DOCK2 Mutation and Recurrent Hemophagocytic Lymphohistiocytosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data
2.2. DOCK2 Wild-Type and Mutant DNA Constructs
2.3. Viral Preparation and Transduction
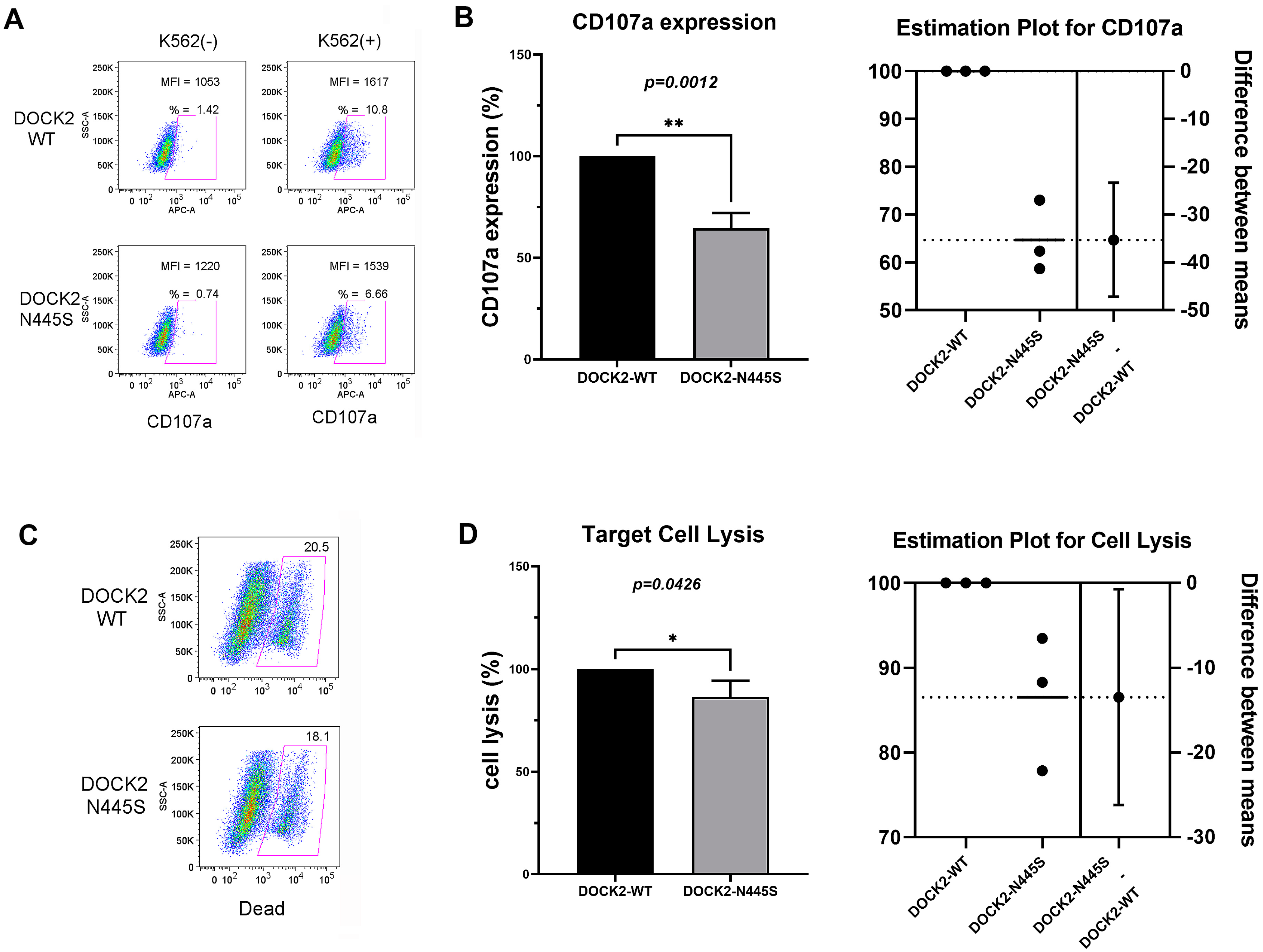
2.4. Degranulation and Cytotoxicity Assays
3. Clinical History
4. Results
DOCK2 Mutation (p.Asn445Ser) Decreases NK Cell Degranulation and Cell Lysis
5. Discussion
5.1. DOCK2 and Immunodeficiency
5.2. DOCK2 and HLH
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Saint Basile, G.; Ménasché, G.; Fischer, A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat. Rev. Immunol. 2010, 8, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Canna, S.W.; Marsh, R.A. Pediatric hemophagocytic lymphohistiocytosis. Blood 2020, 135, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.J.; Tattersall, R.S.; Ramanan, A.V. Macrophage activation syndrome in adults: Recent advances in pathophysiology, diagnosis and treatment. Rheumatology 2019, 58, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, A.; Kukimoto-Niino, M.; Yokoyama, S.; Fukui, Y. Immune regulatory functions of DOCK family proteins in health and disease. Exp. Cell. Res. 2013, 319, 2343–2349. [Google Scholar] [CrossRef]
- Kunimura, K.; Uruno, T.; Fukui, Y. DOCK family proteins: Key players in immune surveillance mechanisms. Int. Immunol. 2020, 32, 5–15. [Google Scholar] [CrossRef]
- Dobbs, K.; Conde, C.D.; Zhang, S.-Y.; Parolini, S.; Audry, M.; Chou, J.; Haapaniemi, E.; Keles, S.; Bilic, I.; Okada, S.; et al. Inherited DOCK2 deficiency in patients with early-onset invasive infections. N. Engl. J. Med. 2015, 372, 2409–2422. [Google Scholar] [CrossRef]
- Sakai, Y.; Tanaka, Y.; Yanagihara, T.; Watanabe, M.; Duan, X.; Terasawa, M.; Nishikimi, A.; Sanematsu, F.; Fukui, Y. The Rac activator DOCK2 regulates natural killer cell-mediated cytotoxicity in mice through the lytic synapse formation. Blood 2013, 122, 386–393. [Google Scholar] [CrossRef]
- Vagrecha, A.; Zhang, M.; Acharya, S.; Lozinsky, S.; Singer, A.; Levine, C.; Al-Ghafry, M.; Levy, C.F.; Cron, R.Q. Hemophagocytic lymphohistiocytosis gene variants in multisystem inflammatory syndrome in children. Biology 2022, 11, 417. [Google Scholar] [CrossRef]
- Selliah, N.; Zhang, M.; White, S.; Zoltick, P.; Sawaya, B.E.; Finkel, T.H.; Cron, R.Q. FOXP3 inhibits HIV-1 infection of CD4 T-cells via inhibition of LTR transcriptional activity. Virology 2008, 381, 161–167. [Google Scholar] [CrossRef]
- Reiff, D.D.; Zhang, M.; Smitherman, E.A.; Mannion, M.L.; Stoll, M.L.; Weiser, P.; Cron, R.Q. A Rare STXBP2 mutation in severe COVID-19 and secondary cytokine storm syndrome. Life 2022, 12, 149. [Google Scholar] [CrossRef]
- Henter, J.-I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef]
- Eloseily, E.M.; Weiser, P.; Crayne, C.B.; Haines, H.; Mannion, M.L.; Stoll, M.L.; Beukelman, T.; Atkinson, T.P.; Cron, R.Q. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthr. Rheumatol. 2020, 72, 326–334. [Google Scholar] [CrossRef]
- Henderson, L.A.; Cron, R.Q. Macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in childhood inflammatory disorders: Diagnosis and management. Paediatr. Drugs 2020, 22, 29–44. [Google Scholar] [CrossRef]
- Schulert, G.S.; Cron, R.Q. The genetics of macrophage activation syndrome. Genes Immun. 2020, 21, 169–181. [Google Scholar] [CrossRef]
- Mellor-Heineke, S.; Villanueva, J.; Jordan, M.B.; Marsh, R.; Zhang, K.; Bleesing, J.J.; Filipovich, A.H.; Risma, K.A. Elevated granzyme B in cytotoxic lymphocytes is a signature of immune activation in hemophagocytic lymphohistiocytosis. Front. Immunol. 2013, 4, 72. [Google Scholar] [CrossRef]
- Rubin, T.S.; Zhang, K.; Gifford, C.; Lane, A.; Choo, S.; Bleesing, J.J.; Marsh, R.A. Perforin and CD107a testing is superior to NK cell function testing for screening patients for genetic HLH. Blood 2017, 129, 2993–2999. [Google Scholar] [CrossRef]
- Fukui, Y.; Hashimoto, O.; Sanui, T.; Oono, T.; Koga, H.; Abe, M.; Inayoshi, A.; Noda, M.; Oike, M.; Shirai, T.; et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature 2001, 412, 826–831. [Google Scholar] [CrossRef]
- Gotoh, K.; Tanaka, Y.; Nishikimi, A.; Nakamura, R.; Yamada, H.; Maeda, N.; Ishikawa, T.; Hoshino, K.; Uruno, T.; Cao, Q.; et al. Selective control of type I IFN induction by the Rac activator DOCK2 during TLR-mediated plasmacytoid dendritic cell activation. J. Exp. Med. 2010, 207, 721–730. [Google Scholar] [CrossRef]
- Moens, L.; Gouwy, M.; Bosch, B.; Pastukhov, O.; Nieto-Patlàn, A.; Siler, U.; Bucciol, G.; Mekahli, D.; Vermeulen, F.; Desmet, L.; et al. Human DOCK2 deficiency: Report of a novel mutation and evidence for neutrophil dysfunction. J. Clin. Immunol. 2019, 39, 298–308. [Google Scholar] [CrossRef]
- Freij, B.J.; Hanrath, A.T.; Chen, R.; Hambleton, S.; Duncan, C.J.A. Life-threatening influenza, hemophagocytic lymphohistiocytosis and probable vaccine-strain varicella in a novel case of homozygous STAT2 deficiency. Front. Immunol. 2021, 11, 624415. [Google Scholar] [CrossRef]
- Aytekin, E.S.; Çağdaş, D.; Tan, Ç.; Çavdarlı, B.; Bilgiç, I.; Tezcan, İ. Hematopoietic stem cell transplantation complicated with EBV associated hemophagocytic lymphohistiocytosis in a patient with DOCK2 deficiency. Turk. J. Pediatr. 2021, 63, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, H.; Edahiro, R.; Takano, T.; Nishihara, H.; Shirai, Y.; Sonehara, K.; Tanaka, H.; Azekawa, S.; Mikami, Y.; Lee, H.; et al. DOCK2 is involved in the host genetics and biology of severe COVID-19. Nature 2022, 609, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, D.; Freeman, A.F. Current status of dedicator of cytokinesis-associated immunodeficiency: DOCK8 and DOCK2. Dermatol. Clin. 2017, 35, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Reiff, D.D.; Cron, R.Q. Who would have predicted multisystem inflammatory syndrome in children? Curr. Rheumatol. Rep. 2022, 24, 1–11. [Google Scholar] [CrossRef]
- Schulert, G.S.; Zhang, M.; Fall, N.; Husami, A.; Kissell, D.; Hanosh, A.; Zhang, K.; Davis, K.; Jentzen, J.M.; Napolitano, L.; et al. Whole-exome sequencing reveals mutations in genes linked to hemophagocytic lymphohistiocytosis and macrophage activation syndrome in fatal cases of H1N1 influenza. J. Infect. Dis. 2016, 213, 1180–1188. [Google Scholar] [CrossRef]
- Zhang, K.; Johnson, J.A.; Biroschak, J.; Villanueva, J.; Lee, S.M.; Bleesing, J.J.; Risma, K.A.; Wenstrup, R.J.; Filipovich, A.H. Familial haemophagocytic lymphohistiocytosis in patients who are heterozygous for the A91V perforin variation is often associated with other genetic defects. Int. J. Immunogenet. 2007, 34, 231–233. [Google Scholar] [CrossRef]
- Zhang, M.; Behrens, E.M.; Atkinson, T.P.; Shakoory, B.; Grom, A.A.; Cron, R.Q. Genetic defects in cytolysis in macrophage activation syndrome. Curr. Rheumatol. Rep. 2014, 16, 439. [Google Scholar] [CrossRef]
- Spessott, W.A.; SanMillan, M.L.; McCormick, M.E.; Patel, N.; Villanueva, J.; Zhang, K.; Nichols, K.E.; Giraudo, C.G. Hemophagocytic lymphohistiocytosis caused by dominant-negative mutations in STXBP2 that inhibit SNARE-mediated membrane fusion. Blood 2015, 125, 1566–1577. [Google Scholar] [CrossRef]
- Zhang, M.; Bracaglia, C.; Prencipe, G.; Bemrich-Stolz, C.J.; Beukelman, T.; Dimmitt, R.A.; Chatham, W.W.; Zhang, K.; Li, H.; Walter, M.R.; et al. A heterozygous RAB27A mutation associated with delayed cytolytic granule polarization and hemophagocytic lymphohistiocytosis. J. Immunol. 2016, 196, 2492–2503. [Google Scholar] [CrossRef]
- Anft, M.; Netter, P.; Urlaub, D.; Prager, I.; Schaffner, S.; Watzl, C. NK cell detachment from target cells is regulated by successful cytotoxicity and influences cytokine production. Cell. Mol. Immunol. 2020, 17, 347–355. [Google Scholar] [CrossRef]
- Jenkins, M.R.; Rudd-Schmidt, J.A.; Lopez, J.A.; Ramsbottom, K.M.; Mannering, S.I.; Andrews, D.M.; Voskoboinik, I.; Trapani, J.A. Failed CTL/NK cell killing and cytokine hypersecretion are directly linked through prolonged synapse time. J. Exp. Med. 2015, 212, 307–317. [Google Scholar] [CrossRef]
- Strippoli, R.; Caiello, I.; De Benedetti, F. Reaching the threshold: A multilayer pathogenesis of macrophage activation syndrome. J. Rheumatol. 2013, 40, 761–767. [Google Scholar] [CrossRef]
- Brisse, E.; Wouters, C.H.; Matthys, P. Advances in the pathogenesis of primary and secondary haemophagocytic lymphohis-tiocytosis: Differences and similarities. Br. J. Haematol. 2016, 174, 203–217. [Google Scholar] [CrossRef]
- Kernan, K.F.; Ghaloul-Gonzalez, L.; Vockley, J.; Lamb, J.; Hollingshead, D.; Chandran, U.; Sethi, R.; Park, H.-J.; Berg, R.A.; Wessel, D.; et al. Prevalence of pathogenic and potentially pathogenic inborn error of immunity associated variants in children with severe sepsis. J. Clin. Immunol. 2022, 42, 350–364. [Google Scholar] [CrossRef]
| Age (Years) | Admission | WBC Count | ANC | ALC | Hgb | Platelet Count |
|---|---|---|---|---|---|---|
| ×103/μL | ×103/μL | ×103/μL | g/dL | ×103/μL | ||
| 7 | Fever, emesis Negative infectious workup | Min: 1.53 Max: 7.24 (nml 4.31–11.0) | Min: 0.72 (nml 1.63–7.55) | Min: 0.34 (nml 0.97–3.96) | Min: 6.5 (nml 10.7–13.4) | Min: 108 Max: 426 (nml 140–440) |
| 7 | Positive blood cultures: Staphylococcus aureus Candida albicans | Min: 1.82 Max: 8.57 (4.31–11.0) | Min: 1.09 (1.63–7.55) | Min: 0.43 (0.97–3.96) | Min: 6.3 (10.7–13.4) | Min: 55 Max: 471 (140–440) |
| 7 | Fever, cough, congestion Negative infectious workup | Min: 1.73 Max: 6.55 (4.31–11.0) | Min: 1.28 (1.63–7.55) | Min: 0.19 (0.97–3.96) | Min: 9.2 (10.7–13.4) | Min: 90 Max: 277 (140–440) |
| 8 | Positive blood cultures: Candida albicans | Min: 2.90 Max: 9.09 (4.31–11.0) | Min: 1.61 (1.63–7.55) | Min: 0.42 (0.97–3.96) | Min: 6.3 (10.7–13.4) | Min: 34 Max: 112 (140–440) |
| 8 | Fever, lethargy Negative infectious workup | Min: 0.39 Max: 2.47 (4.31–11.0) | Min: 0.23 (1.63–7.55) | Min: 0.14 (0.97–3.96) | Min: 6.9 (10.7–13.4) | Min: 94 Max: 400 (140–440) |
| 10 | Positive blood cultures: Bacillus licheniformis | Min: 0.73 Max: 4.82 (4.31–11.0) | Min: 0.45 (1.63–7.55) | Min: 0.24 (0.97–3.96) | Min: 5.8 (10.7–13.4) | Min: 25 Max: 394 (140–440) |
| 10 | Fever, lethargy Negative infectious workup | Min: 1.05 Max: 2.86 (4.31–11.0) | Min: 0.61 (1.63–7.55) | Min: 0.41 (0.97–3.96) | Min: 6.4 (10.7–13.4) | Min: 63 Max: 155 (140–440) |
| 10 | Positive blood cultures: Staphylococcus aureus | Min: 2.72 Max: 5.01 (4.31–11.0) | Min: 1.97 (1.63–7.55) | Min: 0.44 (0.97–3.96) | Min: 8.8 (10.7–13.4) | Min: 40 Max: 124 (140–440) |
| 10 | Positive blood cultures: Staphylococcus aureus | Min: 0.96 Max: 1.07 (4.31–11.0) | Min: 0.76 (1.63–7.55) | Min: 0.26 (0.97–3.96) | Min: 8.1 (10.7–13.4) | Min: 197 Max: 236 (140–440) |
| 11 | Fever, lethargy Negative infectious workup | Min: 2.40 Max: 3.81 (4.31–11.0) | Min: 1.26 (1.63–7.55) | Min: 0.59 (0.97–3.96) | Min: 9.6 (10.7–13.4) | Min: 109 Max: 262 (140–440) |
| 11 | Positive blood cultures: Klebsiella pneumoniae, Staphylococcus aureus | Min: 1.98 Max: 5.93 (4.31–11.0) | Min: 1.04 (1.63–7.55) | Min: 0.26 (0.97–3.96) | Min: 7.5 (10.7–13.4) | Min: 51 Max: 239 (140–440) |
| Age (Years) | Admission | AST | ALT | Ferritin | CRP | ESR | Fibrinogen | LDH |
|---|---|---|---|---|---|---|---|---|
| U/L | U/L | ng/mL | mg/dL | mm/hr | mg/dL | U/L | ||
| 7 | Fever, emesis Negative infectious workup | Max: 399 (nml 15–40) | Max: 104 (nml 10–35) | Max: 52,650 (nml 22–340) | Max: 6.61 (nml < 0.5) | Min: 6 Max: 76 (nml 0–15) | Max/Min: 323 (nml 156–400) | Max: 10,884 (nml 420–750) |
| 7 | Positive blood cultures: Staphylococcus aureus Candida albicans | Max: 392 (15–40) | Max: 118 (10–35) | Max: 36,972 (22–340) | Max: 21.12 (<0.5) | Min: 11 Max: 73 (0–15) | Min: 128 Max: 559 (156–400) | – |
| 7 | Fever, cough, congestion Negative infectious workup | Max: 229 (15–40) | Max: 316 (10–35) | Max: 13,194 (22–340) | Max: 8.84 (<0.5) | Min: 7 Max: 16 (0–15) | Max/Min: 195 (nml 156–400) | Max: 4272 (420–750) |
| 8 | Positive blood cultures: Candida albicans | Max: 76 (18–36) | Max: 45.5 (9.0–25.0) | Max: 1957 (13.7–78.8) | Max: 13.23 (<0.5) | Min: 14 Max: 52 (0–15) | Min: 309 Max: 532 (156–400) | – |
| 8 | Fever, lethargy Negative infectious workup | Max: 175 (18–36) | Max: 72.5 (9.0–25.0) | Max: 20,455.6 (13.7–78.8) | Max: 3.6 (<0.5) | Min: 11 Max: 30 (0–15) | – | – |
| 10 | Positive blood cultures: Bacillus licheniformis | Max: 127 (18–36) | Max: 121.3 (9.0–25.0) | Max: 4429.6 (13.7–78.8) | Max: 7.88 (<0.5) | Min: 9 Max: 30 (0–15) | Min: 84 Max: 197 (156–400) | Max: 1630 (170–283) |
| 10 | Fever, lethargy Negative infectious workup | Max: 225 (18–36) | Max: 168.9 (9.0–25.0) | Max: 2727.1 (13.7–78.8) | Max: 4.42 (<0.5) | Min: 9 Max: 24 (0–15) | Max/Min: 160 (nml 156–400) | – |
| 10 | Positive blood cultures: Staphylococcus aureus | Max: 100 (18–36) | Max: 100 (9.0–25.0) | Max: 896.1 (13.7–78.8) | Max: 15.52 (<0.5) | Min: 33 Max: 51 (0–15) | – | – |
| 10 | Positive blood cultures: Staphylococcus aureus | Max: 171 (18–36) | Max: 134.5 (9.0–25.0) | Max: 827.1 (13.7–78.8) | Max: 1.88 (<0.5) | Min: 15 Max: 28 (0–15) | – | – |
| 11 | Fever, lethargy Negative infectious workup | Max: 95 (18–36) | Max: 83.5 (9.0–25.0) | Max: 239.1 (13.7–78.8) | Max: 13.46 (<0.5) | Min/Max: 13 (0–15) | – | – |
| 11 | Positive blood cultures: Klebsiella pneumoniae, Staphylococcus aureus | Max: 44 (18–36) | Max: 39 (9.0–25.0) | Max: 196 (13.7–78.8) | Max: 14.3 (<0.5) | Min: 48 Max: 77 (0–15) | – | Max: 241 (170–283) |
| Gene | Variant | Zygosity | Variant Classification | Disease Association |
|---|---|---|---|---|
| G6PD | c.[202G>A;376A>G] (p.[Val68Met;ASn126Asp]) | hemizygous | Pathogenic | X-linked G6PD deficiency |
| PEPD | Deletion (Exon 1) | heterozygous | Pathogenic | Autosomal recessive prolidase deficiency |
| PEPD | c.932G>A (p.Arg311Gln) | heterozygous | Uncertain Significance | Autosomal recessive prolidase deficiency |
| DOCK2 | c.1334A>G (p.Asn445Ser) | heterozygous | Uncertain Significance | Autosomal recessive combined immunodeficiency due to DOCK2 deficiency |
| ERCC6L2 | c.4089T>G (p.Asn1363Lys) | heterozygous | Uncertain Significance | Autosomal recessive ERCC6L2 deficiency |
| FANCA | c.753_755delinsAG (p.Asp252Ser) | heterozygous | Uncertain Significance | Autosomal recessive Fanconi anemia type A |
| FCHO1 | c.529C>T (p.Arg177Cys) | heterozygous | Uncertain Significance | Autosomal recessive combined immunodeficiency due to FCHO1 deficiency |
| G6PC3 | c.50A>C (p.Asn17Thr) | heterozygous | Uncertain Significance | Autosomal recessive severe congenital neutropenia |
| RBCK1 | c.1028C>T (p.Ala343Val) | heterozygous | Uncertain Significance | Autosomal recessive polyglucosan body myopathy with or without immunodeficiency |
| RTEL1 | c.3499+7C>T (intronic) | heterozygous | Uncertain Significance | Autosomal recessive dyskeratosis congenital spectrum disorders |
| SAMD9L | c.251dup (p.Asn84Lysfs * 3) | heterozygous | Uncertain Significance | Autosomal dominant ataxia-pancytopenia syndrome |
| UNC45A | c.2003C>A (p.Ser668Tr) | heterozygous | Uncertain Significance | No well-established disease association |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiff, D.D.; Zhang, M.; Cron, R.Q. DOCK2 Mutation and Recurrent Hemophagocytic Lymphohistiocytosis. Life 2023, 13, 434. https://doi.org/10.3390/life13020434
Reiff DD, Zhang M, Cron RQ. DOCK2 Mutation and Recurrent Hemophagocytic Lymphohistiocytosis. Life. 2023; 13(2):434. https://doi.org/10.3390/life13020434
Chicago/Turabian StyleReiff, Daniel D., Mingce Zhang, and Randy Q. Cron. 2023. "DOCK2 Mutation and Recurrent Hemophagocytic Lymphohistiocytosis" Life 13, no. 2: 434. https://doi.org/10.3390/life13020434
APA StyleReiff, D. D., Zhang, M., & Cron, R. Q. (2023). DOCK2 Mutation and Recurrent Hemophagocytic Lymphohistiocytosis. Life, 13(2), 434. https://doi.org/10.3390/life13020434








