Microwave-Assisted Hydrodistillation of Essential Oil from Plectranthus amboinicus: Evaluation of Its Antifungal Effect and Chemical Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
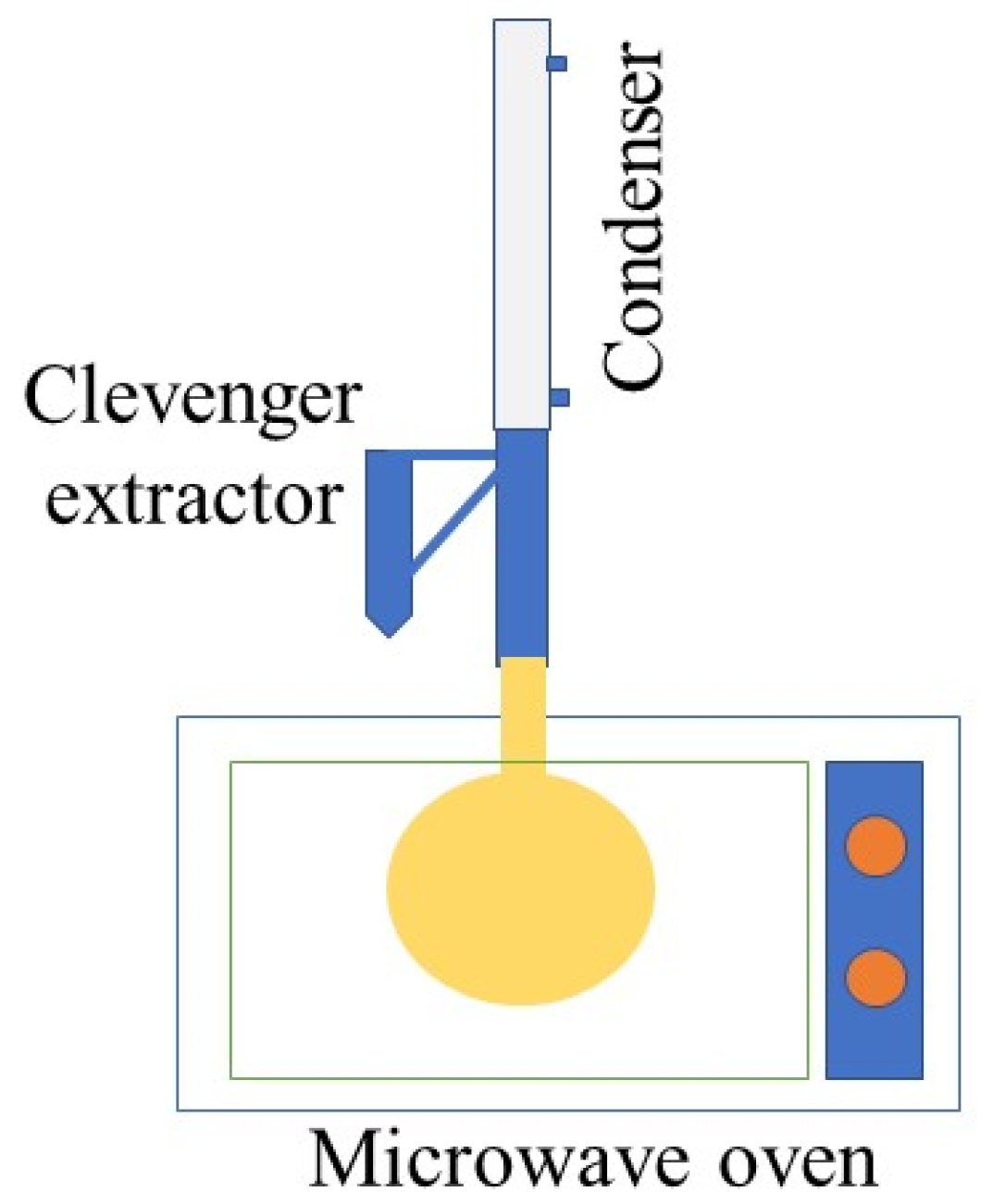
2.3. EOs Extraction
2.4. Antifungal Activity of EOs
2.5. GC-MS and Identification of Volatile Compounds
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayeni, E.A.; Gong, Y.; Yuan, H.; Hu, Y.; Bai, X.; Liao, X. Medicinal plants for anti-neurodegenerative diseases in West Africa. J. Ethnopharmacol. 2022, 285, 114468. [Google Scholar] [CrossRef] [PubMed]
- Chebbac, K.; Ghneim, H.K.; El Moussaoui, A.; Bourhia, M.; El Barnossi, A.; Benziane Ouaritini, Z.; Salamatullah, A.M.; Alzahrani, A.; Aboul-Soud, M.A.M.; Giesy, J.P.; et al. Antioxidant and Antimicrobial Activities of Chemically-Characterized Essential Oil from Artemisia aragonensis Lam. against Drug-Resistant Microbes. Molecules 2022, 27, 1136. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, C.; Alabouvette, C.; Edel-Hermann, V.; Robert, F.; Steinberg, C. Biological control of ornamental plant diseases caused by Fusarium oxysporum: A review. Biol. Control 2016, 101, 17–30. [Google Scholar] [CrossRef]
- Joshi, R. A review of Fusarium oxysporum on its plant interaction and industrial use. J. Med. Plants Stud. 2018, 6, 112–115. [Google Scholar] [CrossRef]
- Symoneaux, R.; Segond, N.; Maignant, A. Sensory and consumer sciences applicated on ornamental plants. In Nonfood Sensory Practices, 1st ed.; Pensé-Lhéritier, A.M., Delarue, J., Eds.; Woodhead Publishing: Sawston, UK, 2022; Volume 2, pp. 291–311. [Google Scholar]
- Gullino, M.L.; Daughtrey, M.L.; Garibaldi, A.; Elmer, W.H. Fusarium wilts of ornamental crops and their management. Crop Prot. 2015, 73, 50–59. [Google Scholar] [CrossRef]
- Hassan, M.A.; El-Saadony, M.T.; Mostafa, N.G.; El-Tahan, A.M.; Mesiha, P.K.; El-Saadony, F.M.; Hassan, A.; El-Shehawi, A.; Ashry, N.M. The use of previous crops as sustainable and eco-friendly management to fight Fusarium oxysporum in sesame plants. Saudi J. Biol. Sci. 2021, 28, 5849–5859. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Basaid, K.; Chebli, B.; Mayad, E.H.; Furze, J.N.; Bouharroud, R.; Krier, F.; Paulitz, T. Biological activities of essential oils and lipopeptides applied to control plant pests and diseases: A review. Int. J. Pest Manag. 2021, 67, 155–177. [Google Scholar] [CrossRef]
- Sharma, A.; Rajendran, S.; Srivastava, A.; Sharma, S.; Kundu, B. Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J. Biosci. Bioeng. 2017, 123, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Manganyi, M.C.; Regnier, T.; Olivier, E.I. Antimicrobial activities of selected essential oils against Fusarium oxysporum isolates and their biofilms. S. Afr. J. Bot. 2015, 99, 115–121. [Google Scholar] [CrossRef]
- Golmakani, M.T.; Rezaei, K. Comparison of microwave-assisted hydrodistillation with the traditional hydrodistillation method in the extraction of essential oils from Thymus vulgaris L. Food Chem. 2008, 109, 925–930. [Google Scholar] [CrossRef]
- Sharma, P.; Zalpouri, R. Microwave-assisted extraction of proteins and carbohydrates from marine resources. Innov. Emerg. Technol. Bio-Mar. Food Sect. 2022, 12, 361–374. [Google Scholar]
- Pérez-Serradilla, J.A.; De Castro, M.L. Microwave-assisted extraction of phenolic compounds from wine lees and spray-drying of the extract. Food Chem. 2011, 124, 1652–1659. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; Juárez-Becerra, G.P.; Sosa Morales, M.E.; López-Malo, A. Microwave-assisted extraction of essential oils from herbs. J. Microw. Power Electromagn. Energy 2013, 47, 63–72. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Cui, G.; Wei, M.; Zou, Z.; Ni, H. Efficient extraction of essential oil from Cinnamomum burmannii leaves using enzymolysis pretreatment and followed by microwave-assisted method. LWT 2021, 147, 111497. [Google Scholar] [CrossRef]
- Zhao, R.; Ben, A.; Wei, M.; Ruan, M.; Gu, H.; Yang, L. Essential oil obtained from Thlaspi arvense L. leaves and seeds using microwave-assisted hydrodistillation and extraction in situ by vegetable oil and its antifungal activity against Penicillium expansum. LWT 2022, 165, 113718. [Google Scholar]
- Davari, M.; Ezazi, R. Mycelial inhibitory effects of antagonistic fungi, plant essential oils and propolis against five phytopathogenic Fusarium species. Arch. Microbiol. 2022, 204, 480. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Cerón-Bustamante, M.; Ward, T.J.; Kelly, A.; Vaughan, M.M.; McCormick, S.P.; Cowger, C.; Villaseñor-Mird, E.; Ayala-Escobara, V.; Nava-Díaz, C. Regional differences in the composition of Fusarium head blight pathogens and mycotoxins associated with wheat in Mexico. Int. J. Food Microbiol. 2018, 273, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Jasso de Rodríguez, D.; Rodríguez, R.G.; Hernández, F.D.N.; Aguilar, C.N.G.; Sáenz, A.G.; Villareal, J.A.Q.; Moreno, L.E.Z. In vitro antifungal activity of extracts of Mexican Chihuahuan Desert plants against postharvest fruit fungi. Ind. Crops Prod. 2011, 34, 960–966. [Google Scholar] [CrossRef]
- Vincent, J.M. Distortion of fungal hyphae in the presence of certain inhibitors. Nature 1947, 159, 850. [Google Scholar] [CrossRef] [PubMed]
- Priya, N.V.; Vinitha, U.G.; Sundaram, M.M. Preparation of chitosan-based antimicrobial active food packaging film incorporated with Plectranthus amboinicus essential oil. Biocatal. Agric. Biotechnol. 2021, 34, 102021. [Google Scholar] [CrossRef]
- Sawant, S.; Baldwin, T.C.; Khan, H.; Rahman, A. Evaluation of the effect of leaf development in Plectranthus amboinicus L. on antimicrobial activity and virulence factors of Pseudomonas aeruginosa PAO1 and Staphylococcus aureus NCTC8325. Curr. Microbiol. 2023, 80, 24. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.H.; Sharma, R.; Susanto, D.; Di Maso, M.; Kwong, E. Microwave-assisted extraction of active pharmaceutical ingredient from solid dosage forms. J. Chromatogr. A 2007, 1156, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Linde, J.H.; Combrinck, S.; Regnier, T.J.C.; Virijevic, S. Chemical composition and antifungal activity of the essential oils of Lippia rehmannii from South Africa. S. Afr. J. Bot. 2010, 76, 37–42. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Maisanaba, S.; Puerto, M.; Prieto, A.I.; Gutierrez-Praena, D.; Camean, A.M. In vitro toxicological evaluation of essential oils and their main compounds used in active food packaging: A review. Food Chem. Toxicol. 2015, 81, 9–27. [Google Scholar] [CrossRef]
- Murthy, P.S.; Ramalakshmi, K.; Srinivas, P. Fungitoxic activity of Indian borage (Plectranthus amboinicus) volatiles. Food Chem. 2009, 114, 1014–1018. [Google Scholar] [CrossRef]
- Ibrahim, E.I.; Yagi, S.; Tzanova, T.; Schohn, H.; Uba, A.I.; Zengin, G. Chemical profile, antiproliferative and antibacterial activities and docking studies of essential oil and hexane fraction of hydrosol from fresh leaf of Plectranthus amboinicus (Lour.) Spreng. Biochem. Syst. Ecol. 2023, 107, 104595. [Google Scholar] [CrossRef]
- Marwah, R.G.; Fatope, M.O.; Deadman, M.L.; Ochei, J.E.; Al-Saidi, S.H. Antimicrobial activity and the major components of the essential oil of Plectranthus cylindraceus. J. Appl. Microbiol. 2007, 103, 1220–1226. [Google Scholar] [CrossRef]
- Silva, A.V.; Yerena, L.R.; Necha, L.L.B. Chemical profile and antifungal activity of plant extracts on Colletotrichum spp. isolated from fruits of Pimenta dioica (L.) Merr. Pestic. Biochem. Physiol. 2021, 179, 104949. [Google Scholar] [CrossRef] [PubMed]
- Marzoug, H.N.B.; Romdhane, M.; Lebrihi, A.; Mathieu, F.; Couderc, F.; Abderraba, M.; Bouajila, J. Eucalyptus oleosa essential oils: Chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules 2011, 16, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, G.; Sinniah, U.R.; Swamy, M.K.; Lynch, P.T. Micropropagation and essential oil characterization of Plectranthus amboinicus (Lour.) Sprengel, an aromatic medicinal plant. In Vitro Cell. Dev. Biol. Plant 2020, 56, 491–503. [Google Scholar] [CrossRef]
- Vasconcelos, S.E.C.B.; Melo, H.M.; Cavalcante, T.T.A.; Júnior, F.E.A.C.; de Carvalho, M.G.; Menezes, F.G.R.; Costa, R.A. Plectranthus amboinicus essential oil and carvacrol bioactive against planktonic and biofilm of oxacillin-and vancomycin-resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2017, 17, 462. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, A.; Venkatesalu, V. Chemical composition and larvicidal activity of the essential oil of Plectranthus am boinicus (Lour.) Spreng against Anopheles stephensi: A malarial vector mosquito. Parasitol. Res. 2010, 107, 1275–1278. [Google Scholar] [CrossRef]
- Da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Giannotti, J.D.G.; Roberto, C.D. Plectranthus amboinicus (Lour.) Spreng. essential oil as a natural alternative for the conservation of beef patties stored under refrigeration. Food Biosci. 2022, 49, 101896. [Google Scholar] [CrossRef]


| Inhibition (%) at Different Doses (µL/L) | |||||
|---|---|---|---|---|---|
| Essential Oil | 100 | 200 | 300 | 400 | 500 |
| P. amboinicus | 27.2 ± 0.2 f | 42.4 ± 0.3 d | 55.7 ± 0.4 e | 100.0 ± 0.1 a | 100.1 ± 0.1 a |
| S. aromaticum | 23.1 ± 0.3 f | 84.1 ± 0.1 c | 87.3 ± 0.2 d | 93.3 ± 0.2 b | 100.0 ± 0.0 a |
| L. alba | 0.0 ± 0.1 i | 6.2 ± 0.1 hi | 5.3 ± 0.3 i | 7.7 ± 0.2 i | 14.5 ± 0.1 g |
| R. officinalis | 0.0 ± 0.0 i | 7.4 ± 0.0 h | 9.9 ± 0.1 h | 11.0 ± 0.1 h | 14.7 ± 0.3 g |
| No | RT 1 | Compound | % 2 |
|---|---|---|---|
| 1 | 3.489 | (E)-beta-Famesene | 0.45 |
| 2 | 3.774 | beta-Myrcene | 0.97 |
| 3 | 3.95 | o-Cymene | 11.61 |
| 4 | 4.15 | gamma-Terpinene | 6.91 |
| 5 | 4.325 | 5-Isopropyl-2-methylbicyclohexan-2-ol | 0.43 |
| 6 | 4.795 | Terpinen-4-ol | 1.35 |
| 7 | 5.401 | 3-Methyl-2-(2-methyl-2-butenyl)-furan | 1.26 |
| 8 | 5.581 | Carvacrol | 41.20 |
| 9 | 5.708 | Ascaridole epoxide | 0.19 |
| 10 | 6.045 | Phenol, 2-methoxy-3-(2-propenyl)- | 0.07 |
| 11 | 6.165 | Cyclohexene, 2-ethenyl-1,3,3-trimethyl- | 1.03 |
| 12 | 6.784 | 11,11-Dimethyl-4,8-dimethylenebicycloundecan-3-ol | 0.21 |
| 13 | 7.032 | Caryophyllene | 11.45 |
| 14 | 7.102 | α-Bergamotene | 7.71 |
| 15 | 7.224 | (1R,7S,E)-7-Isopropyl-4,10-dimethylenecyclodec-5-enol | 0.38 |
| 16 | 7.361 | 1,4,7, -Cycloundecatriene, 1,5,9,9-tetramethyl-, Z, Z,Z- | 3.76 |
| 17 | 7.499 | (1R,7S, E)-7-Isopropyl-4,10-dimethylenecyclodec-5-enol-Dup1 | 1.03 |
| 18 | 7.611 | (E)-beta-Famesene-Dup1 | 0.41 |
| 19 | 7.797 | beta-Bisabolene | 0.87 |
| 20 | 7.963 | 3,8,8-Trimethyl-6-methyleneoctahydro-1H-3a,7-methanoazulen | 0.50 |
| 21 | 8.66 | Caryophyllene oxide | 4.62 |
| 22 | 8.915 | (1R,3E,7E,11R)-1,5,5,8-Tetramethyl-12-oxabicyclo dodeca-3,7-diene | 0.88 |
| 23 | 9.176 | 11,11-Dimethyl-4,8-dimethylenebicyclo [7.2.0] undecan-3-ol-Dup1 | 0.63 |
| 24 | 9.39 | (1R,7S, E)-7-Isopropyl-4,10-dimethylenecyclodec-5-enol-Dup2 | 0.53 |
| 25 | 9.53 | (1R,7S, E)-7-Isopropyl-4,10-dimethylenecyclodec-5-enol-Dup3 | 1.19 |
| 26 | 11.64 | Naphthalene, 1,2,3,4,4a,5,6,7-octahydro-4a-methyl- | 0.19 |
| 27 | 12.24 | Naphthalene, 1,2,3,4,4a,5,6,7-octahydro-4a-methyl--Dup1 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonio-Gutiérrez, O.; Alvízar-Martínez, J.A.; Solano, R.; Vásquez-López, A.; Hernández-Valladolid, S.L.; Lustre-Sánchez, H.; Flores-Moctezuma, H.E.; de Jesús de Luna-Santillana, E.; Lagunez-Rivera, L. Microwave-Assisted Hydrodistillation of Essential Oil from Plectranthus amboinicus: Evaluation of Its Antifungal Effect and Chemical Composition. Life 2023, 13, 528. https://doi.org/10.3390/life13020528
Antonio-Gutiérrez O, Alvízar-Martínez JA, Solano R, Vásquez-López A, Hernández-Valladolid SL, Lustre-Sánchez H, Flores-Moctezuma HE, de Jesús de Luna-Santillana E, Lagunez-Rivera L. Microwave-Assisted Hydrodistillation of Essential Oil from Plectranthus amboinicus: Evaluation of Its Antifungal Effect and Chemical Composition. Life. 2023; 13(2):528. https://doi.org/10.3390/life13020528
Chicago/Turabian StyleAntonio-Gutiérrez, Oscar, José Antonio Alvízar-Martínez, Rodolfo Solano, Alfonso Vásquez-López, Sandra Luz Hernández-Valladolid, Hermes Lustre-Sánchez, Hilda Elizabet Flores-Moctezuma, Erick de Jesús de Luna-Santillana, and Luicita Lagunez-Rivera. 2023. "Microwave-Assisted Hydrodistillation of Essential Oil from Plectranthus amboinicus: Evaluation of Its Antifungal Effect and Chemical Composition" Life 13, no. 2: 528. https://doi.org/10.3390/life13020528
APA StyleAntonio-Gutiérrez, O., Alvízar-Martínez, J. A., Solano, R., Vásquez-López, A., Hernández-Valladolid, S. L., Lustre-Sánchez, H., Flores-Moctezuma, H. E., de Jesús de Luna-Santillana, E., & Lagunez-Rivera, L. (2023). Microwave-Assisted Hydrodistillation of Essential Oil from Plectranthus amboinicus: Evaluation of Its Antifungal Effect and Chemical Composition. Life, 13(2), 528. https://doi.org/10.3390/life13020528








