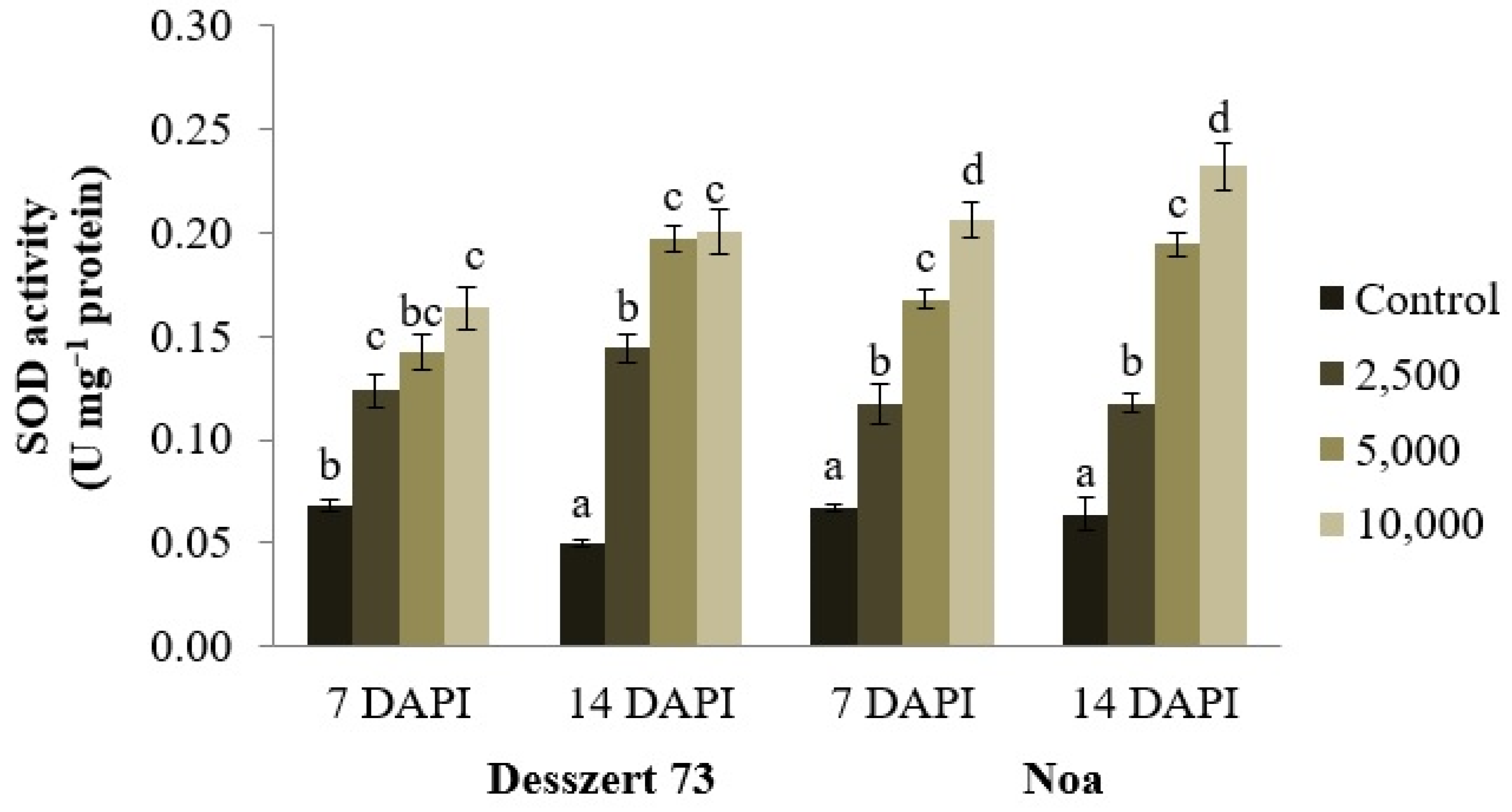
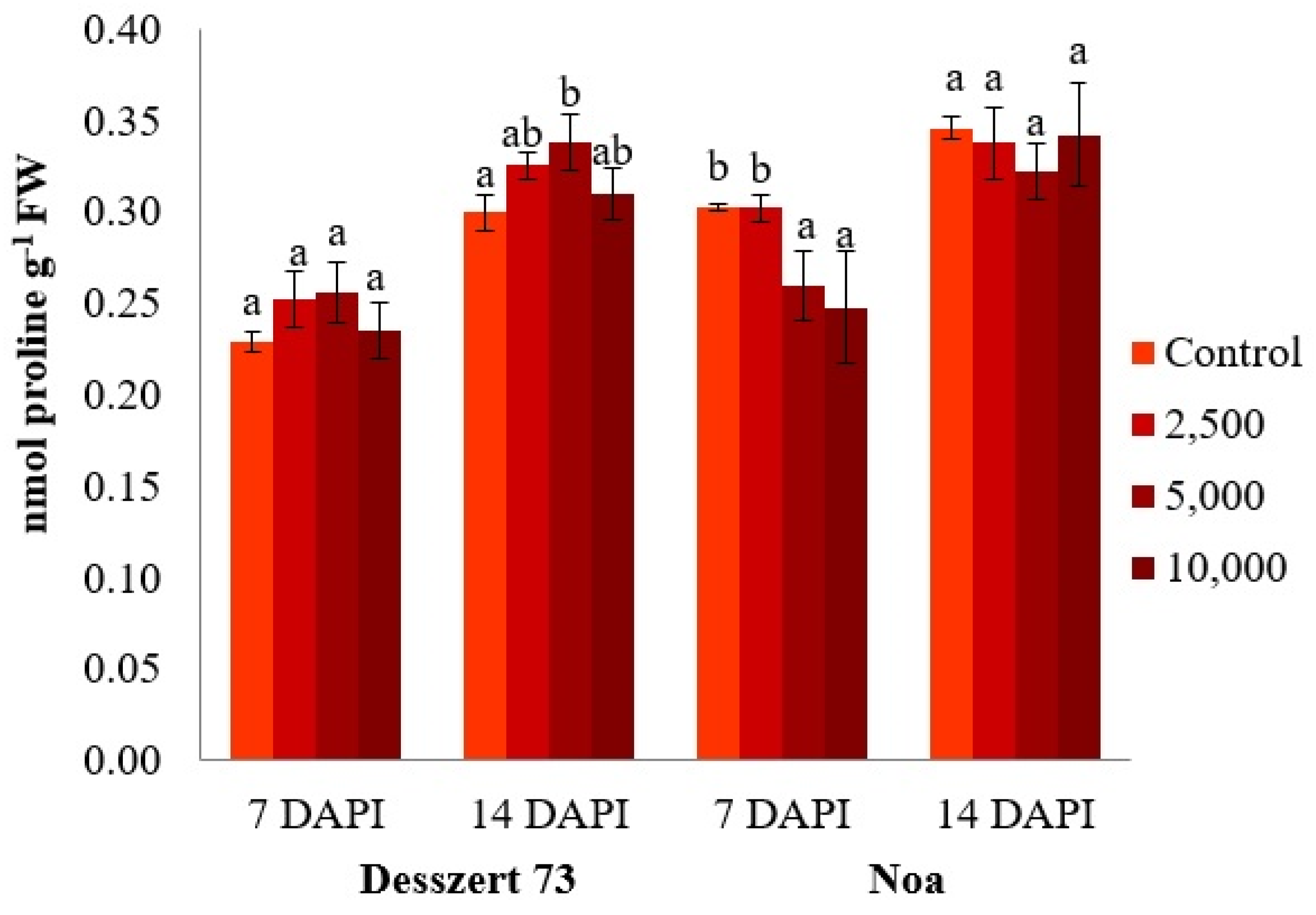
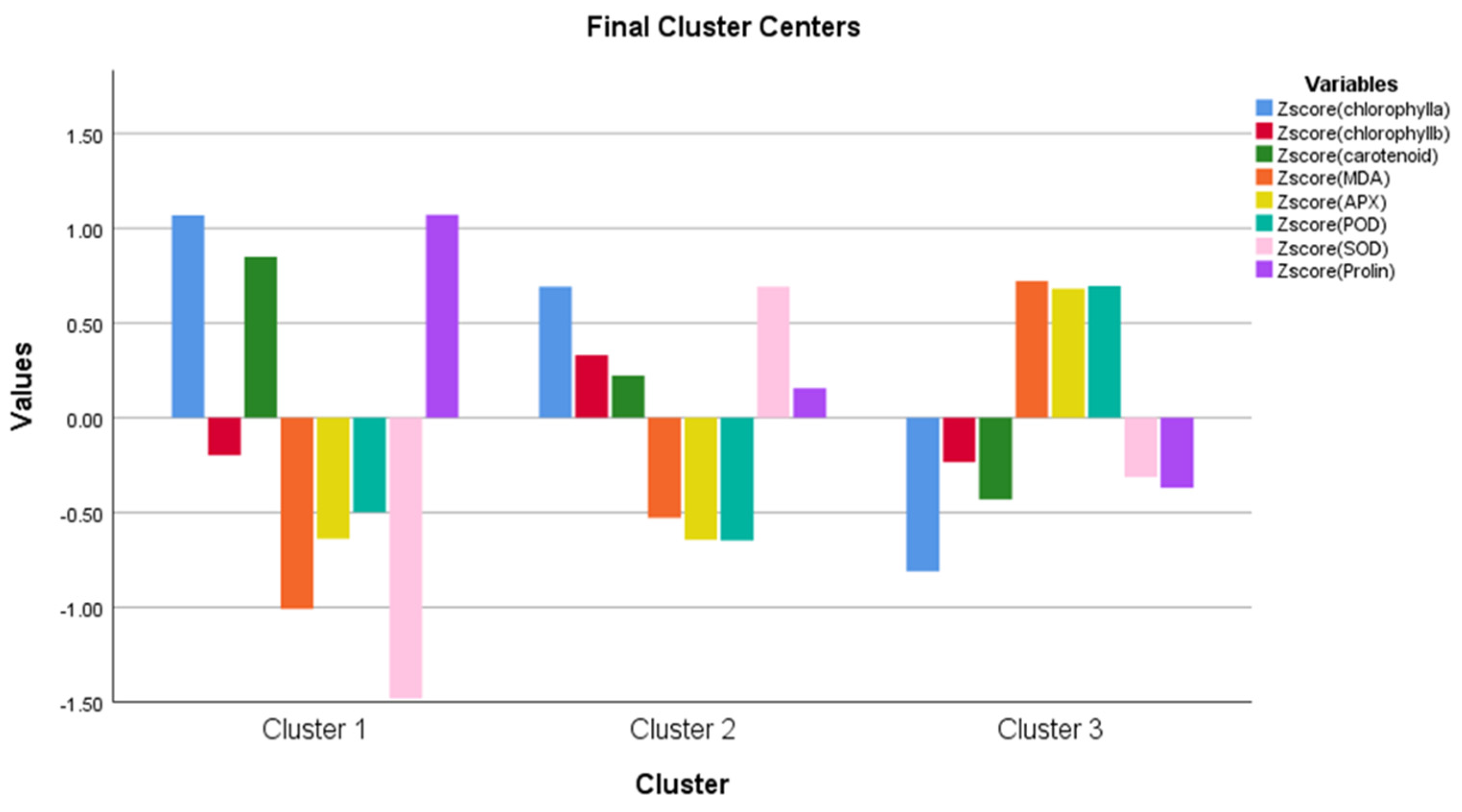

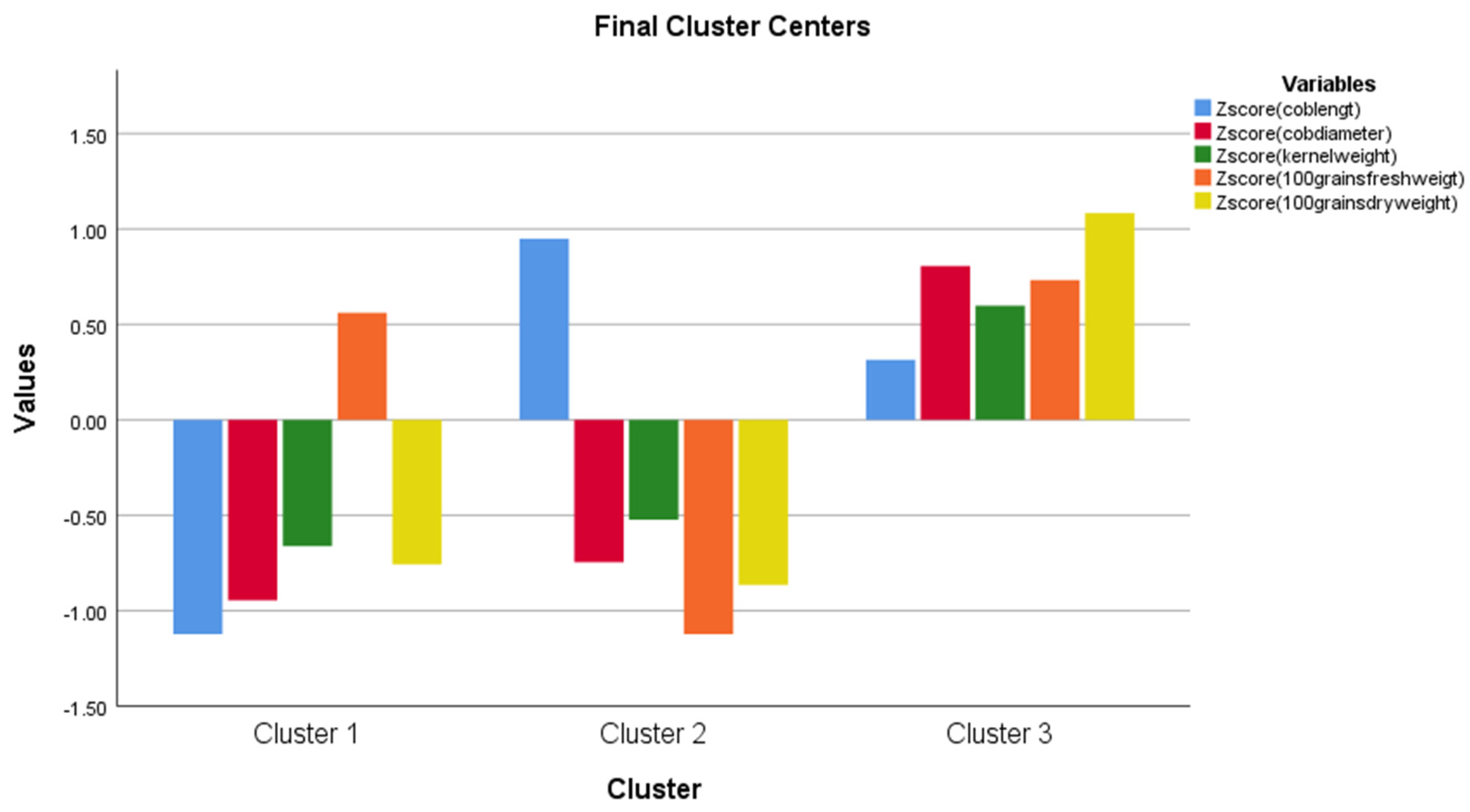
| Hybrid (H) | Treatment (T) | Sampling Time (S) | HxT | HxS | TxS | HxTxS | |
|---|---|---|---|---|---|---|---|
| SPAD | 2.693 | 732.922 * | 84.791 * | 9.333 | 4.18 | 58.41 | 23.831 |
| Chl-a | 3.158 | 2.158 * | 1.589 | 16.52 | 11.150 * | 7.119 | 7.1331 * |
| Chl-b | 4.335 * | 2.778 | 4.598 | 12.891 | 14.516 | 11.010 | 8.987 * |
| Car | 5.112 | 1.889 | 5.115 | 20.150 * | 12.260 | 9.116 | 7.668 * |
| MDA | 799.322 * | 4257.701 * | 14,799.288 * | 3119.895 * | 2648.025 * | 2204.624 * | 232.441 |
| APX | 0.219 * | 1.497 * | 0.116 * | 0.031 * | 0.007 | 0.054 * | 0.020 * |
| POX | 0.020 * | 0.536 * | 0.265 * | 0.001 | 0.028 * | 0.031 * | 0.011 |
| SOD | 0.597 | 28.101 * | 3.135 * | 0.930 * | 0.037 | 0.967 * | 0.084 |
| Proline | 0.013 | 0.001 * | 0.078 * | 0.003 | 0.001 | 0.001 * | 0.001 * |
| Height | 78,375.168 * | 87,860.924 * | 8455.813 * | 913.530 * | 1391.294 * | 10,121.012 * | 2547.550 * |
| Diameter | 374.978 * | 165.483 * | 107.648 * | 1.041 | 107.648 * | 0.919 | 0.919 |
| Cluster | Error | F | Sig. | |||
|---|---|---|---|---|---|---|
| Mean Square | df | Mean Square | df | |||
| Zscore (chlorophylla) | 24.716 | 2 | 0.394 | 75 | 62.765 | 0.000 |
| Zscore (chlorophyllb) | 2.939 | 2 | 0.948 | 75 | 3.099 | 0.051 |
| Zscore (carotenoid) | 7.162 | 2 | 0.814 | 75 | 8.797 | 0.000 |
| Zscore (MDA) | 18.332 | 2 | 0.525 | 75 | 34.932 | 0.000 |
| Zscore (APX) | 17.036 | 2 | 0.598 | 75 | 28.475 | 0.000 |
| Zscore (POD) | 16.822 | 2 | 0.577 | 75 | 29.141 | 0.000 |
| Zscore (SOD) | 18.260 | 2 | 0.546 | 75 | 33.471 | 0.000 |
| Zscore (Proline) | 7.567 | 2 | 0.844 | 75 | 8.970 | 0.000 |
| Chl-a | Chl-b | Car | MDA | APX | POX | SOD | Proline | |
|---|---|---|---|---|---|---|---|---|
| Chl-a | 1 | ns | 0.673 ** | −0.679 ** | −0.479 ** | −0.421 ** | ns | 0.412 ** |
| Chl-b | ns | 1 | ns | ns | −0.291 ** | −0.291 ** | ns | ns |
| Car | 0.673 ** | ns | 1 | −0.382 ** | ns | ns | ns | ns |
| MDA | −0.679 ** | ns | −0.382 ** | 1 | 0.562 ** | 0.424 ** | −0.226 * | −0.691 ** |
| APX | −0.479 ** | −0.291 ** | ns | 0.562 ** | 1 | 0.802 ** | −0.180 | −0.243 * |
| POX | −0.421 ** | −0.291 ** | ns | 0.424 ** | 0.802 ** | 1 | −0.246 * | ns |
| SOD | ns | ns | ns | −0.226 * | ns | −0.246 * | 1 | ns |
| Proline | 0.412 ** | ns | ns | −0.691 ** | −0.243 * | ns | ns | 1 |
| Plant Height | Stem Diameter | |||||||
|---|---|---|---|---|---|---|---|---|
| Desszert 73 | Noa | Desszert 73 | Noa | |||||
| 7 DAPI | 14 DAPI | 7 DAPI | 14 DAPI | 7 DAPI | 14 DAPI | 7 DAPI | 14 DAPI | |
| Control | 453 ± 17.08 b | 640 ± 18.26 c | 544 ± 15.17 b | 647 ± 5.77 c | 22.92 ± 1.44 a | 27.36 ± 0.77 b | 25.34 ± 0.85 a | 28.82 ± 0.94 b |
| 2500 | 375 ± 19.15 ab | 445 ± 19.15 b | 450 ± 10 a | 528 ± 12.58 b | 26.26 ± 0.70 b | 31.84 ± 0.81 c | 27.88 ± 0.84 ab | 33.66 ± 1.46 c |
| 5000 | 345 ± 12.91 a | 403 ± 15.28 ab | 444 ± 23.02 a | 473 ± 20.82 a | 28.42 ± 0.75 b | 31.94 ± 1.11 c | 28.18 ± 0.95 b | 34.92 ± 0.93 c |
| 10,000 | 365 ± 12.91 a | 383 ± 9.57 a | 422 ± 13.04 a | 470 ± 15.81 a | 28.2 ± 0.70 b | 33.94 ± 1.09 c | 29.58 ± 0.68 b | 35.72 ± 0.91 c |
| Desszert 73 | Noa | |||
|---|---|---|---|---|
| Control | Infected | Control | Infected | |
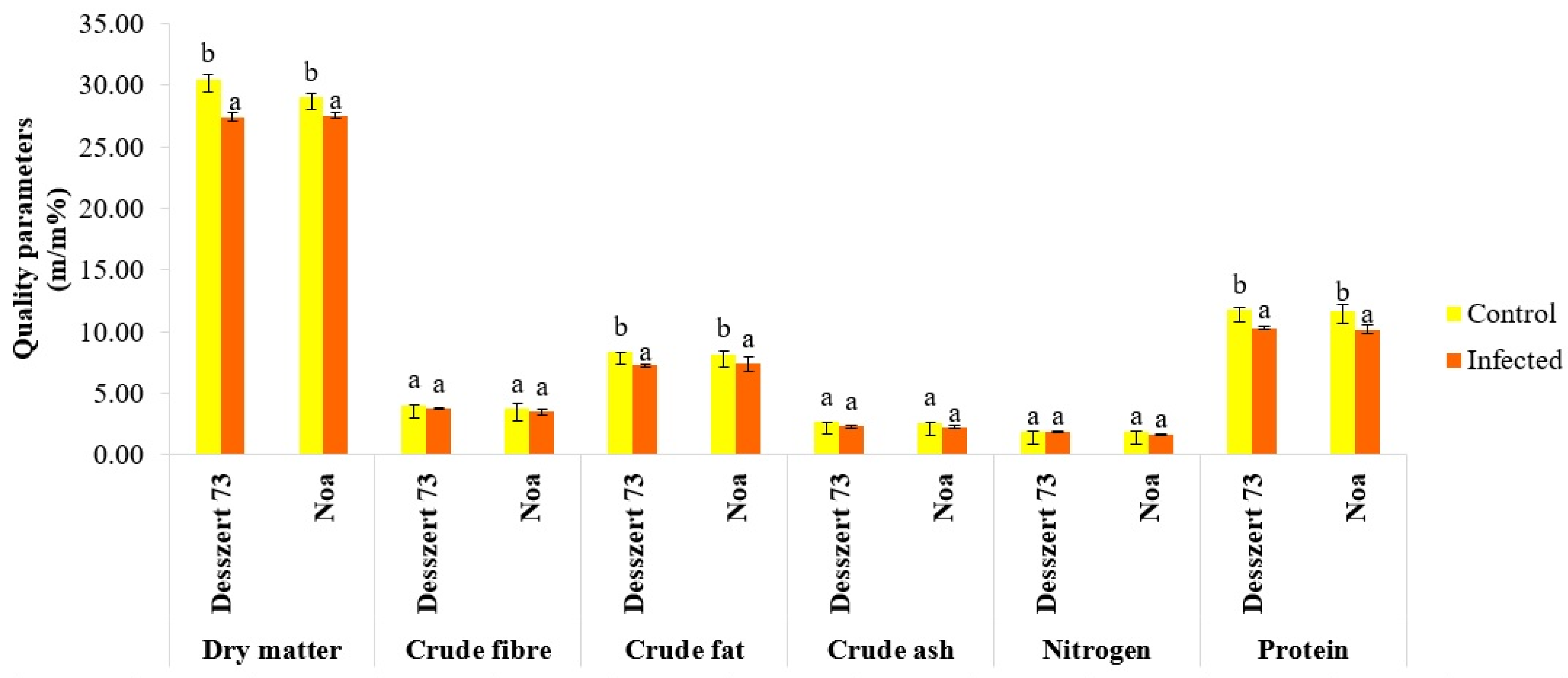
| Cob length (cm) | 21.34 ±1.31 b | 17.32 ± 3.33 a | 23.59 ± 0.19 b | 16.51 ± 0.99 a |
| Cob diameter (cm) | 4.40 ± 0.45 b | 3.62 ± 0.41 a | 4.68 ± 0.16 a | 3.72 ± 0.18 a |
| Kernel weight (g) | 275.81 ± 32.07 b | 161.42 ± 29.44 a | 311.80 ± 24.43 b | 259.30 ± 12.22 a |
| 100 grains fresh weight (g) | 42.69 ± 1.31 b | 34.42 ± 3.30 a | 45.46 ± 4.01 b | 37.92 ± 1.04 a |
| 100 grains dry weight (g) | 9.04 ± 1.15 b | 4.39 ± 0.72 a | 7.99 ± 0.27 b | 3.29 ± 0.15 a |
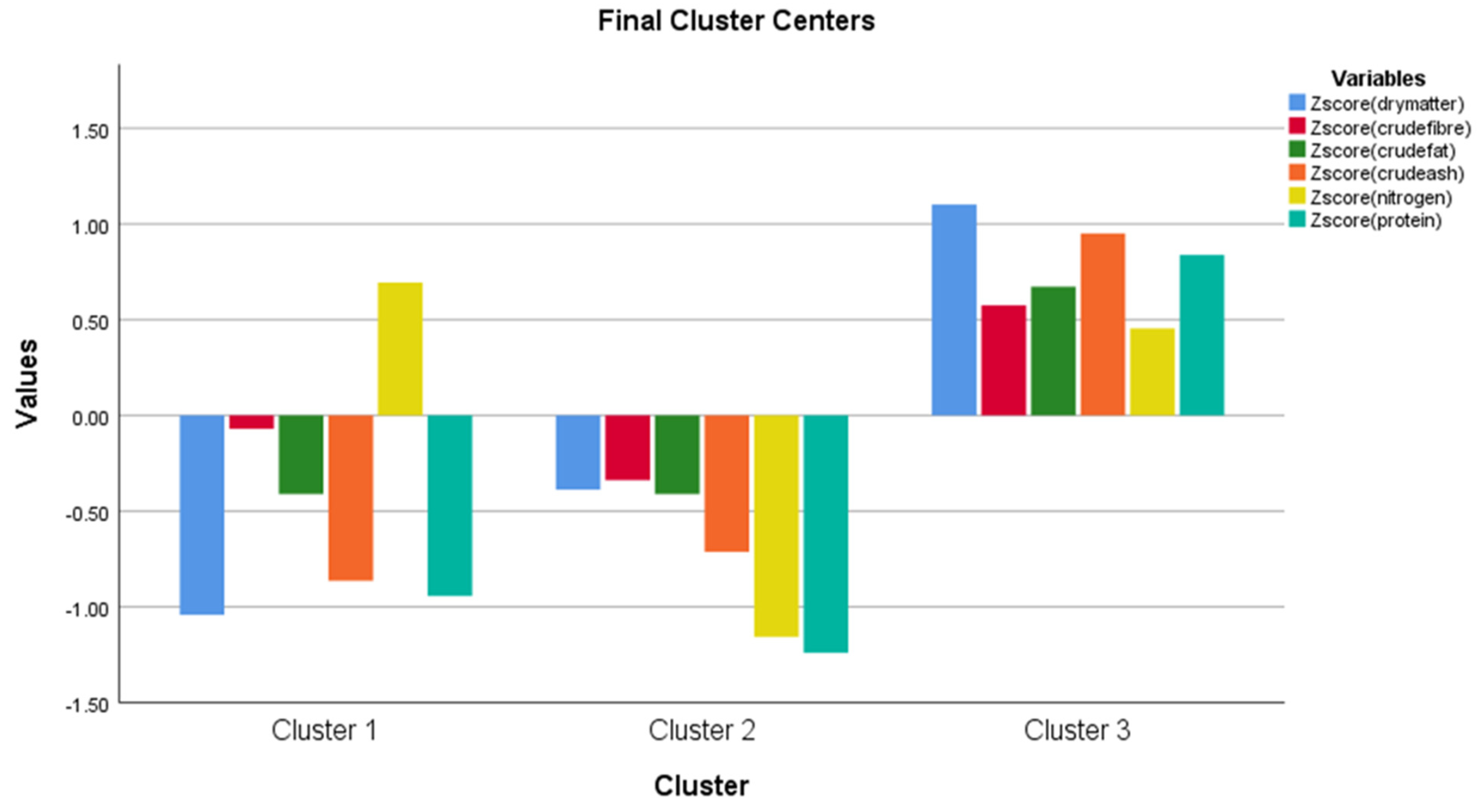
| Cluster Mean Square | df | Error Mean Square | df | F | Sig. | |
|---|---|---|---|---|---|---|
| Zscore (coblenght) | 2.865 | 2 | 0.610 | 7 | 4.697 | 0.051 |
| Zscore (cobdiameter) | 3.170 | 2 | 0.150 | 7 | 21.077 | 0.001 |
| Zscore (kernelweight) | 1.650 | 2 | 0.233 | 7 | 7.067 | 0.021 |
| Zscore (stograinweigt) | 3.900 | 2 | 0.685 | 7 | 5.693 | 0.034 |
| Zscore (stograinsusa) | 4.395 | 2 | 0.032 | 7 | 136.657 | 0.000 |
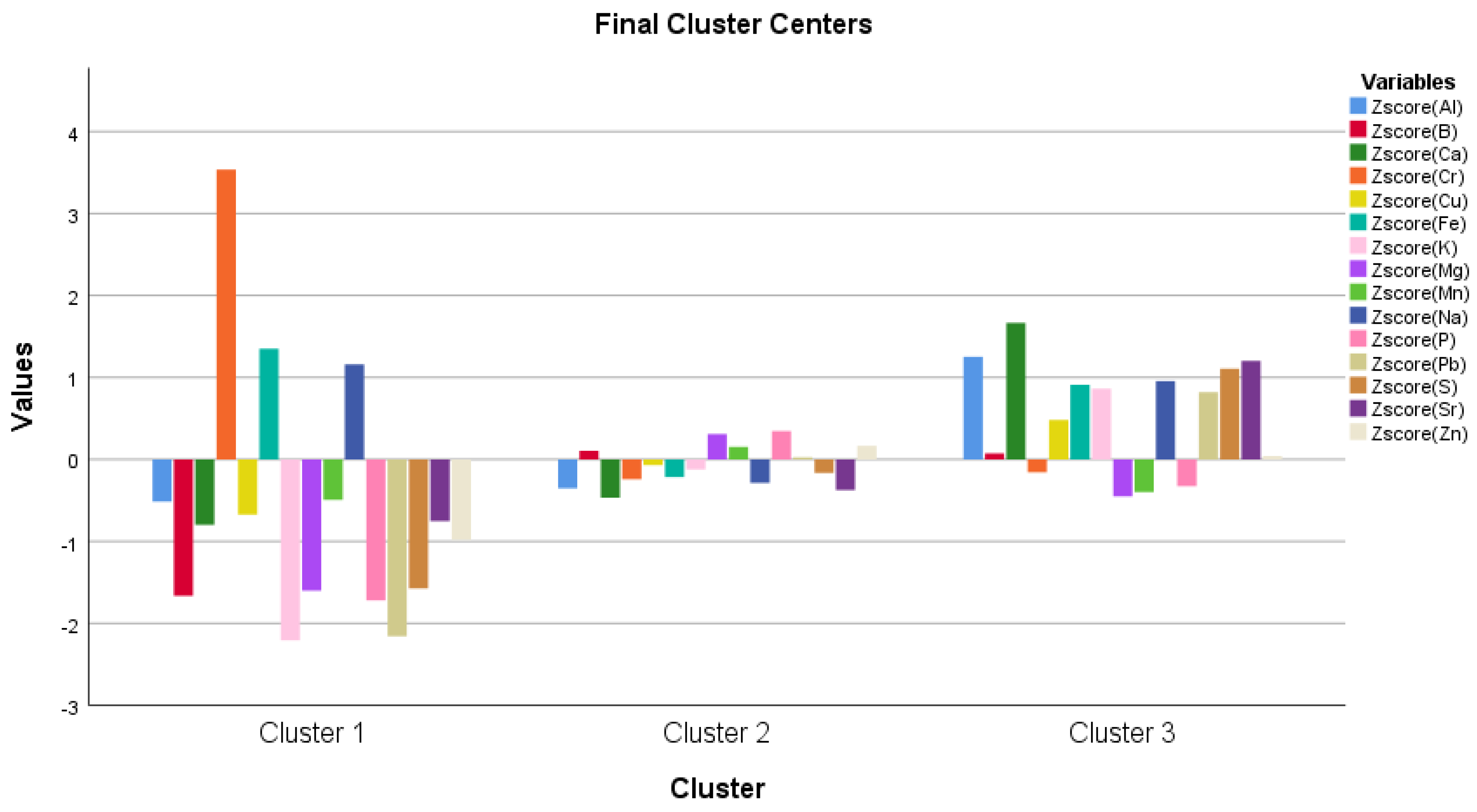
| Desszert 73 | Noa | |||
|---|---|---|---|---|
| Control | Infected Plants | Control | Infected Plants | |
| Al | 0.38 ± 0.20 a | 1.07 ± 0.33 b | 0.81 ± 0.08 a | 1.66 ± 0.42 b |
| B | 3.21 ± 0.02 b | 2.88 ± 0.13 a | 3.38 ± 0.14 a | 3.00 ± 0.21 a |
| Ca | 67.31 ± 2.64 a | 75.48 ± 4.80 b | 67.16 ± 1.62 a | 72.60 ± 4.61 b |
| Cr | 0.11 ± 0.003 a | 0.11 ± 0.008 a | 0.12 ± 0.01 a | 0.14 ± 0.005 a |
| Cu | 2.85 ± 0.15 a | 2.55 ± 0.21 a | 2.34 ± 0.09 a | 2.46 ± 0.14 a |
| Fe | 12.34 ± 0.26 a | 12.12 ± 0.51 a | 12.59 ± 0.48 a | 13.37 ± 0.75a |
| K | 6132.94 ± 77.68 a | 6093.44 ± 113.27 a | 5966.32 ± 202.62 b | 5716.64 ± 148.06 a |
| Mg | 789.62 ± 16.81 b | 686.91 ± 7.66 a | 746.868 ± 12.12 b | 690.20 ± 10.74 a |
| Mn | 9.41 ± 0.40 b | 7.52 ± 0.52 a | 8.45 ± 0.40 b | 7.34 ± 0.53 a |
| Na | 21.35 ± 1.35 a | 22.65 ± 0.84 a | 21.73 ± 1.03 a | 28.05 ± 1.96 b |
| P | 2998.66 ± 151.78 b | 2687.77 ± 63.30 a | 2777.86 ± 62.22 a | 2677.32 ± 50.32 a |
| Pb | 0.11 ± 0.003 a | 0.18 ± 0.006 b | 0.13 ± 0.023 a | 0.13 ± 0.007 a |
| S | 870.61 ± 42.84 a | 930.92 ± 54.18 b | 824.195 ± 18.56 a | 940.99 ± 64.51 b |
| Sr | 0.62 ± 0.09 a | 0.79 ± 0.04 a | 0.56 ± 0.03 a | 0.47 ± 0.11 a |
| Zn | 28.38 ± 0.25 b | 26.86 ± 1.94 a | 24.35 ± 0.90 a | 23.71 ± 1.54 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szőke, L.; Moloi, M.J.; Kaczur, D.; Radócz, L.; Tóth, B. Examination of Different Sporidium Numbers of Ustilago maydis Infection on Two Hungarian Sweet Corn Hybrids’ Characteristics at Vegetative and Generative Stages. Life 2023, 13, 433. https://doi.org/10.3390/life13020433
Szőke L, Moloi MJ, Kaczur D, Radócz L, Tóth B. Examination of Different Sporidium Numbers of Ustilago maydis Infection on Two Hungarian Sweet Corn Hybrids’ Characteristics at Vegetative and Generative Stages. Life. 2023; 13(2):433. https://doi.org/10.3390/life13020433
Chicago/Turabian StyleSzőke, Lóránt, Makoena Joyce Moloi, Dávid Kaczur, László Radócz, and Brigitta Tóth. 2023. "Examination of Different Sporidium Numbers of Ustilago maydis Infection on Two Hungarian Sweet Corn Hybrids’ Characteristics at Vegetative and Generative Stages" Life 13, no. 2: 433. https://doi.org/10.3390/life13020433
APA StyleSzőke, L., Moloi, M. J., Kaczur, D., Radócz, L., & Tóth, B. (2023). Examination of Different Sporidium Numbers of Ustilago maydis Infection on Two Hungarian Sweet Corn Hybrids’ Characteristics at Vegetative and Generative Stages. Life, 13(2), 433. https://doi.org/10.3390/life13020433