Continuous Positive Airway Pressure Reduces Plasma Neurochemical Levels in Patients with OSA: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
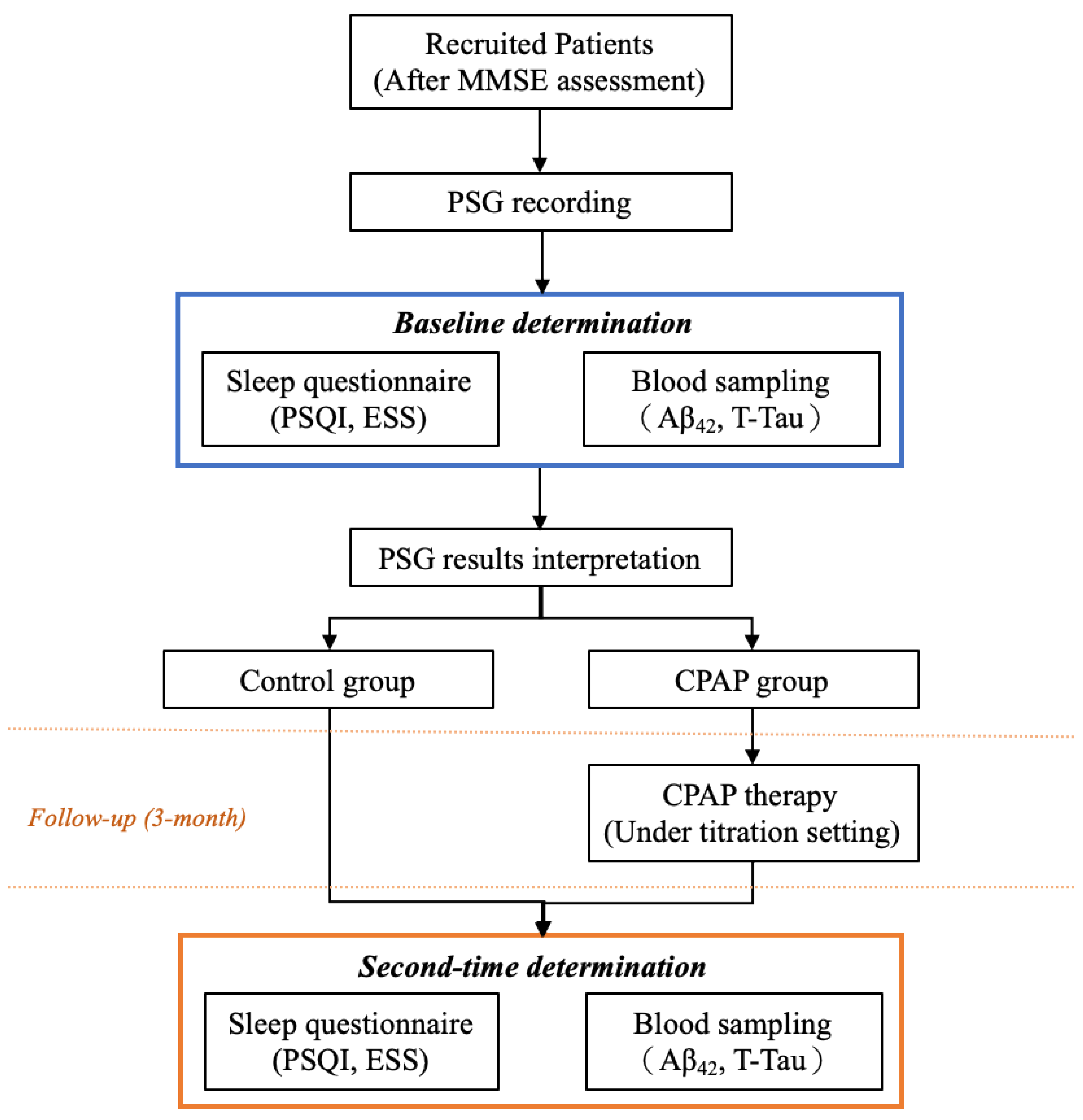
2.1. Participants and Study Flow
2.2. PSG Parameters
2.3. Determination of Neurochemical Biomarker Levels in Plasma
2.4. CPAP Treatment
2.5. Statistics
3. Results
3.1. Demographic Characteristics
3.2. Details of CPAP Titration
3.3. Alterations in the Neurochemical Biomarker Levels
3.4. Effect on CPAP on Neurochemical Biomarker Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maspero, C.; Giannini, L.; Galbiati, G.; Rosso, G.; Farronato, G. Obstructive sleep apnea syndrome: A literature review. Minerva Stomatol. 2015, 64, 97–109. [Google Scholar] [PubMed]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.; Strange, C.; Bachman, D. Neurocognitive impairment in obstructive sleep apnea. Chest 2012, 141, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Kastin, A.J. Can sleep apnea cause Alzheimer’s disease? Neurosci. Biobehav. Rev. 2014, 47, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Hsu, W.H.; Lin, Y.T.; Liu, Y.S.; Lo, K.; Lin, S.Y.; Majumdar, A.; Cheng, W.H.; Lee, K.Y.; Wu, D.; et al. Associations among sleep-disordered breathing, arousal response, and risk of mild cognitive impairment in a northern Taiwan population. J. Clin. Sleep Med. 2022, 18, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Durgan, D.J.; Bryan, R.M., Jr. Cerebrovascular consequences of obstructive sleep apnea. J. Am. Heart Assoc. 2012, 1, e000091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Chen, P.; Peng, Y.; Ouyang, R. Role of Oxidative Stress in the Neurocognitive Dysfunction of Obstructive Sleep Apnea Syndrome. Oxid. Med. Cell Longev. 2016, 2016, 9626831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavie, L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: Implications to the heart and brain. Sleep Med. Rev. 2015, 20, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Daulatzai, M.A. Pathogenesis of cognitive dysfunction in patients with obstructive sleep apnea: A hypothesis with emphasis on the nucleus tractus solitarius. Sleep Disord. 2012, 2012, 251096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holth, J.K.; Fritschi, S.K.; Wang, C.; Pedersen, N.P.; Cirrito, J.R.; Mahan, T.E.; Finn, M.B.; Manis, M.; Geerling, J.C.; Fuller, P.M.; et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 2019, 363, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Holtzman, D.M. Bidirectional relationship between sleep and Alzheimer’s disease: Role of amyloid, tau, and other factors. Neuropsychopharmacology 2020, 45, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, E.N.; Miyazaki, K.; Maruo, K.; Yagihara, H.; Fujita, H.; Wada, K.; Nagai, Y. Chronic sleep fragmentation exacerbates amyloid β deposition in Alzheimer’s disease model mice. Neurosci. Lett. 2017, 653, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, V.; Kanefsky, R.; Matsangas, P.; Mithani, S.; Jeromin, A.; Brock, M.S.; Mysliwiec, V.; Gill, J. Elevated tau and interleukin-6 concentrations in adults with obstructive sleep apnea. Sleep Med. 2018, 43, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Spicuzza, L.; Caruso, D.; Di Maria, G. Obstructive sleep apnoea syndrome and its management. Ther. Adv. Chronic. Dis. 2015, 6, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio, R.S.; Gumb, T.; Pirraglia, E.; Varga, A.W.; Lu, S.E.; Lim, J.; Wohlleber, M.E.; Ducca, E.L.; Koushyk, V.; Glodzik, L.; et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015, 84, 1964–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troussière, A.C.; Charley, C.M.; Salleron, J.; Richard, F.; Delbeuck, X.; Derambure, P.; Pasquier, F.; Bombois, S. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1405–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, J.R.; Ayalon, L.; Palmer, B.W.; Loredo, J.S.; Corey-Bloom, J.; Natarajan, L.; Liu, L.; Ancoli-Israel, S. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: A preliminary study. J. Clin. Sleep Med. 2009, 5, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds III, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef] [PubMed]
- Quan, S.; Gillin, J.C.; Littner, M.; Shepard, J. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. editorials. Sleep 1999, 22, 662–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, M.J.; Yang, S.Y.; Horng, H.E.; Yang, C.C.; Chen, T.F.; Chieh, J.J.; Chen, H.H.; Chen, T.C.; Ho, C.S.; Chang, S.F.; et al. Combined plasma biomarkers for diagnosing mild cognition impairment and Alzheimer’s disease. ACS Chem. Neurosci. 2013, 4, 1530–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, M.J.; Chen, Y.F.; Chen, T.F.; Yang, S.Y.; Yang, F.P.; Tseng, T.W.; Chieh, J.J.; Chen, J.C.; Tzen, K.Y.; Hua, M.S.; et al. Plasma tau as a window to the brain-negative associations with brain volume and memory function in mild cognitive impairment and early Alzheimer’s disease. Hum. Brain Mapp. 2014, 35, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Lue, L.F.; Sabbagh, M.N.; Chiu, M.J.; Jing, N.; Snyder, N.L.; Schmitz, C.; Guerra, A.; Belden, C.M.; Chen, T.F.; Yang, C.C.; et al. Plasma Levels of Aβ42 and Tau Identified Probable Alzheimer’s Dementia: Findings in Two Cohorts. Front. Aging Neurosci. 2017, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.B.; Lee, Y.J.; Lin, S.Y.; Chen, J.P.; Hu, C.J.; Wang, P.N.; Cheng, I.H. Plasma Aβ42 and Total Tau Predict Cognitive Decline in Amnestic Mild Cognitive Impairment. Sci. Rep. 2019, 9, 13984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Tian, Z.; Wei, J.; Mu, Z.; Liang, J.; Li, M. Association between obstructive sleep apnea and Alzheimer’s disease-related blood and cerebrospinal fluid biomarkers: A meta-analysis. J. Clin. Neurosci. 2022, 102, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, E.N.; Wada, K.; Nagai, Y. Sleep Disturbance as a Potential Modifiable Risk Factor for Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.C.; Lin, C.M.; Lee, M.B.; Chou, P. The relationship between pre-sleep arousal and spontaneous arousals from sleep in subjects referred for diagnostic polysomnograms. J. Chin. Med. Assoc. 2011, 74, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.-Y.; Wu, S.-M.; Kuan, Y.-C.; Lin, Y.-T.; Hsu, C.-R.; Hsu, W.-H.; Liu, Y.-S.; Majumdar, A.; Stettler, M.; Yang, C.-M.; et al. Associations between risk of Alzheimer’s disease and obstructive sleep apnea, intermittent hypoxia, and arousal responses: A pilot study. Front. Neurol. 2022, 13, 1038735. [Google Scholar] [CrossRef]
- Bubu, O.M.; Umasabor-Bubu, O.Q.; Turner, A.D.; Parekh, A.; Mullins, A.E.; Kam, K.; Birckbichler, M.K.; Mukhtar, F.; Mbah, A.K.; Williams, N.J.; et al. Self-reported obstructive sleep apnea, amyloid and tau burden, and Alzheimer’s disease time-dependent progression. Alzheimers Dement 2020, 17, 226–245. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, R.K.; Berry, R.B. Positive Airway Pressure Treatment for Obstructive Sleep Apnea. Chest 2007, 132, 1057–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Im, S.; Park, J.I.; Kim, Y.; Sohn, M.K.; Jee, S. Improvement of Cognitive Function after Continuous Positive Airway Pressure Treatment for Subacute Stroke Patients with Obstructive Sleep Apnea: A Randomized Controlled Trial. Brain Sciences 2019, 9, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redline, S.; Adams, N.; Strauss, M.E.; Roebuck, T.; Winters, M.; Rosenberg, C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am. J. Respir. Crit. Care Med. 1998, 157 Pt 1, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Dunietz, G.L.; Chervin, R.D.; Burke, J.F.; Conceicao, A.S.; Braley, T.J. Obstructive sleep apnea treatment and dementia risk in older adults. Sleep 2021, 44, zsab076. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Hsiao, H.T.; Lo, I.H.; Nikolai, T. Association Between Obstructive Sleep Apnea, Its Treatment, and Alzheimer’s Disease: Systematic Mini-Review. Front. Aging Neurosci. 2020, 12, 591737. [Google Scholar] [CrossRef] [PubMed]
- Mullins, A.E.; Kam, K.; Parekh, A.; Bubu, O.M.; Osorio, R.S.; Varga, A.W. Obstructive Sleep Apnea and Its Treatment in Aging: Effects on Alzheimer’s disease Biomarkers, Cognition, Brain Structure and Neurophysiology. Neurobiol. Dis. 2020, 145, 105054. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Chiaravalloti, A.; Izzi, F.; Nuccetelli, M.; Bernardini, S.; Schillaci, O.; Mercuri, N.B.; Placidi, F. Sleep apnoeas may represent a reversible risk factor for amyloid-β pathology. Brain 2017, 140, e75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, C.; Mercuri, N.B.; Izzi, F.; Romigi, A.; Cordella, A.; Sancesario, G.; Placidi, F. Obstructive Sleep Apnea is Associated With Early but Possibly Modifiable Alzheimer’s Disease Biomarkers Changes. Sleep 2017, 40, zsx011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Gao, Y.; Li, M.; Zhang, S.; Shen, T.; Yuan, X.; Shang, X.; Li, Z.; Zhang, J. Altered amyloid-β and tau proteins in neural-derived plasma exosomes in obstructive sleep apnea. Sleep Med. 2022, 94, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Thompson, C.; Martineau-Dussault, M.; André, C.; Baril, A.A.; Martinez Villar, G.; Carrier, J.; Gosselin, N. Obstructive Sleep Apnea and Cognitive Decline: A Review of Potential Vulnerability and Protective Factors. Brain Sci. 2021, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Li, H.Y.; Huang, C.G.; Wang, R.Y.L.; Chuang, L.P.; Chen, N.H.; Liu, C.H.; Yang, Y.H.; Liu, C.Y.; Hsu, C.M.; et al. Risk of Alzheimer’s Disease in Obstructive Sleep Apnea Patients With or Without Treatment: Real-World Evidence. Laryngoscope 2020, 130, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Dulewicz, M.; Kulczyńska-Przybik, A.; Słowik, A.; Borawska, R.; Mroczko, B. Fatty Acid Binding Protein 3 (FABP3) and Apolipoprotein E4 (ApoE4) as Lipid Metabolism-Related Biomarkers of Alzheimer’s Disease. J. Clin. Med. 2021, 10, 3009. [Google Scholar] [CrossRef] [PubMed]
- Leuzy, A.; Cullen, N.C.; Mattsson-Carlgren, N.; Hansson, O. Current advances in plasma and cerebrospinal fluid biomarkers in Alzheimer’s disease. Curr. Opin. Neurol. 2021, 34, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Leuzy, A.; Chiotis, K.; Hasselbalch, S.G.; Rinne, J.O.; de Mendonça, A.; Otto, M.; Lleó, A.; Castelo-Branco, M.; Santana, I.; Johansson, J.; et al. Pittsburgh compound B imaging and cerebrospinal fluid amyloid-β in a multicentre European memory clinic study. Brain 2016, 139 Pt 9, 2540–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, M.W.; Veitch, D.P.; Aisen, P.S.; Beckett, L.A.; Cairns, N.J.; Cedarbaum, J.; Green, R.C.; Harvey, D.; Jack, C.R.; Jagust, W.; et al. 2014 Update of the Alzheimer’s Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimers Dement 2015, 11, e1–e120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcolea, D.; Martínez-Lage, P.; Izagirre, A.; Clerigué, M.; Carmona-Iragui, M.; Alvarez, R.M.; Fortea, J.; Balasa, M.; Morenas-Rodríguez, E.; Lladó, A.; et al. Feasibility of lumbar puncture in the study of cerebrospinal fluid biomarkers for Alzheimer’s disease: A multicenter study in Spain. J. Alzheimers Dis. 2014, 39, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Popa-Wagner, A.; Dumitrascu, D.I.; Capitanescu, B.; Petcu, E.B.; Surugiu, R.; Fang, W.H.; Dumbrava, D.A. Dietary habits, lifestyle factors and neurodegenerative diseases. Neural Regen. Res. 2020, 15, 394–400. [Google Scholar] [CrossRef] [PubMed]

| Categorical Variables | Control Group (n = 10) | CPAP Group (n = 20) | p |
|---|---|---|---|
| Age (year) a | 50.4 ± 11.84 | 53.85 ± 10.53 | 0.43 |
| Sex (male/female) b | 9/1 | 16/4 | 0.48 |
| BMI (kg/m2) c | 27.78 ± 3.86 | 30.42 ± 4.41 | 0.12 |
| Neck circumference (cm) c | 39.7 ± 3.13 | 40.4 ± 4.0 | 0.63 |
| Waist circumference (cm) c | 98.5 ± 10.3 | 100.32 ± 12.15 | 0.69 |
| MMSE (score) a | 29.2 ± 1.03 | 28.5 ± 1.93 | 0.43 |
| Sleep architecture | |||
| Sleep efficiency (%) a | 81.62 ± 10.36 | 74.78 ± 16.17 | 0.23 |
| Wake (% of SPT) a | 12.48 ± 8.13 | 20.29 ± 14.7 | 0.17 |
| NREM (% of SPT) c | 77.86 ± 6.14 | 68.03 ± 12.34 | <0.05 |
| REM (% of SPT) c | 9.64 ± 5.75 | 11.68 ± 5.73 | 0.37 |
| WASO (min) c | 42.58 ± 27.79 | 67.65 ± 43.63 | 0.11 |
| Sleep disorder index (events/h) | |||
| ODI-3% c | 41.24 ± 24.14 | 44.62 ± 20.67 | 0.69 |
| AHI c | 42.02 ± 22.42 | 47.08 ± 20.38 | 0.54 |
| Snoring index c | 299.8 ± 292.06 | 374.7 ± 154.82 | 0.36 |
| Arousal index c | 29.19 ± 18.0 | 27.42 ± 11.51 | 0.75 |
| OSA severity b | 0.05 | ||
| Normal, n (%) | - | - | |
| Mild, n (%) | 2 (20%) | - | |
| Moderate, n (%) | - | 4 (20%) | |
| Severe, n (%) | 8 (80%) | 16 (80%) |
| Categorical Variables. | CPAP Group (n = 20) |
|---|---|
| CPAP setting (cmH2O) | |
| Optimal CPAP level | 8.95 ± 3.65 |
| Pressure 95th centile | 9.06 ± 3.6 |
| Maximum pressure | 10.58 ± 4.65 |
| Sleep architecture | |
| Sleep efficiency (%) | 79.78 ± 11.96 |
| Wake (% of SPT) | 16.6 ± 10.82 |
| NREM (% of SPT) | 67.73 ± 8.25 |
| REM (% of SPT) | 15.66 ± 6.03 |
| WASO (min) | 58.76 ± 36.52 |
| Sleep disorder index (events/h) | |
| ODI-3% | 6.22 ± 5.4 |
| AHI | 5.39 ± 4.19 |
| Snoring index | 73.4 ± 113.06 |
| Arousal index | 9.02 ± 5.87 |
| CPAP usage time (n, %) | |
| 4–6 h | 14 (70%) |
| 6–8 h | 6 (30%) |
| Categorical Variables | Control Group (n = 10) | CPAP Group (n = 20) | ||||
|---|---|---|---|---|---|---|
| Baseline | After 3-Month | p | Baseline | After 3-Month | p | |
| Questionnaire | ||||||
| ESS (score) a | 10.5 ± 7.07 | 9.2 ± 6.7 | 0.32 | 13.25 ± 6.15 | 9.5 ± 6.13 | <0.05 |
| PSQI (score) a | 8.6 ± 3.57 | 7.7 ± 2.98 | 0.56 | 10.05 ± 4.05 | 6.85 ± 3.56 | <0.01 |
| Neurochemical biomarker | ||||||
| T-Tau (pg/mL) b | 20.17 ± 3.48 | 22.48 ± 2.78 | <0.05 | 24.69 ± 5.26 | 22.47 ± 2.38 | <0.05 |
| Aβ42 (pg/mL) b | 15.84 ± 1.03 | 16.43 ± 0.64 | <0.05 | 16.53 ± 1.06 | 16.36 ± 0.43 | 0.73 |
| Aβ42 × T-Tau (pg2/mL2) b | 321.21 ± 66.36 | 369.94 ± 51.73 | <0.01 | 412.56 ± 117.73 | 368.21 ± 46.21 | <0.05 |
| Aβ42/T-Tau a | 0.8 ± 0.12 | 0.74 ± 0.09 | 0.07 | 0.69 ± 0.09 | 0.73 ± 0.07 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-T.; Huang, H.-T.; Hung, H.-Y.; Lin, S.-Y.; Hsu, W.-H.; Lee, F.-Y.; Kuan, Y.-C.; Lin, Y.-T.; Hsu, C.-R.; Stettler, M.; et al. Continuous Positive Airway Pressure Reduces Plasma Neurochemical Levels in Patients with OSA: A Pilot Study. Life 2023, 13, 613. https://doi.org/10.3390/life13030613
Liu W-T, Huang H-T, Hung H-Y, Lin S-Y, Hsu W-H, Lee F-Y, Kuan Y-C, Lin Y-T, Hsu C-R, Stettler M, et al. Continuous Positive Airway Pressure Reduces Plasma Neurochemical Levels in Patients with OSA: A Pilot Study. Life. 2023; 13(3):613. https://doi.org/10.3390/life13030613
Chicago/Turabian StyleLiu, Wen-Te, Huei-Tyng Huang, Hsin-Yi Hung, Shang-Yang Lin, Wen-Hua Hsu, Fang-Yu Lee, Yi-Chun Kuan, Yin-Tzu Lin, Chia-Rung Hsu, Marc Stettler, and et al. 2023. "Continuous Positive Airway Pressure Reduces Plasma Neurochemical Levels in Patients with OSA: A Pilot Study" Life 13, no. 3: 613. https://doi.org/10.3390/life13030613





