Clinical Manifestation, Transmission, Pathogenesis, and Diagnosis of Monkeypox Virus: A Comprehensive Review
Abstract
1. Introduction
2. Features of Monkeypox Epidemics
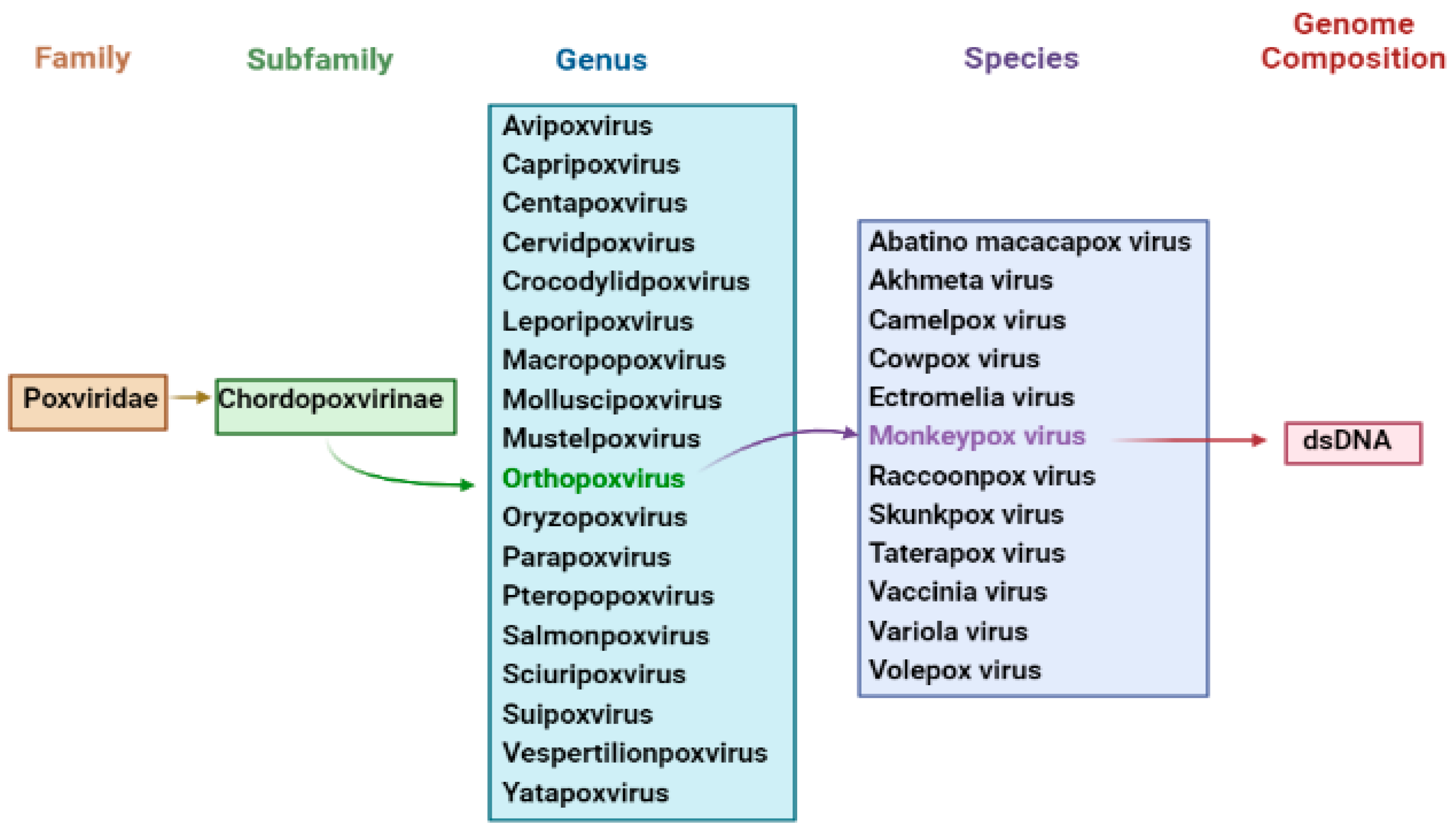
3. Nomenclature of Monkeypox Virus
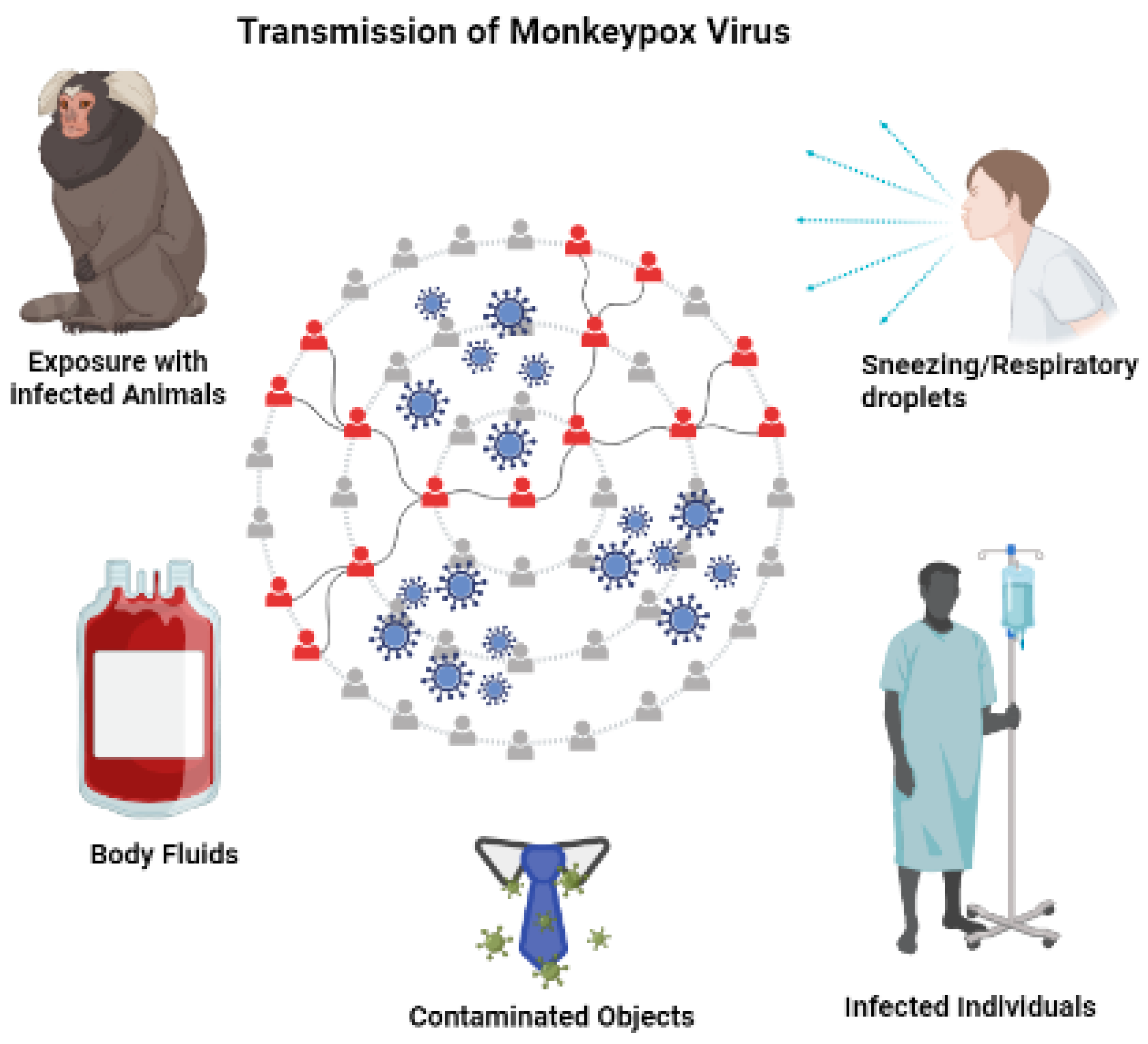
4. Transmission
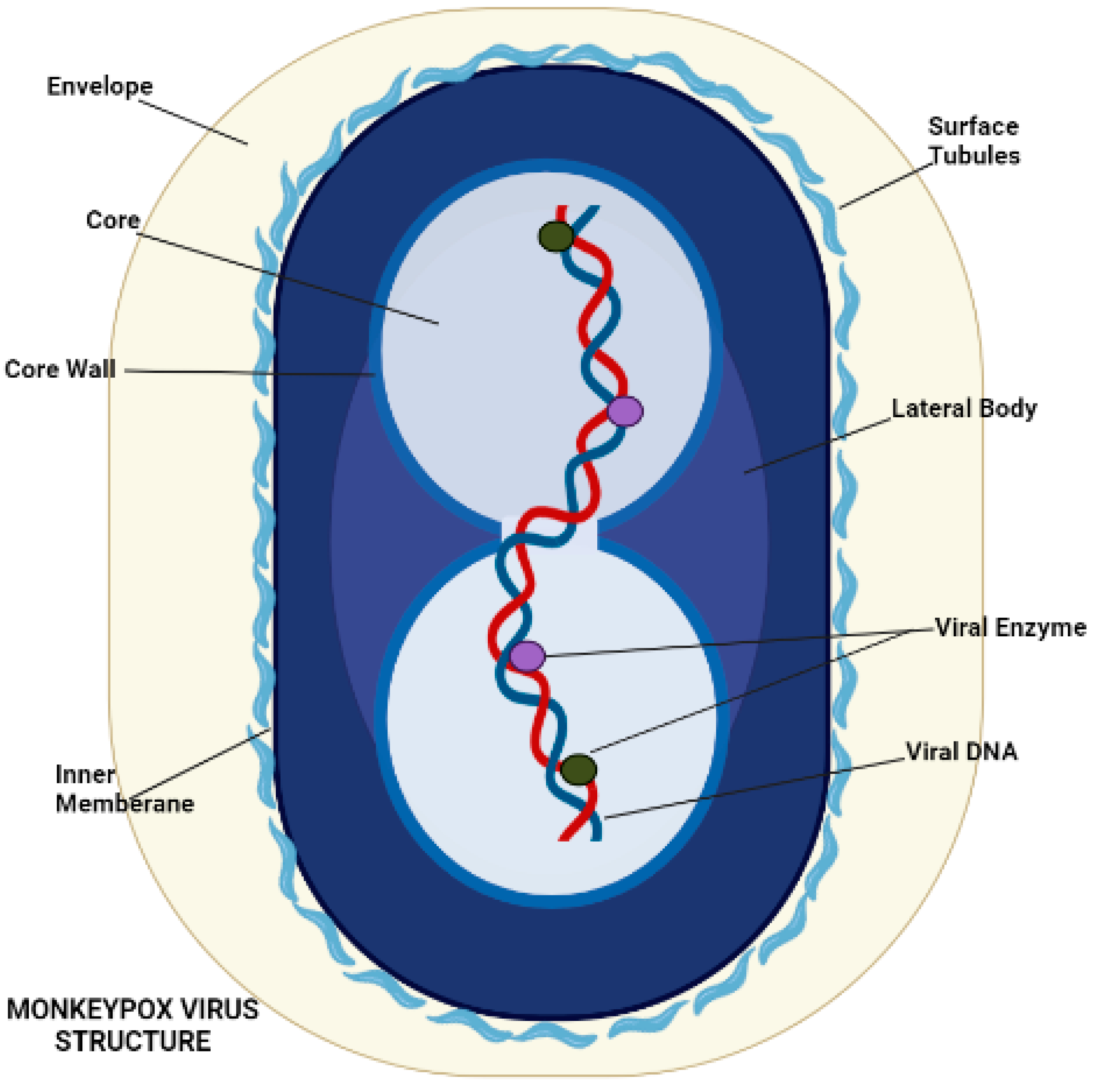
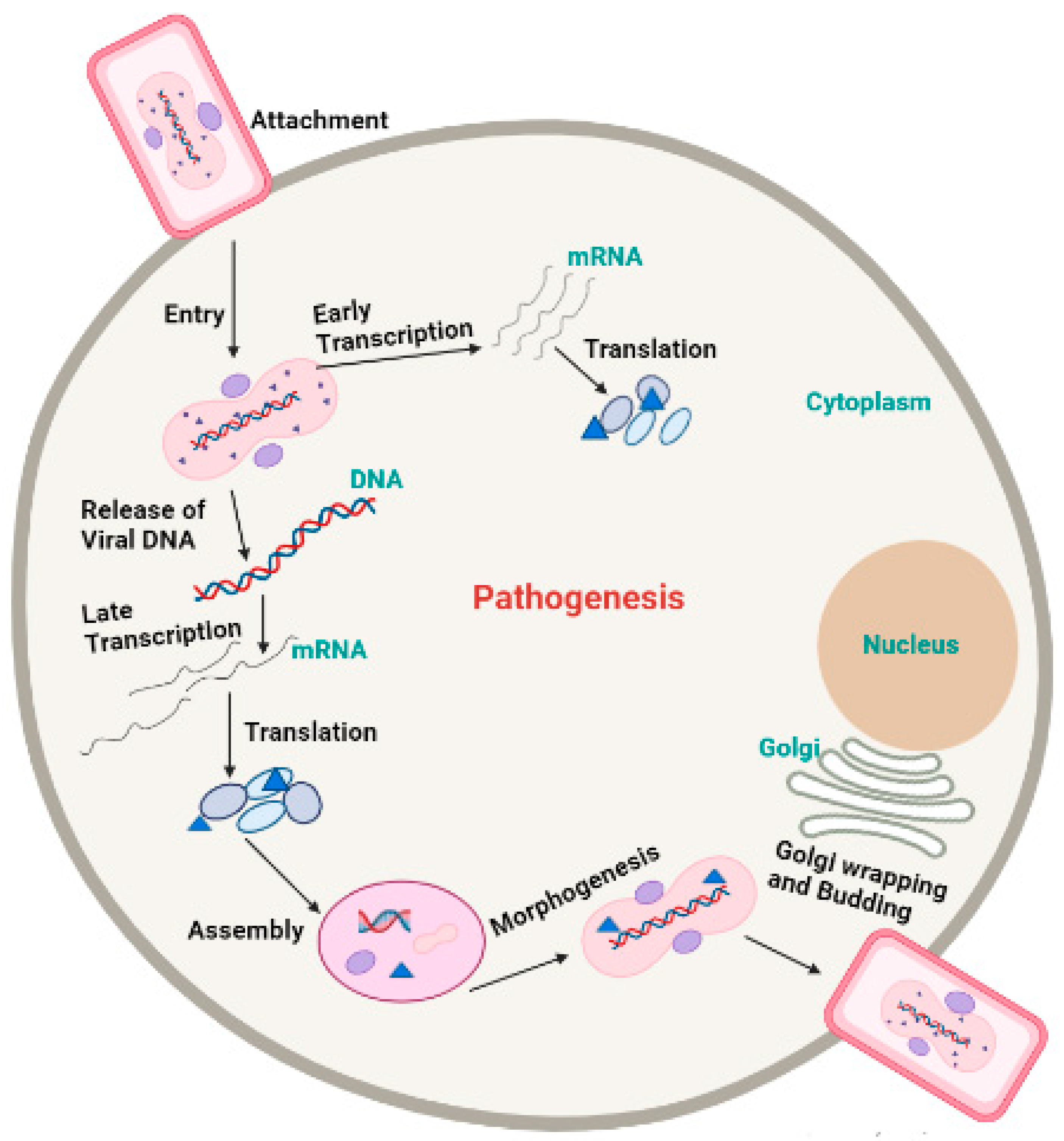
5. Morphology and Pathogenesis of Monkeypox
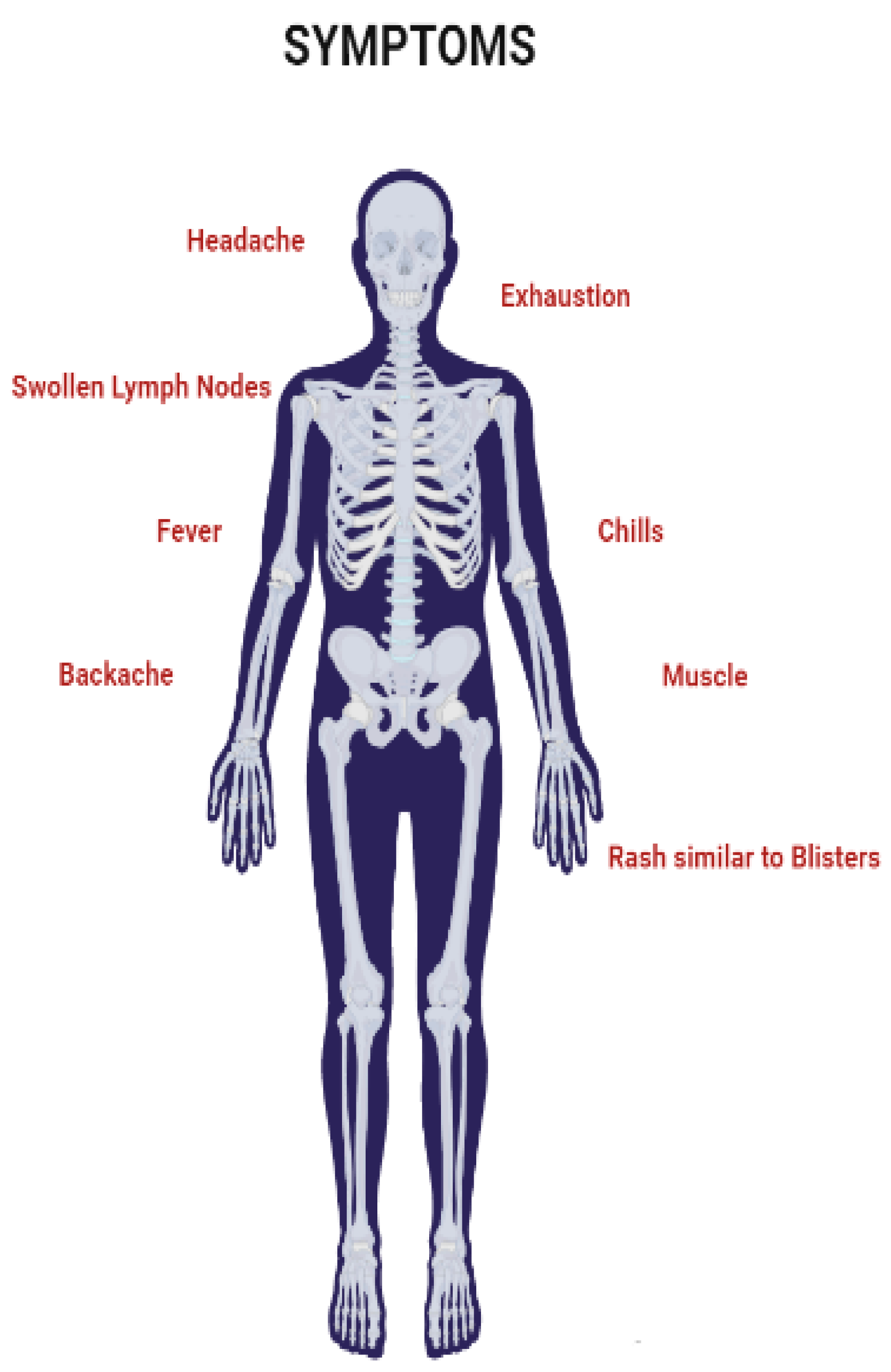
6. Symptoms
7. Diagnostic Tests
8. Immune Response
9. Prevention, Treatment, and Therapeutics
9.1. Vaccine
9.2. Vaccinia Immune Globulin Intravenous
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pal, M.; Mengstie, F.; Kandi, V. Epidemiology, Diagnosis, and Control of Monkeypox Disease. Acta Path. Mtcrobiol. Scand. 1959, 46, 156–176. [Google Scholar]
- Khattak, S.; Qaisar, M.; Zaman, S.; Khan, T.A.; Ali, Y.; Wu, D.D.; Ji, X.Y. Monkeypox virus preparation in Paki-stan-Next viral zoonotic disease outbreak after COVID-19? Biomed. Lett. 2022, 8, 196–201. [Google Scholar]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar]
- Kindrachuk, J.; Arsenault, R.; Kusalik, A.; Kindrachuk, K.N.; Trost, B.; Napper, S.; Jahrling, P.B.; Blaney, J.E. Systems Kinomics Demonstrates Congo Basin Monkeypox Virus Infection Selectively Modulates Host Cell Signaling Responses as Compared to West African Monkeypox Virus. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef]
- Jezek, Z.; Szczeniowski, M.; Paluku, K.M.; Mutombo, M. Human Monkeypox: Clinical Features of 282 Patients. J. Infect. Dis. 1987, 156, 293–298. [Google Scholar] [CrossRef]
- Kmiec, D.; Kirchhoff, F. Monkeypox: A new threat? Int. J. Mol. Sci. 2022, 23, 7866. [Google Scholar] [CrossRef]
- Anwar, F.; Waris, A. Monkeypox virus outbreak: A brief timeline. New Microbes New Infect. 2022, 48, 101004. [Google Scholar] [CrossRef] [PubMed]
- Nolen, L.D.; Tamfum, J.-J.M.; Kabamba, J.; Likofata, J.; Katomba, J.; McCollum, A.M.; Monroe, B.; Kalemba, L.; Mukadi, D.; Bomponda, P.L.; et al. Introduction of Monkeypox into a Community and Household: Risk Factors and Zoonotic Reservoirs in the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2015, 93, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Ng, O.T.; Lee, V.; Marimuthu, K.; Vasoo, S.; Chan, G.; Lin, R.T.P.; Leo, Y.S. A case of imported Monkeypox in Singapore. Lancet Infect. Dis. 2019, 19, 1166. [Google Scholar] [CrossRef]
- Mauldin, M.R.; McCollum, A.M.; Nakazawa, Y.J.; Mandra, A.; Whitehouse, E.R.; Davidson, W.; Zhao, H.; Gao, J.; Li, Y.; Doty, J.; et al. Exportation of Monkeypox Virus From the African Continent. J. Infect. Dis. 2020, 225, 1367–1376. [Google Scholar] [CrossRef]
- Díez-Fuertes, F.; Iglesias-Caballero, M.; Garcia-Perez, J.; Monzón, S.; Jiménez, P.; Varona, S.; Cuesta, I.; Zaballos, A.; Jimenez, M.; Checa, L.; et al. A founder effect led early SARS-CoV-2 transmission in Spain. J. Virol. 2021, 95, e01583-20. [Google Scholar] [CrossRef]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A tale of two clades: Monkeypox viruses. J. Gen. Virol. 2005, 86, 2661–2672. [Google Scholar] [CrossRef]
- Doshi, R.H.; Guagliardo, S.A.J.; Doty, J.B.; Babeaux, A.D.; Matheny, A.; Burgado, J.; Townsend, M.B.; Morgan, C.N.; Satheshkumar, P.S.; Ndakala, N.; et al. Epidemiologic and Ecologic Investigations of Monkeypox, Likouala Department, Republic of the Congo, 2017. Emerg. Infect. Dis. 2019, 25, 281–289. [Google Scholar] [CrossRef]
- Ray, S.; Maunsell, J. Different Origins of Gamma Rhythm and High-Gamma Activity in Macaque Visual Cortex. PLoS Biol. 2011, 9, e1000610. [Google Scholar] [CrossRef]
- Heymann, D.L.; Szczeniowski, M.; Esteves, K. Re-emergence of monkeypox in Africa: A review of the past six years. Br. Med Bull. 1998, 54, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Hutin, Y.J.; Williams, R.J.; Malfait, P.; Pebody, R.; Loparev, V.N.; Ropp, S.L.; Rodriguez, M.; Knight, J.C.; Tshioko, F.K.; Khan, A.S.; et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The Detection of Monkeypox in Humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef]
- Sale, T.A.; Melski, J.W.; Stratman, E.J. Monkeypox: An epidemiologic and clinical comparison of African and US disease. J. Am. Acad. Dermatol. 2006, 55, 478–481. [Google Scholar] [CrossRef]
- Formenty, P.; Muntasir, M.O.; Damon, I.; Chowdhary, V.; Opoka, M.L.; Monimart, C.; Mutasim, E.M.; Manuguerra, J.-C.; Davidson, W.B.; Karem, K.L.; et al. Human Monkeypox Outbreak Caused by Novel Virus Belonging to Congo Basin Clade, Sudan, 2005. Emerg. Infect. Dis. 2010, 16, 1539–1545. [Google Scholar] [CrossRef]
- Rimoin, A.W.; Mulembakani, P.M.; Johnston, S.C.; Lloyd Smith, J.O.; Kisalu, N.K.; Kinkela, T.L.; Blumberg, S.; Thomassen, H.A.; Pike, B.L.; Fair, J.N.; et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. USA 2010, 107, 16262–16267. [Google Scholar] [CrossRef]
- Chapman, J.L.; Nichols, D.K.; Martinez, M.J.; Raymond, J.W. Animal Models of Orthopoxvirus Infection. Vet. Pathol. 2010, 47, 852–870. [Google Scholar] [CrossRef] [PubMed]
- Zaucha, G.M.; Jahrling, P.B.; Geisbert, T.W.; Swearengen, J.R.; Hensley, L. The pathology of experimental aerosolized mon-keypox virus infection in cynomolgus monkeys (Macaca fascicularis). Lab. Investig. 2001, 81, 1581–1600. [Google Scholar] [CrossRef] [PubMed]
- Hahon, N.; Wilson, B.J. Pathogenesis of variola in Macaca irus monkeys. Am. J. Epidemiol. 1960, 71, 69–80. [Google Scholar] [CrossRef]
- Rubins, K.H.; Hensley, L.E.; Relman, D.A.; Brown, P.O. Stunned Silence: Gene Expression Programs in Human Cells Infected with Monkeypox or Vaccinia Virus. PLoS ONE 2011, 6, e15615. [Google Scholar] [CrossRef]
- Joklik, W.K. The poxviruses. Bacteriol. Rev. 1966, 30, 33–66. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommends New Name for Monkeypox Disease; WHO News Release; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Haller, S.L.; Peng, C.; McFadden, G.; Rothenburg, S. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. 2014, 21, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.; Irausquin, S.; Friedman, R. The evolutionary biology of poxviruses. Infect. Genet. Evol. 2010, 10, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Norby, R.J.; Ledford, J.; Reilly, C.D.; Miller, N.E.; O’Neill, E.G. Fine-root production dominates response of a deciduous forest to atmospheric CO 2 enrichment. Proc. Natl. Acad. Sci. USA 2004, 101, 9689–9693. [Google Scholar] [CrossRef]
- Johnson, R.F.; Dyall, J.; Ragland, D.R.; Huzella, L.; Byrum, R.; Jett, C.; St Claire, M.; Smith, A.L.; Paragas, J.; Blaney, J.E.; et al. Comparative Analysis of Monkeypox Virus Infection of Cynomolgus Macaques by the Intravenous or Intrabronchial Inoculation Route. J. Virol. 2011, 85, 2112–2125. [Google Scholar] [CrossRef]
- Saijo, M.; Ami, Y.; Suzaki, Y.; Nagata, N.; Iwata, N.; Hasegawa, H.; Iizuka, I.; Shiota, T.; Sakai, K.; Ogata, M.; et al. Virulence and pathophysiology of the Congo Basin and West African strains of monkeypox virus in non-human primates. J. Gen. Virol. 2009, 90, 2266–2271. [Google Scholar] [CrossRef]
- McConnell, S.; Hickman, R.L.; Wooding Jr, W.L.; Huxsoll, D.L. Monkeypox: Experimental infection in chimpanzee (Pan satyrus) and immunization with vaccinia virus. Am. J. Vet. Res. 1968, 29, 1675–1680. [Google Scholar] [PubMed]
- Esposito, J.J.; Sammons, S.A.; Frace, A.M.; Osborne, J.D.; Olsen-Rasmussen, M.; Zhang, M.; Govil, D.; Damon, I.K.; Kline, R.; Laker, M.; et al. Genome Sequence Diversity and Clues to the Evolution of Variola (Smallpox) Virus. Science 2006, 313, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.E.; Ng, O.T.; Ho, Z.J.; Mak, T.M.; Marimuthu, K.; Vasoo, S.; Yeo, T.W.; Ng, Y.K.; Cui, L.; Ferdous, Z.; et al. Im-ported Monkeypox, Singapore. Emerg. Infect. Dis. 2020, 26, 1826. [Google Scholar] [CrossRef]
- Vaughan, A.; Aarons, E.; Astbury, J.; Balasegaram, S.; Beadsworth, M.; Beck, C.R.; Chand, M.; O’Connor, C.; Dunning, J.; Ghebrehewet, S.; et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Eurosurveillance 2018, 23, 1800509. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.; Heymann, D.; Brown, C.S.; Edmunds, W.J.; Elsgaard, J.; Fine, P.; Hochrein, H.; Hoff, N.A.; Green, A.; Ihekweazu, C.; et al. Human monkeypox—After 40 years, an unintended consequence of smallpox eradication. Vaccine 2020, 38, 5077–5081. [Google Scholar] [CrossRef]
- Bartlett, J.G. Review of Literature: General Infectious Diseases; Canadian Press: Toronto, ON, Canada, 2002. [Google Scholar]
- Hutson, C.L.; Olson, V.A.; Carroll, D.S.; Abel, J.A.; Hughes, C.M.; Braden, Z.H.; Weiss, S.; Self, J.; Osorio, J.E.; Hudson, P.N.; et al. A prairie dog animal model of systemic orthopoxvirus disease using West African and Congo Basin strains of monkeypox virus. J. Gen. Virol. 2009, 90, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Rathish, B.; Zahra, F. Mpox (Monkeypox); StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Vivancos, R.; Anderson, C.; Blomquist, P.; Balasegaram, S.; Bell, A.; Bishop, L.; Brown, C.S.; Chow, Y.; Edeghere, O.; Florence, I.; et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Eurosurveillance 2022, 27, 2200422. [Google Scholar] [CrossRef]
- McCollum, A.M.; Damon, I.K. Human monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [CrossRef]
- Iñigo Martínez, J.; Gil Montalbán, E.; Jiménez Bueno, S.; Martín Martínez, F.; Nieto Juliá, A.; Sánchez Díaz, J.; García Marín, N.; Córdoba Deorador, E.; Nunziata Forte, A.; Alonso García, M.; et al. Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Eurosurveillance 2022, 27, 2200471. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, S.; Bondy, L.; Hanage, W.P. Monkeypox Virus Infections in Humans. Clin. Microbiol. Rev. 2022, 35, e00092-22. [Google Scholar] [CrossRef]
- Selb, R.; Werber, D.; Falkenhorst, G.; Steffen, G.; Lachmann, R.; Ruscher, C.; McFarland, S.; Bartel, A.; Hemmers, L.; Koppe, U.; et al. A shift from travel-associated cases to autochthonous transmission with Berlin as epicentre of the monkeypox outbreak in Germany, May to June 2022. Eurosurveillance 2022, 27, 2200499. [Google Scholar] [CrossRef]
- Ogoina, D.; Yinka-Ogunleye, A. Sexual history of human monkeypox patients seen at a tertiary hospital in Bayelsa, Nigeria. Int. J. STD AIDS 2022, 33, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Resch, W.; Hixson, K.K.; Moore, R.J.; Lipton, M.S.; Moss, B. Protein composition of the vaccinia virus mature virion. Virology 2006, 358, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Manes, N.P.; Estep, R.D.; Mottaz, H.M.; Moore, R.J.; Clauss, T.R.W.; Monroe, M.E.; Du, X.; Adkins, J.N.; Wong, S.W.; Smith, R.D. Comparative Proteomics of Human Monkeypox and Vaccinia Intracellular Mature and Extracellular Enveloped Virions. J. Proteome Res. 2008, 7, 960–968. [Google Scholar] [CrossRef]
- Shchelkunov, S.N.; Totmenin, A.V.; Babkin, I.V.; Safronov, P.F.; Ryazankina, O.I.; Petrov, N.A.; Gutorov, V.V.; Uvarova, E.; Mikheev, M.V.; Sisler, J.R.; et al. Human monkeypox and smallpox viruses: Genomic comparison. FEBS Lett. 2001, 509, 66–70. [Google Scholar] [CrossRef]
- Kugelman, J.R.; Johnston, S.C.; Mulembakani, P.M.; Kisalu, N.; Lee, M.S.; Koroleva, G.; McCarthy, S.E.; Gestole, M.C.; Wolfe, N.D.; Fair, J.N.; et al. Genomic Variability of Monkeypox Virus among Humans, Democratic Republic of the Congo. Emerg. Infect. Dis. 2014, 20, 232–239. [Google Scholar] [CrossRef]
- Lalani, A.S.; Graham, K.; Mossman, K.; Rajarathnam, K.; Clark-Lewis, I.; Kelvin, D.; McFadden, G. The purified myxoma virus gamma interferon receptor homolog M-T7 interacts with the heparin-binding domains of chemokines. J. Virol. 1997, 71, 4356–4363. [Google Scholar] [CrossRef]
- Moss, B. Membrane fusion during poxvirus entry. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Challberg, M.; Englund, P. Purification and properties of the deoxyribonucleic acid polymerase induced by vaccinia virus. J. Biol. Chem. 1979, 254, 7812–7819. [Google Scholar] [CrossRef]
- Smith, G.L.; Vanderplasschen, A.; Law, M. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 2002, 83, 2915–2931. [Google Scholar] [CrossRef]
- Pickup, D.J. Extracellular Virions: The Advance Guard of Poxvirus Infections. PLoS Pathog. 2015, 11, e1004904. [Google Scholar] [CrossRef]
- Farahat, R.A.; Sah, R.; El-Sakka, A.A.; Benmelouka, A.Y.; Kundu, M.; Labieb, F.; Shaheen, R.S.; Abdelaal, A.; Abdelazeem, B.; Rodriguez-Morales, A.J.; et al. Human monkeypox disease (MPX). Infez. Med. 2022, 30, 372–391. [Google Scholar]
- Hammerschlag, Y.; MacLeod, G.; Papadakis, G.; Sanchez, A.A.; Druce, J.; Taiaroa, G.; Savic, I.; Mumford, J.; Roberts, J.; Caly, L.; et al. Monkeypox infection presenting as genital rash, Australia, May 2022. Eurosurveillance 2022, 27, 2200411. [Google Scholar] [CrossRef] [PubMed]
- Bjekic, M.; Markovic, M.; Dejanovic, L. Genital rash as an initial presentation of monkeypox. An. Bras. Dermatol. 2023, 98, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Martínez, Y.; Rodríguez-Morales, A.J.; Franco-Paredes, C.; Chastain, D.B.; Gharamti, A.A.; Barahona, L.V.; Henao-Martínez, A.F. Monkeypox—A description of the clinical progression of skin lesions: A case report from Colorado, USA. Ther. Adv. Infect. Dis. 2022, 9. [Google Scholar] [CrossRef]
- Griffiths-Acha, J.; Vela-Ganuza, M.; Sarró-Fuente, C.; López-Estebaranz, J.L. Monkeypox: A new differential diagnosis when addressing genital ulcer disease. Br. J. Dermatol. 2022, 187, 1050–1052. [Google Scholar] [CrossRef]
- Minhaj, F.S.; Ogale, Y.P.; Whitehill, F.; Schultz, J.; Foote, M.; Davidson, W.; Hughes, C.M.; Wilkins, K.; Bachmann, L.; Chate-lain, R.; et al. Monkeypox outbreak—Nine states, May 2022: Weekly/June 10, 2022/71 (23); 764–769. Am. J. Transpl. 2022, 22, 2104–2110. [Google Scholar] [CrossRef]
- Soriano, V.; Corral, O. International outbreak of monkeypox in men having sex with men. Aids Rev. 2022, 24. [Google Scholar] [CrossRef] [PubMed]
- Malacrida, L.M.; Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, Z.; Ladnyi, I.D. Smallpox and Its Eradication; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, C.; Chuai, X.; Chiu, S. Monkeypox virus: A re-emergent threat to humans. Virol. Sin. 2022, 37, 477–482. [Google Scholar] [CrossRef]
- Lum, F.M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.; Lye, D.C.; Rénia, L.; Ng, L.F. Monkeypox: Disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef]
- Arndt, W.D.; Cotsmire, S.; Trainor, K.; Harrington, H.; Hauns, K.; Kibler, K.V.; Huynh, T.P.; Jacobs, B.L. Evasion of the Innate Immune Type I Interferon System by Monkeypox Virus. J. Virol. 2015, 89, 10489–10499. [Google Scholar] [CrossRef]
- Esteban, D.J.; Nuara, A.A.; Buller, R.M.L. Interleukin-18 and glycosaminoglycan binding by a protein encoded by Variola virus. J. Gen. Virol. 2004, 85, 1291–1299. [Google Scholar] [CrossRef]
- Takata, M.A.; Gonçalves-Carneiro, D.; Zang, T.M.; Soll, S.J.; York, A.; Blanco-Melo, D.; Bieniasz, P.D. CG dinucleotide sup-pression enables antiviral defence targeting non-self RNA. Nature 2017, 550, 124–127. [Google Scholar] [CrossRef]
- Kmiec, D.; Nchioua, R.; Sherrill-Mix, S.; Stürzel, C.M.; Heusinger, E.; Braun, E.; Gondim, M.V.P.; Hotter, D.; Sparrer, K.M.J.; Hahn, B.H.; et al. CpG Frequency in the 5′ Third of the env Gene Determines Sensitivity of Primary HIV-1 Strains to the Zinc-Finger Antiviral Protein. Mbio 2020, 11, e02903-19. [Google Scholar] [CrossRef]
- Nchioua, R.; Kmiec, D.; Müller, J.A.; Conzelmann, C.; Groß, R.; Swanson, C.M.; Neil, S.J.; Stenger, S.; Sauter, D.; Münch, J.; et al. SARS-CoV-2 is restricted by zinc finger antiviral protein despite preadaptation to the low-CpG environment in humans. MBio 2020, 11, e01930-20. [Google Scholar] [CrossRef]
- Peng, C.; Wyatt, L.S.; Glushakow-Smith, S.G.; Lal-Nag, M.; Weisberg, A.S.; Moss, B. Zinc-finger antiviral protein (ZAP) is a restriction factor for replication of modified vaccinia virus Ankara (MVA) in human cells. PLoS Pathog. 2020, 16, e1008845. [Google Scholar] [CrossRef] [PubMed]
- Hudson, P.N.; Self, J.; Weiss, S.; Braden, Z.; Xiao, Y.; Girgis, N.M.; Emerson, G.; Hughes, C.; Sammons, S.A.; Isaacs, S.N.; et al. Elucidating the Role of the Complement Control Protein in Monkeypox Pathogenicity. PLoS ONE 2012, 7, e35086. [Google Scholar] [CrossRef]
- Hammarlund, E.; Dasgupta, A.; Pinilla, C.; Norori, P.; Früh, K.; Slifka, M.K. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc. Natl. Acad. Sci. USA 2008, 105, 14567–14572. [Google Scholar] [CrossRef]
- Kinnunen, P.M.; Holopainen, J.M.; Hemmilä, H.; Piiparinen, H.; Sironen, T.; Kivelä, T.; Virtanen, J.; Niemimaa, J.; Nikkari, S.; Järvinen, A.; et al. Severe Ocular Cowpox in a Human, Finland. Emerg. Infect. Dis. 2015, 21, 2261–2263. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, E.R.; Rao, A.K.; Yon, C.Y.; Patricia, A.Y.; Griffin, M.; Gorman, S.; Angel, K.A.; McDonald, E.C.; Manlutac, A.L.; De Perio, M.A.; et al. Novel treatment of a vaccinia virus infection from an occupational needlestick—San Diego, California, 2019. Morb. Mortal. Wkly. Rep. 2019, 68, 943. [Google Scholar] [CrossRef] [PubMed]
- Mileto, D.; Riva, A.; Cutrera, M.; Moschese, D.; Mancon, A.; Meroni, L.; Giacomelli, A.; Bestetti, G.; Rizzardini, G.; Gismondo, M.R.; et al. New challenges in human monkeypox outside Africa: A review and case report from Italy. Travel Med. Infect. Dis. 2022, 49, 102386. [Google Scholar] [CrossRef]
- Sosa, L.E.; Njie, G.J.; Lobato, M.N.; Morris, S.B.; Buchta, W.; Casey, M.L.; Goswami, N.D.; Gruden, M.; Hurst, B.J.; Khan, A.R.; et al. Tuberculosis screening, testing, and treatment of US health care personnel: Recommendations from the National Tuberculosis Controllers Association and CDC, 2019. Am. J. Transplant. 2019, 19, 2383–2387. [Google Scholar] [CrossRef]
- Patauner, F.; Gallo, R.; Durante-Mangoni, E. Monkeypox infection: An update for the practicing physician: Monkeypox infection. Eur. J. Intern. Med. 2022, 104, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.J.; Lane, J.M.; Davis, K.A.; Stewart, J.J.; Crouch, H.K.; Florez, C.E.; Hospenthal, D.R. Clinical Efficacy of Intramuscular Vaccinia Immune Globulin: A Literature Review. Clin. Infect. Dis. 2004, 39, 819–826. [Google Scholar] [CrossRef]
- Vora, S.; Damon, I.; Fulginiti, V.; Weber, S.G.; Kahana, M.; Stein, S.L.; Gerber, S.I.; Garcia-Houchins, S.; Lederman, E.; Hruby, D.; et al. Severe Eczema Vaccinatum in a Household Contact of a Smallpox Vaccinee. Clin. Infect. Dis. 2008, 46, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, D.A.; Fisher, R.D.; Montgomery, J.R.; Davidson, W.; Yu, P.A.; Yu, Y.C.; Burgado, J.; Wilkins, K.; Petersen, B.W.; Okulicz, J.F. Preemptive Tecovirimat Use in an Active Duty Service Member Who Presented with Acute Myeloid Leukemia after Smallpox Vaccination. Clin. Infect. Dis. 2019, 69, 2205–2207. [Google Scholar] [CrossRef]
| Treatments | Route | Dosing | Mode of Action | Common Adverse Events | Ref. |
|---|---|---|---|---|---|
| Tecovirimat | PO, IV (Approved in May 2022) | Adults: 600 mg twice daily for 14 days; pediatrics (13 kg or more), if 13 kg to less than 25 kg: 200 mg BID for 14 days. If 25 kg to less than 40 kg: 400 mg twice daily for 14 days. If 40 kg or more: 600 mg twice daily for 14 days | Orthopoxviral VP37 envelope wrapping protein inhibitor | Headache, nausea, abdominal pain, vomiting. Infusion-site reactions may occur with IV form | [73,74,75,76,77] |
| Brincidofovir | PO (tablets, Oral suspension) | Adults weighing ≥ 48 kg: 200 mg once weekly for two doses; adults and pediatric patients weighing ≥10 kg to less than 48 kg: 4 mg/kg of the oral suspension once weekly for two doses; for pediatrics weighing less than 10 kg, the dose is 6 mg/kg of the oral suspension once weekly for two doses | Phosphorylated to the active metabolite, cidofovir diphosphate, which selectively inhibits Orthopoxvirus DNA polymerase-mediated viral DNA synthesis | Diarrhea, nausea, vomiting, and abdominal pain | [67,68,69,70,71] |
| Cidofovir | IV | 5 mg/kg once weekly for two weeks, followed by 5 mg/kg IV once every other week | Undergoes cellular phosphorylation, then selectively inhibits Orthopoxvirus DNA polymerase-mediated viral DNA synthesis | Decreased serum bicarbonate, proteinuria, neutropenia, infection, hypotony of the eye, iritis, uveitis, nephrotoxicity, fever | [66] |
| Vaccinia immune globulin | IV | 6000 U/kg as soon as symptoms appear; may be repeated based on the severity of symptoms and response to treatment; 9000 U/kg may be considered if the patient does not respond to the initial dose | Antibodies obtained from pooled human the plasma of individuals immunized with the smallpox vaccine provide passive immunity | Headache, nausea, rigors, dizziness | [78,79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwar, F.; Haider, F.; Khan, S.; Ahmad, I.; Ahmed, N.; Imran, M.; Rashid, S.; Ren, Z.-G.; Khattak, S.; Ji, X.-Y. Clinical Manifestation, Transmission, Pathogenesis, and Diagnosis of Monkeypox Virus: A Comprehensive Review. Life 2023, 13, 522. https://doi.org/10.3390/life13020522
Anwar F, Haider F, Khan S, Ahmad I, Ahmed N, Imran M, Rashid S, Ren Z-G, Khattak S, Ji X-Y. Clinical Manifestation, Transmission, Pathogenesis, and Diagnosis of Monkeypox Virus: A Comprehensive Review. Life. 2023; 13(2):522. https://doi.org/10.3390/life13020522
Chicago/Turabian StyleAnwar, Faheem, Fatima Haider, Sarmir Khan, Ibrar Ahmad, Naveed Ahmed, Muhammad Imran, Summya Rashid, Zhi-Guang Ren, Saadullah Khattak, and Xin-Ying Ji. 2023. "Clinical Manifestation, Transmission, Pathogenesis, and Diagnosis of Monkeypox Virus: A Comprehensive Review" Life 13, no. 2: 522. https://doi.org/10.3390/life13020522
APA StyleAnwar, F., Haider, F., Khan, S., Ahmad, I., Ahmed, N., Imran, M., Rashid, S., Ren, Z.-G., Khattak, S., & Ji, X.-Y. (2023). Clinical Manifestation, Transmission, Pathogenesis, and Diagnosis of Monkeypox Virus: A Comprehensive Review. Life, 13(2), 522. https://doi.org/10.3390/life13020522









