Contrasting Autoimmune Comorbidities in Microscopic Colitis and Inflammatory Bowel Diseases
Abstract
:1. Introduction
2. Patients and Methods
3. Results
3.1. General Patient Characteristics of IBD and MC Cohorts
3.2. Autoimmune Diseases in IBD and MC
3.3. Colonic Involvement
3.4. Undifferentiated Connective Tissue Disease (UCTD) in Ulcerative Colitis
3.5. Gluten-Related Disorders in IBD
3.6. Rheumatoid Arthritis (RA) in IBD
3.7. Hepatobiliary Autoimmune Diseases
3.8. Autoimmune Diseases of the Thyroid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burke, K.E.; D’Amato, M.; Ng, S.C.; Pardi, D.S.; Ludvigsson, J.F.; Khalili, H. Microscopic colitis. Nat. Rev. Dis. Prim. 2021, 7, 39. [Google Scholar] [CrossRef]
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Prim. 2020, 6, 22. [Google Scholar] [CrossRef]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Prim. 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Ribaldone, D.G.; Pellicano, R.; Actis, G.; Adriani, A. The gut and the Inflammatory Bowel Diseases inside-out: The extra-intestinal manifestations. Minerva Gastroenterol. Dietol. 2019, 65, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.; Manjer, J.; Ohlsson, B. Microscopic colitis is associated with several concomitant diseases. Drug Target Insights 2013, 2013, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Järnerot, G.; Tysk, C.; Bohr, J.; Eriksson, S. Collagenous Colitis and Fecal Stream Diversion. Gastroenterology 1995, 109, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Münch, A.; Söderholm, J.D.; Wallon, C.; Öst, Å.; Olaison, G.; Ström, M. Dynamics of mucosal permeability and inflammation in collagenous colitis before, during, and after loop ileostomy. Gut 2005, 54, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Holster, S.; Rode, J.; Bohr, J.; Kumawat, A.K.; Veress, G.; Hörnquist, E.H.; Brummer, R.J.; König, J. Faecal microbiota transfer in patients with microscopic colitis–a pilot study in collagenous colitis. Scand. J. Gastroenterol. 2020, 55, 1454–1466. [Google Scholar] [CrossRef]
- Waller, K.M.J.; Leong, R.W.; Paramsothy, S. An update on fecal microbiota transplantation for the treatment of gastrointestinal diseases. J. Gastroenterol. Hepatol. 2022, 37, 246–255. [Google Scholar] [CrossRef]
- Fasullo, M.J.; Al-Azzawi, Y.; Abergel, J. Microscopic Colitis After Fecal Microbiota Transplant. ACG Case Rep. J. 2017, 4, e87. [Google Scholar] [CrossRef]
- Fedor, I.; Zold, E.; Barta, Z. Microscopic colitis: Controversies in clinical symptoms and autoimmune comorbidities. Ann. Med. 2021, 53, 1279–1284. [Google Scholar] [CrossRef]
- Fedor, I.; Zold, E.; Barta, Z. Temporal relationship of extraintestinal manifestations in inflammatory bowel disease. J. Clin. Med. 2021, 10, 5984. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I. Chi-squared and Fisher–Irwin tests of two-by-two tables with small sample recommendations. Stat. Med. 2007, 26, 3661–3675. [Google Scholar] [CrossRef] [PubMed]
- Bodolay, E.; Szegedi, G. Undifferentiated connective tissue disease. Orv. Hetil. 2009, 150, 867–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vries, A.B.; Janse, M.; Blokzijl, H.; Weersma, R.K. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J. Gastroenterol. 2015, 21, 1956–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehgal, K.; Eaton, J.E.; Pardi, D.; Khanna, S. S3234 The Association of Microscopic Colitis and Primary Sclerosing Cholangitis. Am. Coll. Gastroenterol. 2021, 116, S1333. [Google Scholar] [CrossRef]
- Talari, K.; Goyal, M. Retrospective studies—Utility and caveats. J. R. Coll. Physicians Edinb. 2020, 50, 398–402. [Google Scholar] [CrossRef]
- Gueyffier, F.; Cucherat, M. The limitations of observation studies for decision making regarding drugs efficacy and safety. Therapies 2019, 74, 181–185. [Google Scholar] [CrossRef]
- Conway, G.; Velonias, G.; Andrews, E.; Garber, J.J.; Yajnik, V.; Ananthakrishnan, A.N. The impact of co-existing immune-mediated diseases on phenotype and outcomes in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2017, 45, 814–823. [Google Scholar] [CrossRef] [Green Version]
- Alinaghi, F.; Tekin, H.G.; Burisch, J.; Wu, J.J.; Thyssen, J.P.; Egeberg, A. Global Prevalence and Bidirectional Association between Psoriasis and Inflammatory Bowel Disease-A Systematic Review and Meta-analysis. J. Crohns Colitis 2020, 14, 351–360. [Google Scholar] [CrossRef]
- Li, Y.; Guo, J.; Cao, Z.; Wu, J. Causal Association Between Inflammatory Bowel Disease and Psoriasis: A Two-Sample Bidirectional Mendelian Randomization Study. Front. Immunol. 2022, 13, 916645. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Colombel, J.F.; Feagan, B.G.; Reich, K.; Deodhar, A.A.; McInnes, I.B.; Porter, B.; Das Gupta, A.; Pricop, L.; Fox, T. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: A retrospective analysis of pooled data from 21 clinical trials. Ann. Rheum. Dis. 2019, 78, 473–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darch, K.M.; Holland, T.L.; Spelman, L.J. Secukinumab-Induced Inflammatory Bowel Disease in a Patient Treated for Chronic Plaque Psoriasis and Psoriatic Arthritis: A Case Report and Review of the Role of Novel Biologic Agents Targeting the p19 Subunit of IL-23. Case Rep. Med. 2020, 2020, 9404505. [Google Scholar] [CrossRef] [PubMed]
- Onac, I.A.; Clarke, B.D.; Tacu, C.; Lloyd, M.; Hajela, V.; Batty, T.; Thoroughgood, J.; Smith, S.; Irvine, H.; Hill, D.; et al. Secukinumab as a potential trigger of inflammatory bowel disease in ankylosing spondylitis or psoriatic arthritis patients. Rheumatology 2021, 60, 5233–5238. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Casteele, N.V.; Boland, B.S.; Rivera-Nieves, J.; Ernst, P.B.; Eckmann, L.; Barrett, K.E.; Chang, J.T.; Sandborn, W.J. Should We Divide Crohn’s Disease Into Ileum-Dominant and Isolated Colonic Diseases? Clin. Gastroenterol. Hepatol. 2019, 17, 2634–2643. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.M.; Haritunians, T.; Chhina, S.; Liu, Z.; Yang, S.; Landers, C.; Li, D.; Ye, B.D.; Shih, D.; Vasiliauskas, E.A.; et al. Colonic Phenotypes Are Associated with Poorer Response to Anti-TNF Therapies in Patients with IBD. Inflamm. Bowel Dis. 2017, 23, 1382–1393. [Google Scholar] [CrossRef] [Green Version]
- Meisinger, C.; Freuer, D. Rheumatoid arthritis and inflammatory bowel disease: A bidirectional two-sample Mendelian randomization study. Semin. Arthritis Rheum. 2022, 55, 151992. [Google Scholar] [CrossRef]
- Ashrafi, M.; Kuhn, K.A.; Weisman, M.H. The arthritis connection to inflammatory bowel disease (IBD): Why has it taken so long to understand it? RMD Open 2021, 7, e001558. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Xing, C.; Deng, G.; Zeng, F.; Xie, T.; Gu, L.; Yang, H. The risk of rheumatoid arthritis among patients with inflammatory bowel disease: A systematic review and meta-analysis. BMC Gastroenterol. 2020, 20, 192. [Google Scholar] [CrossRef]
- Weaver, K.N.; Herfarth, H. Gluten-Free Diet in IBD: Time for a Recommendation? Mol. Nutr. Food Res. 2021, 65, 1901274. [Google Scholar] [CrossRef]
- Passali, M.; Josefsen, K.; Frederiksen, J.L.; Antvorskov, J.C. Current evidence on the efficacy of gluten-free diets in multiple sclerosis, psoriasis, type 1 diabetes and autoimmune thyroid diseases. Nutrients 2020, 12, 2316. [Google Scholar] [CrossRef]
- Caio, G.; Riegler, G.; Patturelli, M.; Facchiano, A.; de Magistris, L.; Sapone, A. Pathophysiology of non-celiac gluten sensitivity: Where are we now? Minerva Gastroenterol. Dietol. 2017, 63, 16–21. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas-Torres, F.I.; Cabrera-Chávez, F.; Figueroa-Salcido, O.G.; Ontiveros, N. Non-celiac gluten sensitivity: An update. Medicina 2021, 57, 526. [Google Scholar] [CrossRef]
- Gazda, J.; Drazilova, S.; Janicko, M.; Jarcuska, P. The Epidemiology of Primary Biliary Cholangitis in European Countries: A Systematic Review and Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2021, 2021, 9151525. [Google Scholar] [CrossRef]
- Tunio, N.A.; Mansoor, E.; Sheriff, M.Z.; Cooper, G.S.; Sclair, S.N.; Cohen, S.M. Epidemiology of Autoimmune Hepatitis (AIH) in the United States Between 2014 and 2019: A Population-based National Study. J. Clin. Gastroenterol. 2021, 55, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, K.; Morhardt, T.L.; Kamada, N. The role of dietary nutrients in inflammatory bowel disease. Front. Immunol. 2019, 10, 3183. [Google Scholar] [CrossRef] [PubMed]
- Akobeng, A.K.; Elawad, M.; Gordon, M. Glutamine for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2016, 2016, CD007348. [Google Scholar] [CrossRef] [Green Version]
- Severo, J.S.; da Silva Barros, V.J.; Alves da Silva, A.C.; Parente, J.M.L.; Lima, M.M.; Lima, A.M.; dos Santos, A.A.; Neto, E.M.M.; Tolentino, M. Effects of glutamine supplementation on inflammatory bowel disease: A systematic review of clinical trials. Clin. Nutr. ESPEN 2021, 42, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ilchmann-Diounou, H.; Menard, S. Psychological Stress, Intestinal Barrier Dysfunctions, and Autoimmune Disorders: An Overview. Front. Immunol. 2020, 11, 1823. [Google Scholar] [CrossRef]
- Fasano, A. Leaky Gut and Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2012, 42, 71–78. [Google Scholar] [CrossRef]
- Campbell, A.W. Autoimmunity and the gut. Autoimmune Dis. 2014, 2014, 152428. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.C. Intestinal permeability and autoimmune diseases. Biosci. Horiz. Int. J. Stud. Res. 2017, 10, hzx015. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Fang, F.; Tomasson, G.; Arnberg, F.K.; Mataix-Cols, D.; de la Cruz, L.F.; Almqvist, C.; Fall, K.; Valdimarsdóttir, U.A. Association of stress-related disorders with subsequent autoimmune disease. JAMA—J. Am. Med. Assoc. 2018, 319, 2388–2400. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Qu, Y.; Zhu, J. The Relationship Between Inflammation and Post-traumatic Stress Disorder. Front. Psychiatry 2021, 12, 707543. [Google Scholar] [CrossRef]
- Bookwalter, D.B.; Roenfeldt, K.A.; Leardmann, C.A.; Kong, S.Y.; Riddle, M.S.; Rull, R.P. Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry 2020, 20, 23. [Google Scholar] [CrossRef]
- Eccles, J.A.; Ascott, A.; McGeer, R.; Hills, E.; Jones, A.S.; Page, L.A.; Smith, M.A.; Loewenberger, A.; Gregory, J. Inflammatory bowel disease psychological support pilot reduces inflammatory bowel disease symptoms and improves psychological wellbeing. Frontline Gastroenterol. 2021, 12, 154. [Google Scholar] [CrossRef] [Green Version]
- Timmer, A.; Preiss, J.C.; Motschall, E.; Rücker, G.; Jantschek, G.; Moser, G. Psychological interventions for treatment of inflammatory bowel disease. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Claire Wilson, J.; Furlano, R.I.; Jick, S.S.; Meier, C.R. Inflammatory bowel disease and the risk of autoimmune diseases. J. Crohns Colitis 2016, 10, 186–193. [Google Scholar] [CrossRef]
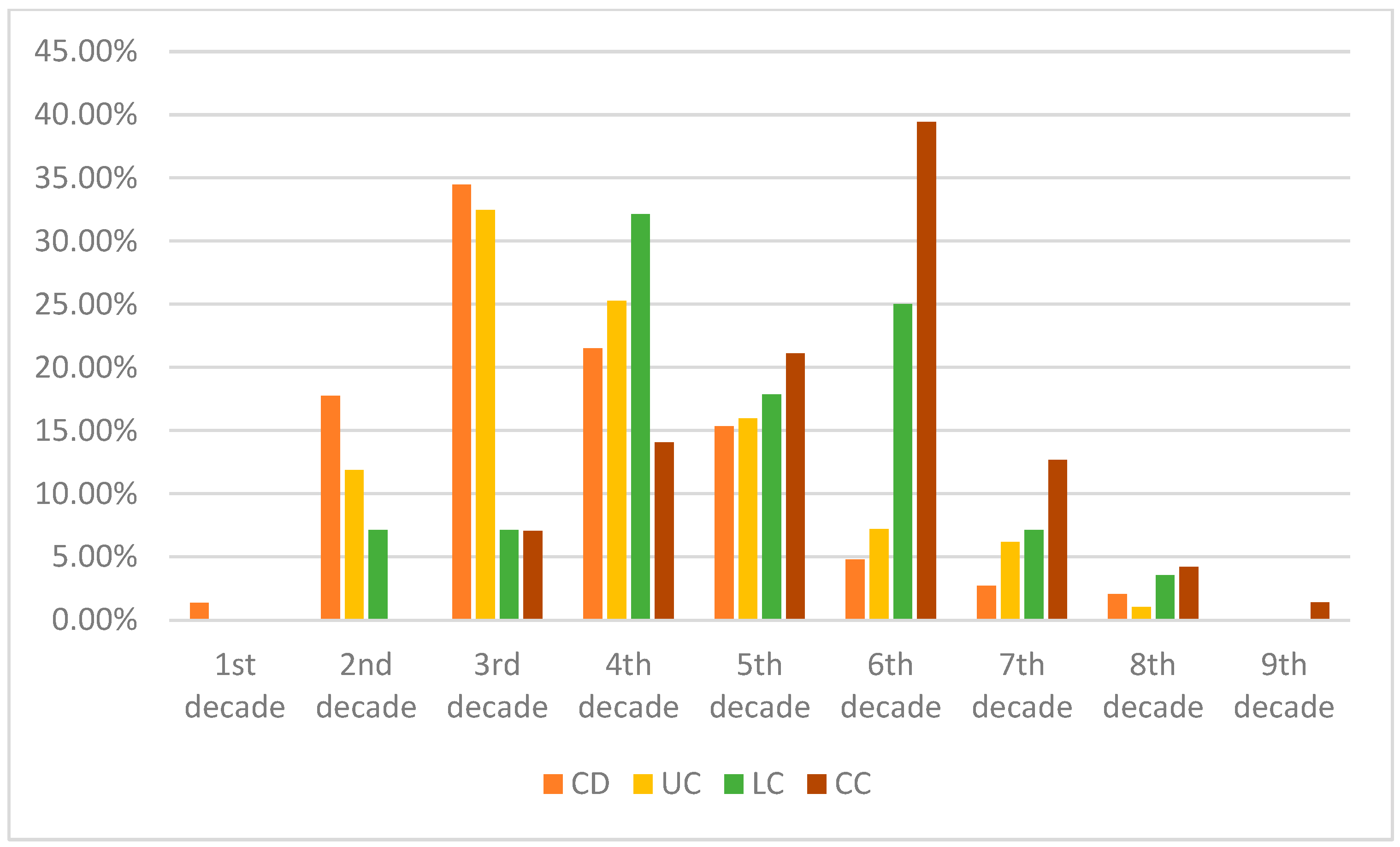
| Microscopic Colitis | Inflammatory Bowel Diseases | |||
| Lymphocytic Colitis | Collagenous Colitis | Crohn’s Disease | Ulcerative Colitis | |
| number of patients | 103 | 508 | ||
| 28 (27.2%) | 75 (72.8%) | 303 | 205 | |
| sex: | 5 male (18%); 23 female (82%) p < 0.0001 | 31 male (41.3%); 44 (58.7%) female p = 0.0337 | 133 male (43.9%); 170 (56.1%) female p = 0.0027 | 89 male (43.4%), 116 (56.6%) female p = 0.0076 |
| age of diagnosis (years) | 44.5 ± 5.3 | 51.9 ± 12.8 | 32.4 ± 12.2 | 35 ± 13.8 |
| Difference: 7.4 years (95% CI: 2.4383% to 12.3617%; p = 0.0038) | Difference: 2.6 years (95% CI: 0.3135% to 4.8865%; p = 0.0259) | |||
| AI diseases and intergroup difference | 10 (36%) | 30 (40%) | 51 (16.8%) | 46 (22.4%) |
| difference: 4 % (95% CI: -17.1256 % to 22.8420 %; p = 0.7124) | difference: 5.6 % (95 % CI: -1.3328% to 12.8528 %; p = 0.1153) | |||
| AI diseases total | 40 (38.8%) | 97 (19.1%) | ||
| Age of Diagnosis | CD | UC | LC | CC |
|---|---|---|---|---|
| 1st decade | 1.36% | 0% | 0.00% | 0.00% |
| 2nd decade | 17.75% | 11.86% | 7.14% | 0.00% |
| 3rd decade | 34.47% | 32.47% | 7.14% | 7.04% |
| 4th decade | 21.50% | 25.26% | 32.14% | 14.08% |
| 5th decade | 15.36% | 15.98% | 17.86% | 21.13% |
| 6th decade | 4.78% | 7.22% | 25.00% | 39.44% |
| 7th decade | 2.73% | 6.19% | 7.14% | 12.68% |
| 8th decade | 2.05% | 1.03% | 3.57% | 4.23% |
| 9th decade | 0.00% | 0% | 0.00% | 1.41% |
| Involved Bowel Segment | Small Intestinal Predominant Crohn (L1, L3, L4) | Colonic Crohn (L2) | Ulcerative Colitis |
| Autoimmune diseases | 29 (204 patients total; 14.2%) | 22 (89 patients total; 24.7%) | 46 (205 patients total; 22.4%) |
| Colonic IBD (CD L2 and UC combined): 68 (294 patients total; 23.1%) | |||
| Differences between subgroups of patients in autoimmune diseases | |||
| Difference between small intestinal and colonic Crohn’s disease: 10.5%; p = 0.0297 | Difference between UC and small intestinal predominant Crohn’s disease: 8.2%; p = 0.0325 | ||
| Difference between small intestinal predominant Crohn’s disease and colonic IBD: 8.9%; p = 0.0135 | |||
| Autoimmune Diseases Total: | Crohn’s | Ulcerative Colitis | Difference (p-Values) |
| 51 (16.8%) | 46 (22.4%) | 5.6% (p = 0.1153) | |
| Rheumatoid arthritis (RA) | 5 (1.66%) | 11 (5.37%) | 3.72% (p = 0.0187) |
| Thyroiditis | 13 (4.29%) | 10 (4.88%) | 0.59% (p = 0.7539) |
| Gluten-sensitive enteropathy (celiac disease) | 12 (3.96%) | 2 (0.98%) | 2.98% (p = 0.0444) |
| Non-celiac gluten sensitivity (NCGS) | 2 (0.66%) | 0 | 0.66% (p= 0.2443) |
| Dermatitis Herpetiformis (DH) | 2 (0.66%) | 1 (0.49%) | 0.17% (p = 0.8065) |
| Systemic Sclerosis | 2 (0.66%) | 0 | 0.66% (p= 0.2443) |
| Sjögren’s syndrome | 4 (1.32%) | 3 (1.46%) | 0.14% (p = 0.8944) |
| Antiphospholipid syndrome (APS) | 2 (0.66%) | 2 (0.98%) | 0.32% (p = 0.6895) |
| Addison’s disease | 2 (0.66%) | 1 (0.49%) | 0.17% (p = 0.8065) |
| Multiple sclerosis (MS) | 1 (0.33%) | 0 | 0.33% (p = 0.4108) |
| Polymyositis | 2 (0.66%) | 0 | 0.66% (p = 0.2443) |
| Ankylosing spondylitis (AS) | 1 (0.33%) | 2 (0.98%) | 0.65% (p = 0.3494) |
| Vitiligo | 2 (0.66%) | 0 | 0.66% (p = 0.2443) |
| Psoriasis | 2 (0.66%) | 3 (1.46%) | 0.8% (p = 0.3703) |
| Primary biliary cholangitis (PBC) | 1 (0.33%) | 0 | 0.33% (p = 0.4108) |
| Autoimmune hepatitis (AIH) | 0 | 2 (0.98%) | 0.98% (p = 0.0845) |
| Undifferentiated connective tissue disease (UCTD) | 4 (1.32%) | 10 (4.88%) | 3.56% (p = 0.0163) |
| Lymphocytic Colitis | Collagenous Colitis |
|---|---|
| 10 (36%) | 30 (40%) |
| In total: 39% of all patients; difference between groups: 4%, p = 0.7124 All autoimmune disorders in both groups combined: | |
| Hashimoto thyroiditis | 14 (13.59%) |
| Rheumatoid arthritis (RA) | 7 (6.79%) |
| Sjögren’s syndrome | 7 (6.79%) |
| Undifferentiated connective tissue disease (UCTD) | 5 (4.85%) |
| Gluten Sensitive Enteropathy (celiac disease, GSE) | 4 (3.88%) |
| Systemic Lupus Erythematosus (SLE) | 4 (3.88%) |
| Mixed connective tissue diseases (MCTD) | 1 (0.97%) |
| Ankylosing spondylitis (AS) | 1 (0.97%) |
| Graves–Basedow thyroiditis | 1 (0.97%) |
| Autoimmune hepatitis (AIH) | 1 (0.97%) |
| Microscopic Colitis | Inflammatory Bowel Disease | |
|---|---|---|
| Typical age of presentation | Above 50 years of age, most commonly above 65 years. | Usually in the first three decades of life, particularly Crohn’s disease. |
| Presence of autoimmune comorbidities | Rather common (affecting more than one-third of patients). Almost exclusively diagnosed before bowel disease. | More common than in the general population, but roughly half of that is seen in microscopic colitis. They can present before or roughly at the same time as bowel symptoms. |
| Sex ratios | Females outnumber male cases by 2.5×. | More balanced ratios, with mild female predominance. |
| Affected bowel segments | Purely colonic, without lesions in upper intestinal segments. | Can present anywhere along the GI tract (Crohn’s disease), and UC may have terminal ileal inflammation (backwash ileitis). |
| Disease course | Usually benign, no risk of malignant transformation, very low risk for complications and strictures–scar tissue. | Greatly depends on management. Increased tendency for colorectal carcinomas, risk (esp. CD) for strictures, and even perforation. |
| Effective management | The vast majority of cases respond to budesonide (topical corticosteroid). | Various agents are shown to be effective. Both systemic and topical corticosteroids, a range of monoclonal antibodies, aminosalicylates, and other immune-modulatory agents. |
| Clinical symptoms | Watery diarrhea without bleeding, though the absence of diarrhea or reduced bowel motility does not rule out the disease.Symptoms frequently during early dawn hours. | Crohn’s disease may present late, only when there are strictures and hindered GI transit, whereas UC is usually detected earlier and more likely to have blood in the stool. Symptoms are random throughout the day and night. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedor, I.; Zold, E.; Barta, Z. Contrasting Autoimmune Comorbidities in Microscopic Colitis and Inflammatory Bowel Diseases. Life 2023, 13, 652. https://doi.org/10.3390/life13030652
Fedor I, Zold E, Barta Z. Contrasting Autoimmune Comorbidities in Microscopic Colitis and Inflammatory Bowel Diseases. Life. 2023; 13(3):652. https://doi.org/10.3390/life13030652
Chicago/Turabian StyleFedor, Istvan, Eva Zold, and Zsolt Barta. 2023. "Contrasting Autoimmune Comorbidities in Microscopic Colitis and Inflammatory Bowel Diseases" Life 13, no. 3: 652. https://doi.org/10.3390/life13030652
APA StyleFedor, I., Zold, E., & Barta, Z. (2023). Contrasting Autoimmune Comorbidities in Microscopic Colitis and Inflammatory Bowel Diseases. Life, 13(3), 652. https://doi.org/10.3390/life13030652





