The Use of Multimodality Imaging for the Diagnosis of Myocardial Outpouchings and Invaginations: A Systematic Review
Abstract
1. Introduction
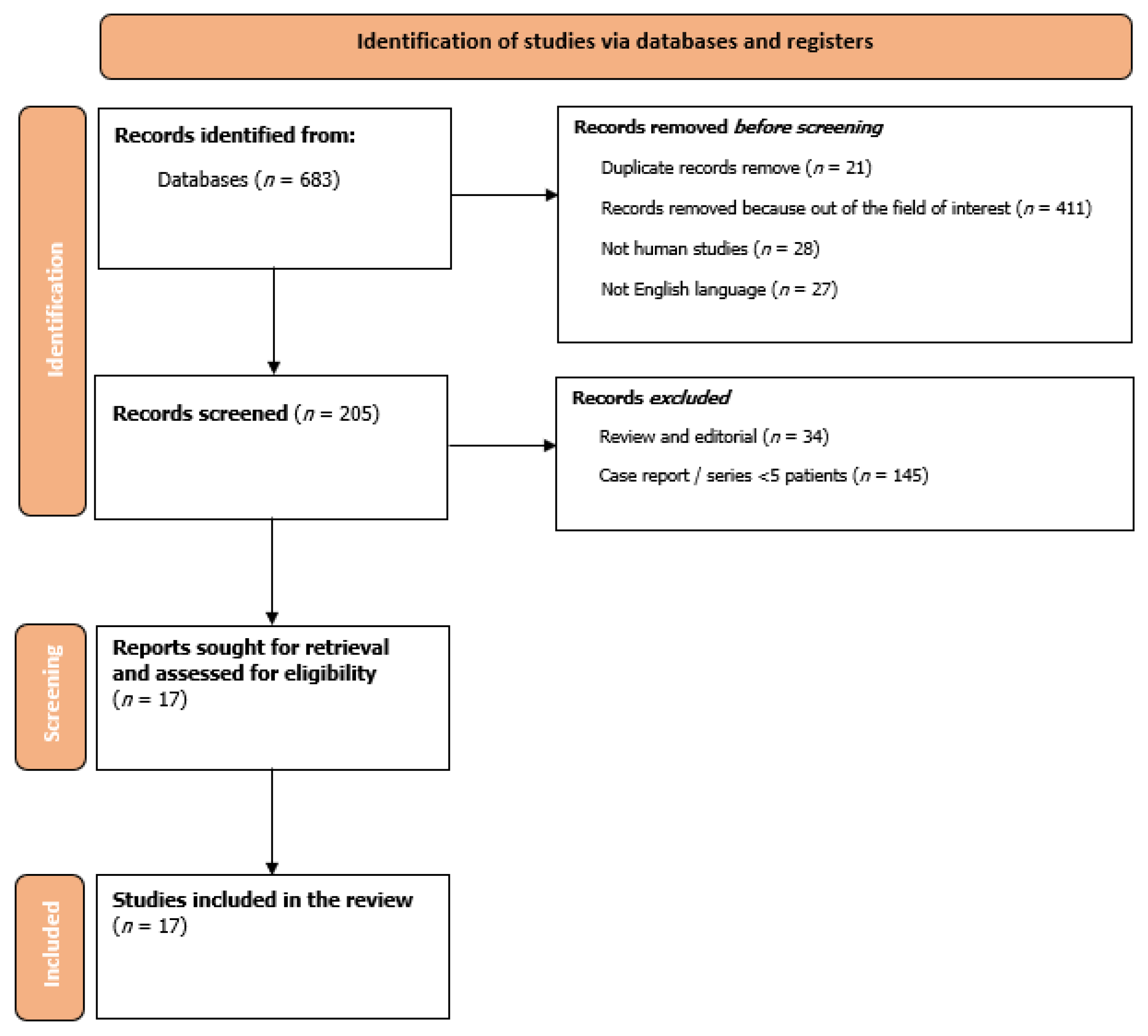
2. Materials and Methods
3. Results
4. Discussion
4.1. Ventricular Outpouchings: Diverticula and Congenital Aneurysms
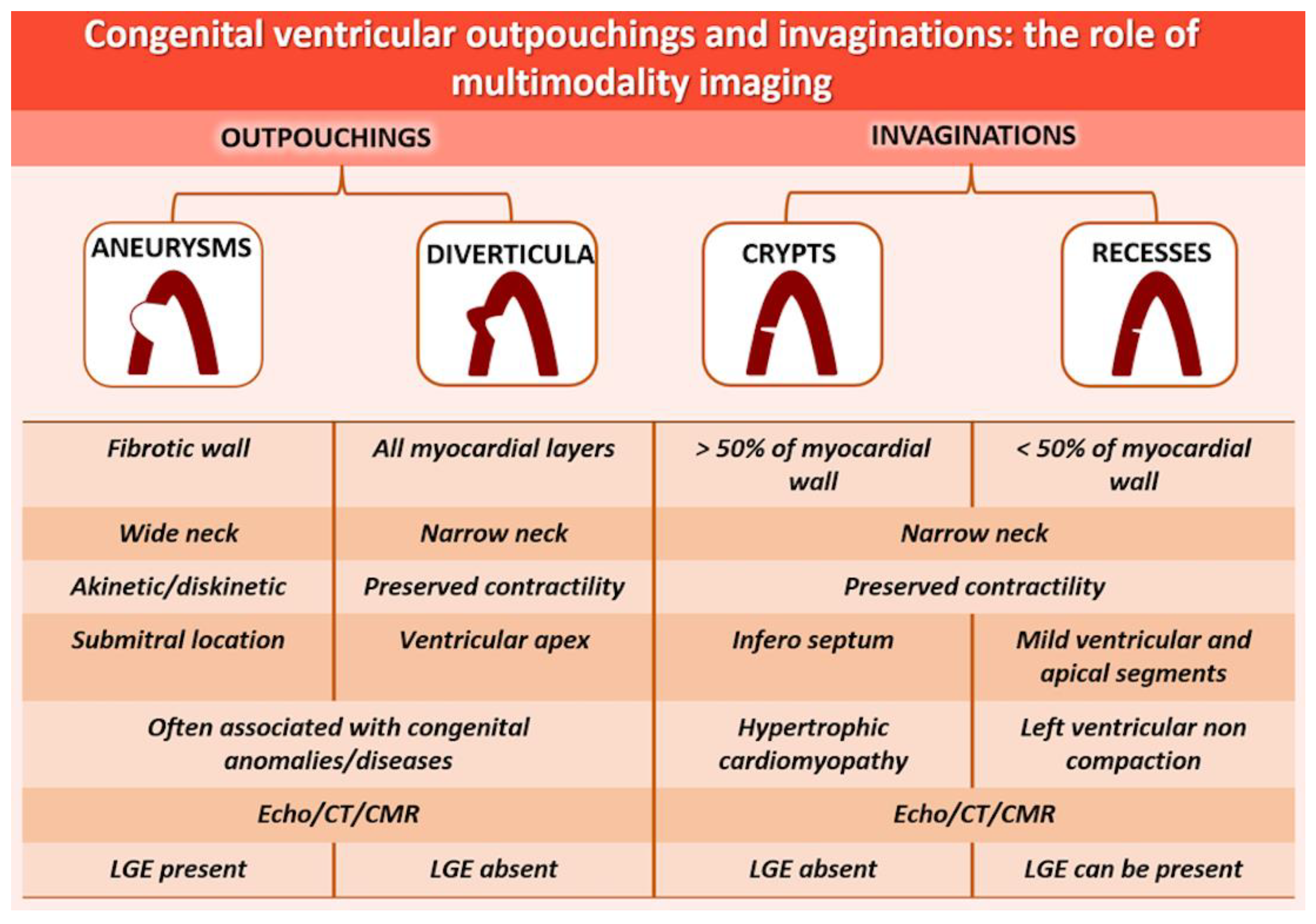
4.1.1. Definitions
4.1.2. Epidemiology
4.1.3. Etiology
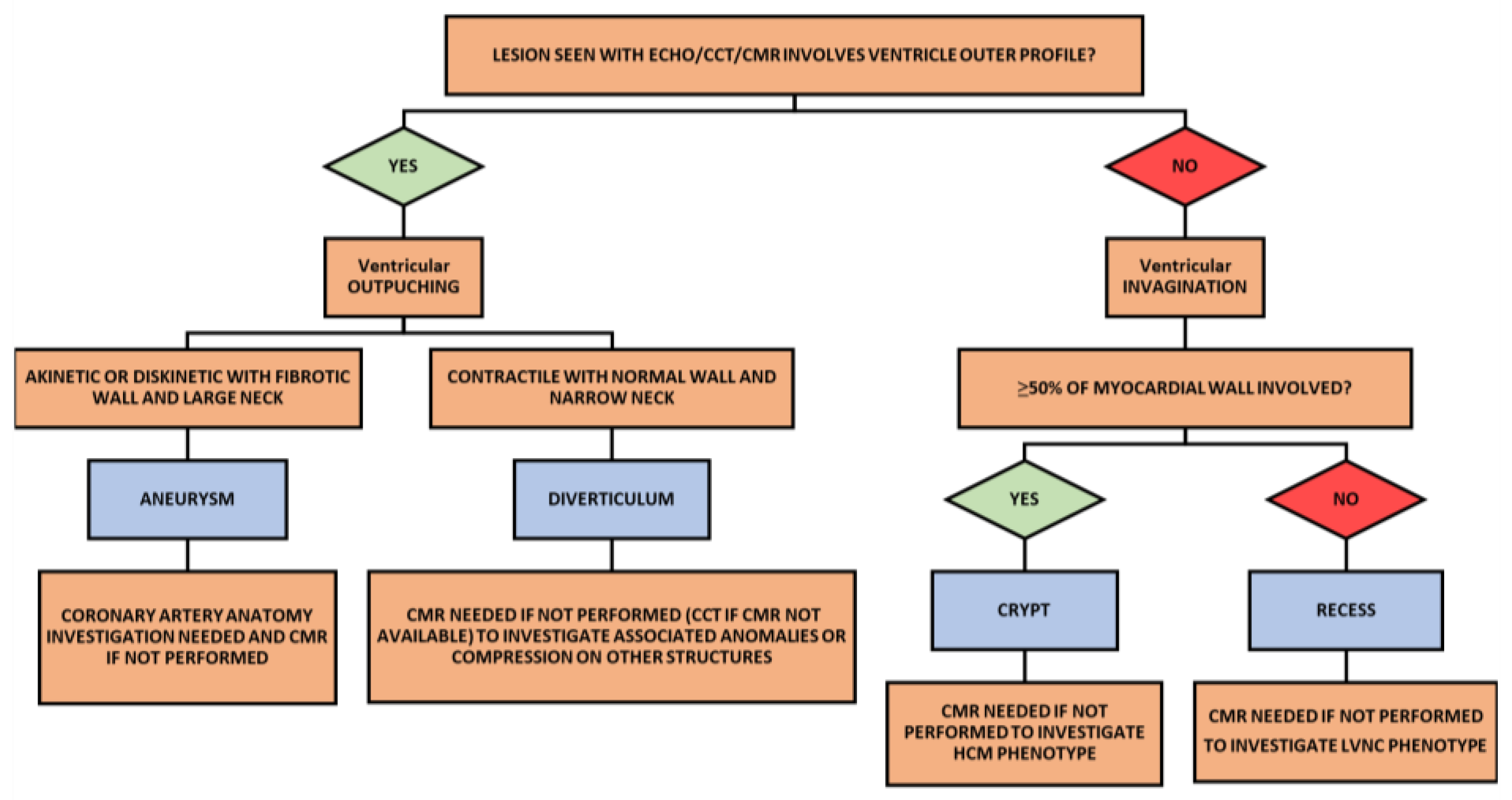
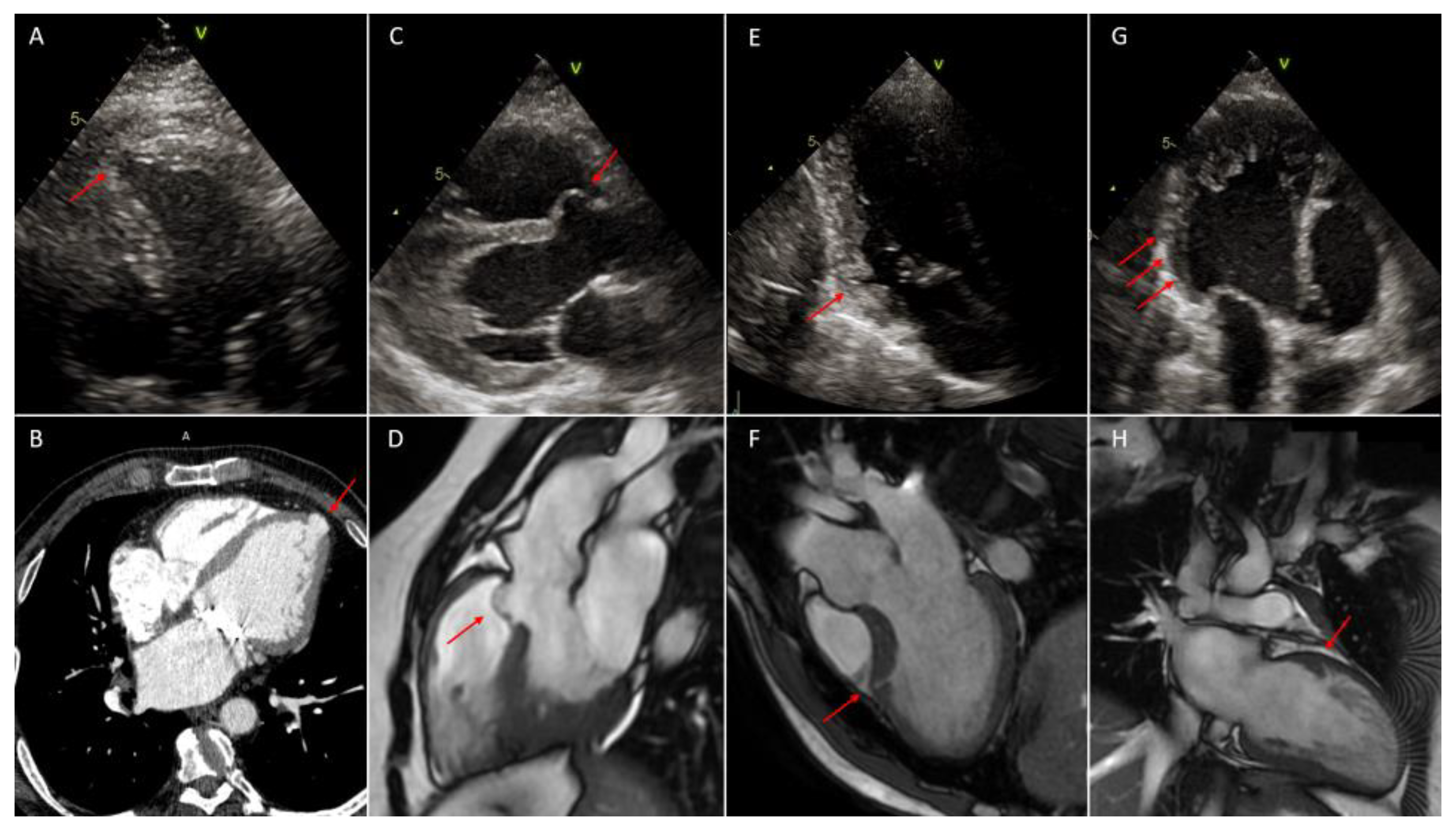
4.1.4. Diagnosis
4.1.5. Prognosis and Treatment
4.2. Ventricular Invaginations: Crypts and Recesses
4.2.1. Definitions
4.2.2. Epidemiology
4.2.3. Etiology
4.2.4. Diagnosis
4.2.5. Prognosis and Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiegand, G.; Rauch, R.; Singer, H.; Koch, A.; Hofbeck, M. Anterosuperior Diverticula of the Right Ventricle: Morphological Spectrum and Long-Term Outcome of a Distinct Cardiac Anomaly. Pediatr. Cardiol. 2014, 35, 983–989. [Google Scholar] [CrossRef]
- Basso, C.; Marra, M.P.; Thiene, G. Myocardial clefts, crypts, or crevices: Once again, you see only what you look for. Circ. Cardiovasc. Imag. 2014, 7, 217–219. [Google Scholar] [CrossRef]
- Høyland, K.; Ali, A.M.; Vegsundvåg, J.; Chambers, J.B.; Saeed, S. Echocardiographic features of left ventricular recess, cleft, diverticulum, and aneurysm: A systematic review. J. Clin. Ultrasound JCU 2022, 50, 339–346. [Google Scholar] [CrossRef]
- Ichida, F. Left ventricular noncompaction. Circ. J. 2009, 73, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Child, N.; Muhr, T.; Sammut, E.; Dabir, D.; Ucar, E.A.; Bueser, T.; Gill, J.; Carr-White, G.; Nagel, E.; O Puntmann, V. Prevalence of myocardial crypts in a large retrospective cohort study by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2014, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, G.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 372, n71. [Google Scholar]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Srichai, M.B.; Hecht, E.M.; Kim, D.; Jacobs, J.E. Ventricular Diverticula on Cardiac CT: More Common Than Previously Thought. Am. J. Roentgenol. 2007, 189, 204–208. [Google Scholar] [CrossRef]
- Marijon, E.; Ou, P.; Fermont, L.; Concordet, S.; Le Bidois, J.; Sidi, D.; Bonnet, D. Diagnosis and outcome in congenital ventricular diver-ticulum and aneurysm. J. Thorac. Cardiovasc. Surg. 2006, 131, 433–437. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.J.; Moniotte, S.; Powell, A.J.; del Nido, P.J.; Geva, T. Usefulness of Magnetic Resonance Imaging Evaluation of Congenital Left Ventricular Aneurysms. Am. J. Cardiol. 2007, 100, 310–315. [Google Scholar] [CrossRef]
- Nakazono, T.; Jeudy, J.; White, C.S. Left and right ventricular diverticula: Incidence and imaging findings on 256-slice multide-tector computed tomography. J. Thorac. Imaging 2012, 27, 179–183. [Google Scholar] [CrossRef] [PubMed]
- De Bruecker, Y.; Janssen, L.; De Somer, F.; Boeren, K.; Ector, B.; Janssens, L.; Perdieus, D. Left ventricular diverticulum: Incidental finding on dual source cardiac CT. J. Belg. De Radiol. 2011, 94, 59–62. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Strata, E.; Di Bella, G.; Todiere, G.; Pugliese, N.; Del Franco, A.; Lombardi, M. Prognostic role of isolated left ventricular di-verticuli detected by cardiovascular magnetic resonance. J. Cardiovasc. Med. 2015, 16, 562–567. [Google Scholar] [CrossRef]
- Gelehrter, S.; Wright, G.; Gless, T.; Ludomirsky, A.; Ohye, R.; Bove, E.; Ensing, G. Left ventricular outflow tract pseudoaneurysms in congenital heart disease. Am. J. Cardiol. 2002, 90, 806–809. [Google Scholar] [CrossRef]
- Deshpande, J.; Vaideeswar, P.; Sivaraman, A. Subvalvular left ventricular aneurysms. Cardiovasc. Pathol. 2000, 9, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Petryka, J.; Baksi, A.J.; Prasad, S.K.; Pennell, D.J.; Kilner, P.J. Prevalence of Inferobasal Myocardial Crypts Among Patients Referred for Cardiovascular Magnetic Resonance. Circ. Cardiovasc. Imag. 2014, 7, 259–264. [Google Scholar] [CrossRef]
- Arow, Z.; Nassar, M.; Monakier, D.; Assali, A.; Vaknin-Assa, H.; Kornowski, R.; Hamdan, A. Prevalence and morphology of myocardial crypts in normal and hypertrophied myocardium by computed tomography. Int. J. Cardiovasc. Imag. 2019, 35, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Germans, T.; Wilde, A.A.; Dijkmans, P.A.; Chai, W.; Kamp, O.; Pinto, Y.M.; van Rossum, A.C. Structural Abnormalities of the Inferoseptal Left Ventricular Wall Detected by Cardiac Magnetic Resonance Imaging in Carriers of Hypertrophic Cardiomyopathy Mutations. J. Am. Coll. Cardiol. 2006, 48, 2518–2523. [Google Scholar] [CrossRef]
- Maron, M.S. Clinical Utility of Cardiovascular Magnetic Resonance in Hypertrophic Cardiomyopathy. J. Cardiovasc. Magn. Reson. 2012, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, W.P.; Germans, T.; Head, M.C.; van der Velden, J.; Heymans, M.; Christiaans, I.; Houweling, A.C.; Wilde, A.A.; Van Rossum, A.C. Multiple myocardial crypts on modified long-axis view are a specific finding in pre-hypertrophic HCM mutation carriers. Eur. Heart J. Cardiovasc. Imag. 2012, 13, 292–297. [Google Scholar] [CrossRef]
- Urbano-Moral, J.A.; Gutierrez-Garcia-Moreno, L.; Rodriguez-Palomares, J.F.; Matabuena-Gomez-Limon, J.; Niella, N.; Maldonado, G.; Valle-Racero, J.I.; Niella, M.; Teixido-Tura, G.; Evangelista-Masip, S.; et al. Structural abnormalities in hypertrophic cardiomyopathy beyond left ventricular hypertrophy by multimodality im-aging evaluation. Echocardiography 2019, 36, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- E Sigvardsen, P.; Pham, M.H.C.; Kühl, J.T.; Fuchs, A.; Afzal, S.; Møgelvang, R.; Nordestgaard, B.G.; Køber, L.; Kofoed, K.F. Left ventricular myocardial crypts: Morphological patterns and prognostic implications. Eur. Heart J. Cardiovasc. Imag. 2020, 22, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Cay, S. Congenital ventricular diverticula: Which type of diagnostic tool is sufficient for diagnosis? Int. J. Cardiol. 2011, 147, 132. [Google Scholar] [CrossRef]
- Ozturk, E.; Saglam, M.; Sivrioglu, A.K.; Kara, K. Left ventricular clefts and diverticula. Eur. J. Radiol. 2013, 82, e628. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qu, H.; Wang, H.; Wang, D.; Li, P.; Liu, T. Ventricular Diverticulum: A Review of the Literature. J. Card. Surg. 2013, 28, 133–138. [Google Scholar] [CrossRef]
- Ohlow, M.-A. Congenital left ventricular aneurysms and diverticula: An entity in search of an identity. J. Geriatr. Cardiol. JGC 2017, 14, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Cresti, A.; Cannarile, P.; Aldi, E.; Solari, M.; Sposato, B.; Franci, L.; Limbruno, U. Multimodality Imaging and Clinical Significance of Con-genital Ventricular Outpouchings: Recesses, Diverticula, Aneurysms, Clefts, and Crypts. J. Cardiovasc. Echogr. 2018, 28, 9–17. [Google Scholar] [CrossRef]
- Erol, C.; Koplay, M.; Olcay, A.; Kivrak, A.S.; Ozbek, S.; Seker, M.; Paksoy, Y. Congenital left ventricular wall abnormalities in adults detected by gated cardiac multidetector computed tomography: Clefts, aneurysms, diverticula and terminology problems. Eur. J. Radiol. 2012, 81, 3276–3281. [Google Scholar] [CrossRef]
- Shemisa, K.; Li, J.; Tam, M.; Barcena, J. Left ventricular noncompaction cardiomyopathy. Cardiovasc. Diagn. Ther. 2013, 3, 170. [Google Scholar] [PubMed]
- Zuccarino, F.; Vollmer, I.; Sanchez, G.; Navallas, M.; Pugliese, F.; Gayete, A. Left Ventricular Noncompaction: Imaging Findings and Diagnostic Criteria. Am. J. Roentgenol. 2015, 204, W519–W530. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Ramirez, G.; Castillo-Castellon, F.; Espinola-Zavaleta, N.; Meave, A.; Kimura-Hayama, E.T. Left ventricular noncom-paction: A proposal of new diagnostic criteria by multidetector computed tomography. J. Cardiovasc. Comput. Tomogr. 2012, 6, 346–354. [Google Scholar] [CrossRef] [PubMed]
| References | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wiegand et al. 2014 [1] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 12 |
| Child et al. 2014 [5] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 20 |
| Srichai et al. 2006 [8] | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 18 |
| Marijon et al. 2006 [9] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 12 |
| McMahon et al. 2007 [10] | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 10 |
| Nakazono et al. 2012 [11] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 18 |
| De Bruecker et al. 2011 [12] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 12 |
| Aquaro et al. 2013 [13] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 20 |
| Gelehrter et al. 2002 [14] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 12 |
| Deshpande et al. 2000 [15] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 20 |
| Petryka et al. 2014 [16] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 20 |
| Arow et al. 2019 [17] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 20 |
| Germans et al. 2006 [18] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 12 |
| Maron et al. 2012 [19] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 22 |
| Brouwer et al. 2012 [20] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 20 |
| Urbano-Moral et al. 2019 [21] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 22 |
| Sigvardsen et al. 2020 [22] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 22 |
| References | Study Type | N | Male n (%) | SA Analyzed | Patients with Confirmed SA | SA Definition | Modality Imaging Used | Outcome |
|---|---|---|---|---|---|---|---|---|
| Wiegand et al., 2014 [1] | Retrospective | 5 | Unknown | 5 | 5 | Diverticula: Ventricular outpouching with synchronous contractions during cardiac systole. | Echocardiography | The rare antero-superior right ventricular diverticula appear to be a specific congenital cardiovascular anomaly and are frequently associated with other congenital defects. |
| Child et al., 2014 [5] | prospective | 1020 | 617 (61) | 64 | 64 | Crypts: invaginations > 50% of the myocardial thickness | CMR | The overall prevalence of crypts was 6,3%. No significant differences were found between NICM and ICM patients. Crypts were more prevalent in patients referred for family screening (23%, p < 0.001) |
| Srichai et al., 2006 [8] | Retrospective | 680 | Unknown | 15 | 15 | Diverticula: Ventricular outpouching with involvement of at least half of the compacted myocardial wall thickness in the diastolic phase. | CT | Prevalence of diverticula in CT performed for other reasons was 2.2%. All patients had normal left ventricular function. |
| Marijon et al., 2006 [9] | Retrospective | 22 | 11 (50) | 22 | 22 | Diverticula: ventricular outpouching with synchronous contractions during cardiac systole and muscular fibers on histological examination. Aneurysms: large akinetic or dyskinetic outpouchings with wide connection to the ventricle and endocardial or transmural fibrosis on histologic examination | Echocardiography, CMR | Diverticula and aneurysms are different pathologies and should not be confused. Aneurysms have poorer prognosis compared to diverticula (p < 0.0001). |
| McMahon et al., 2007 [10] | Retrospective | 23 | 16 (70) | 26 | 26 | Aneurysms: segment of the left ventricle wall with a thin-walled outpouching with a wide communication with the cavity. Diverticula: ventricular outpouchings with narrow communication with ventricular chamber | CMR | Apical and free-wall aneurysms were larger (p = 0.02) and more frequently associated with scar tissue (p = 0.02). Aneurysm volume was associated with left ventricular size (p < 0.0001) and EF (p < 0.0001). |
| Nakazono et al., 2012 [11] | Prospective and Retrospective | 324 | 188 (58) | 18 | 18 | Diverticula: Protruding structure with a sac-like or tube-like shape and narrow orifice with lesion depth at least half of the compacted myocardial wall. | CT | Incidence of right and left ventricular diverticula was 0.6% and 3.4%, respectively, in 256-slice CT performed for other reasons. |
| De Bruecker et al., 2011 [12] | Retrospective | 542 | Unknown | 20 | 20 | Diverticula: Ventricular outpouching with involvement of at least half of the compacted myocardial wall thickness in the diastolic phase. | CT | Incidence of left ventricular diverticula was 4.1% in dual source CT performed for other reasons. |
| Aquaro et al., 2013 [13] | Retrospective | 377 | Unknown | 25 | 25 | NS | CMR | Prevalence of ventricular diverticula in patients undergoing CMR for other reasons and without other cardiac pathologies was 0.76%. |
| Gelehrter et al., 2002 [14] | Retrospective | 9 | Unknown | 9 | 9 | NS | Echocardiography | LVOT pseudoaneurysm is rare and can impinge upon other structures and is frequently located in the area of fibrous continuity between mitral and aortic valve. |
| Deshpande et al., 2000 [15] | Retrospective | 16 | 11 (69) | 16 | 16 | NS | Echocardiography Autoptic | Subvalvular left ventricular aneurysm are rare entities often associated with other congenital cardiac disease. |
| Petryka et al., 2014 [16] | Retrospective | 686 | 377 (55) | 46 | 46 | Crypts: invaginations penetrating > 50% of the myocardial thickness | CMR | Overall prevalence of crypts was 6.7% with a higher detection in HCM patients (16%). Crypts were identified in patients without CMR anomalies (8.7%). |
| Arow et al., 2019 [17] | Prospective | 393 | 239 (61) | 37 | 37 | Crypts: invaginations penetrating > 50% of the compact myocardium | CT | Myocardial crypts were more prevalent in HCM patients (24.7%) with a larger area and deeper penetration. |
| Germans et al., 2006 [18] | Prospective | 32 | 12 (38) | 13 | 13 | NS | Echocardiography, CMR | Crypts were identified in the 81% of carriers (13/16) by CMR in the inferoseptal LV wall and they were not observed in healthy volunteers. 2D-echo was not able to detect crypts in any case. |
| Maron et al., 2012 [19] | Prospective | 390 | 254 (85) | 29 | 29 | Crypts: LV invaginations extending by visual assessment ≥ 50% of wall thickness but not fully penetrant and not visible at end-systole | Echocardiography, CMR | The crypts’ prevalence was higher in carriers than in HCM group (61% vs. 4%, p < 0.001). Any crypts were detected in the control group and by 2D-echo. |
| Brouwer et al., 2012 [20] | Prospective | 295 | 160 (54) | 61 | 61 | Crypts: disruption of compacted myocardium penetrating the LV wall of ≥30% and showing total or subtotal obliteration during systole | CMR | Carrier’s group had a higher number of crypts (p < 0.001) and a higher penetration in the myocardium (p < 0.01). Modified two chamber view was more sensitive for crypt visualization then standard long axis. Detecting ≥ 2 crypts have a 100% positive predictive value to identify carriers in family screening. |
| Urbano-Moral et al., 2019 [21] | Prospective | 100 | 65 (65) | 15 | 15 | Crypts: sharp-edged disruptions penetrating ≥ 30% within the compact myocardium | Standard and contrast echocardiography, CMR | Contrast echocardiography (n = 94) identified crypts in 15 patients (16%) with HCM and appeared non inferior to CMR. |
| Sigvardsen et al., 2020 [22] | Prospective | 10097 | 4411 (44) | 915 | 915 | Crypts: narrow blood-filled invaginations from LV cavity extending > 50% of the compacted myocardial wall by visual inspection | CT | Crypts were detected in 9.1% of patients and more frequently in the general population. During the follow-up (median 4 years), crypts’ detection was not associated with an increased hazard ratio of major adverse cardiovascular events. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavasini, R.; Bianchi, N.; Frascaro, F.; Marchini, F.; Meossi, S.; Zanarelli, L.; Sanguettoli, F.; Cossu, A.; Tonet, E.; Passarini, G.; et al. The Use of Multimodality Imaging for the Diagnosis of Myocardial Outpouchings and Invaginations: A Systematic Review. Life 2023, 13, 650. https://doi.org/10.3390/life13030650
Pavasini R, Bianchi N, Frascaro F, Marchini F, Meossi S, Zanarelli L, Sanguettoli F, Cossu A, Tonet E, Passarini G, et al. The Use of Multimodality Imaging for the Diagnosis of Myocardial Outpouchings and Invaginations: A Systematic Review. Life. 2023; 13(3):650. https://doi.org/10.3390/life13030650
Chicago/Turabian StylePavasini, Rita, Nicola Bianchi, Federica Frascaro, Federico Marchini, Sofia Meossi, Luca Zanarelli, Federico Sanguettoli, Alberto Cossu, Elisabetta Tonet, Giulia Passarini, and et al. 2023. "The Use of Multimodality Imaging for the Diagnosis of Myocardial Outpouchings and Invaginations: A Systematic Review" Life 13, no. 3: 650. https://doi.org/10.3390/life13030650
APA StylePavasini, R., Bianchi, N., Frascaro, F., Marchini, F., Meossi, S., Zanarelli, L., Sanguettoli, F., Cossu, A., Tonet, E., Passarini, G., & Campo, G. (2023). The Use of Multimodality Imaging for the Diagnosis of Myocardial Outpouchings and Invaginations: A Systematic Review. Life, 13(3), 650. https://doi.org/10.3390/life13030650









