Effects of Simulated 5-Ion Galactic Cosmic Radiation on Function and Structure of the Mouse Heart
Abstract
1. Introduction
2. Materials and Methods
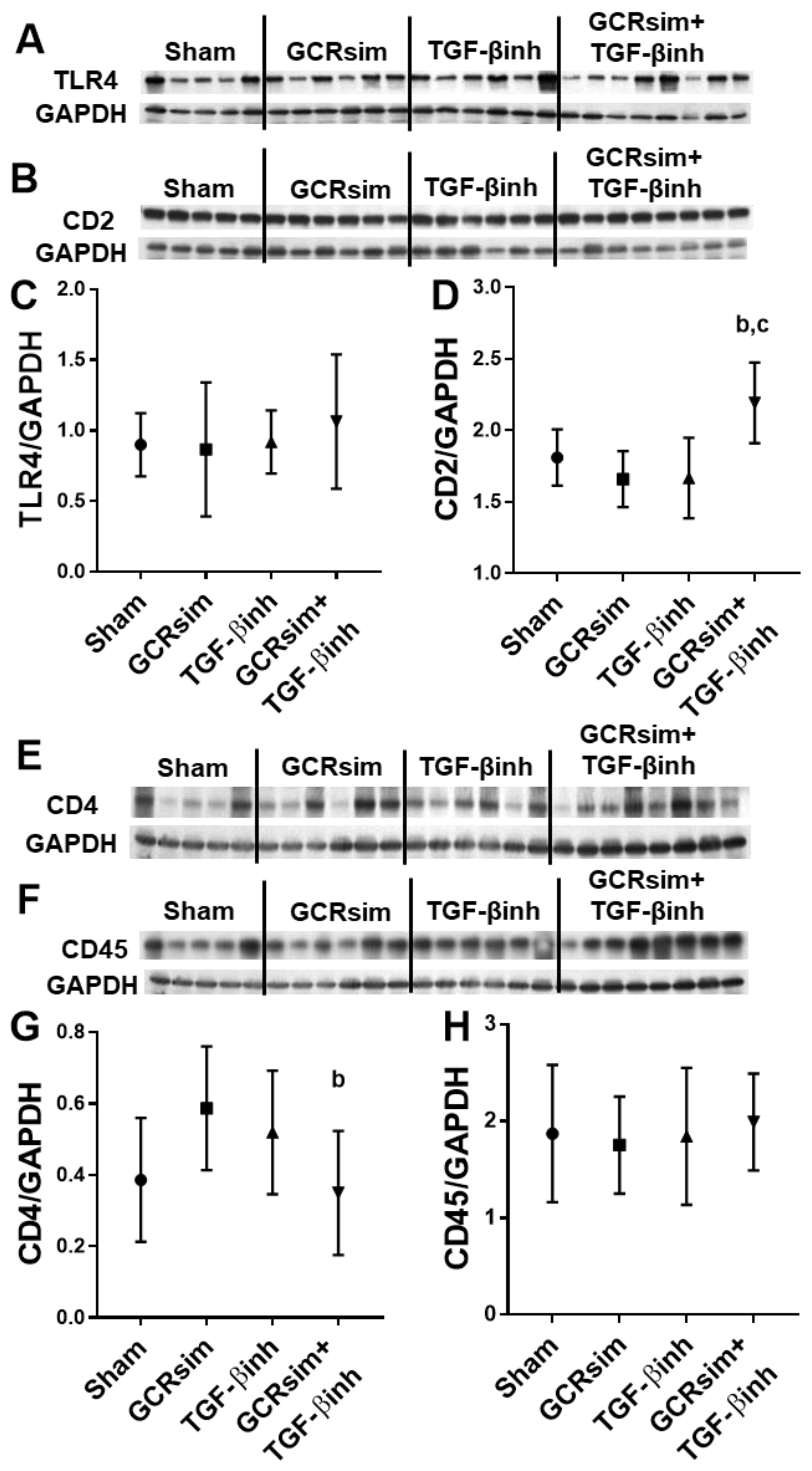
3. Results
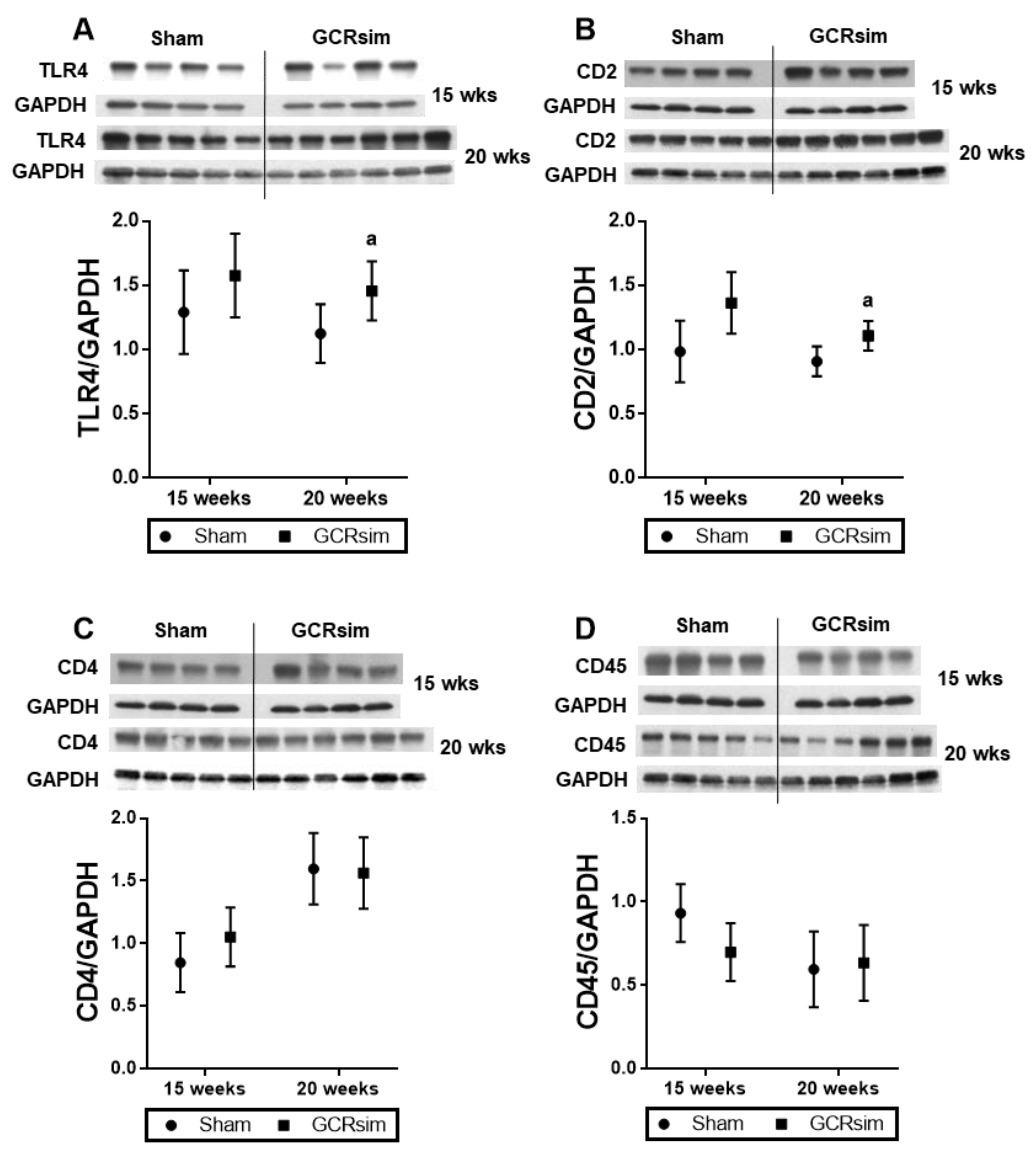
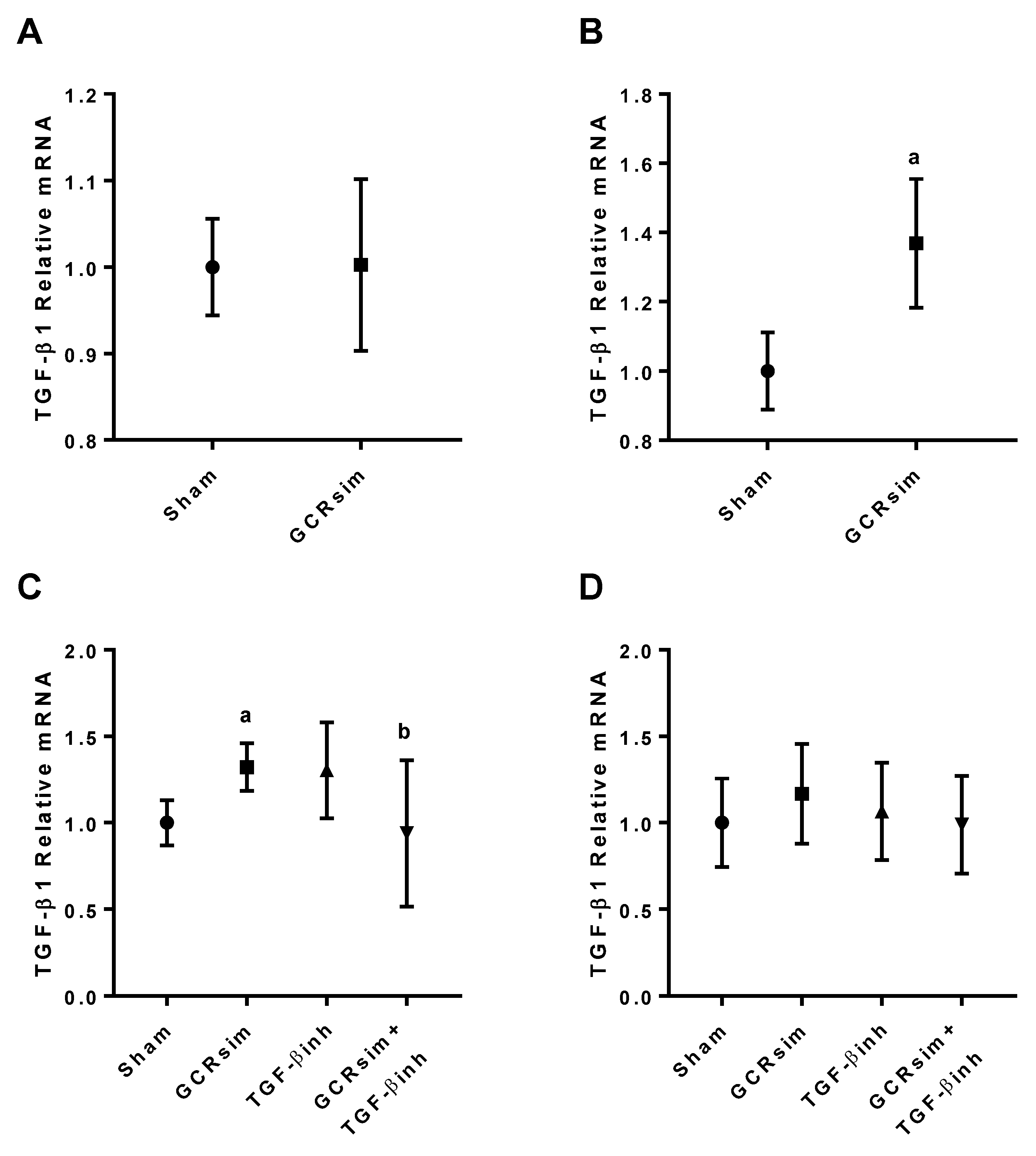
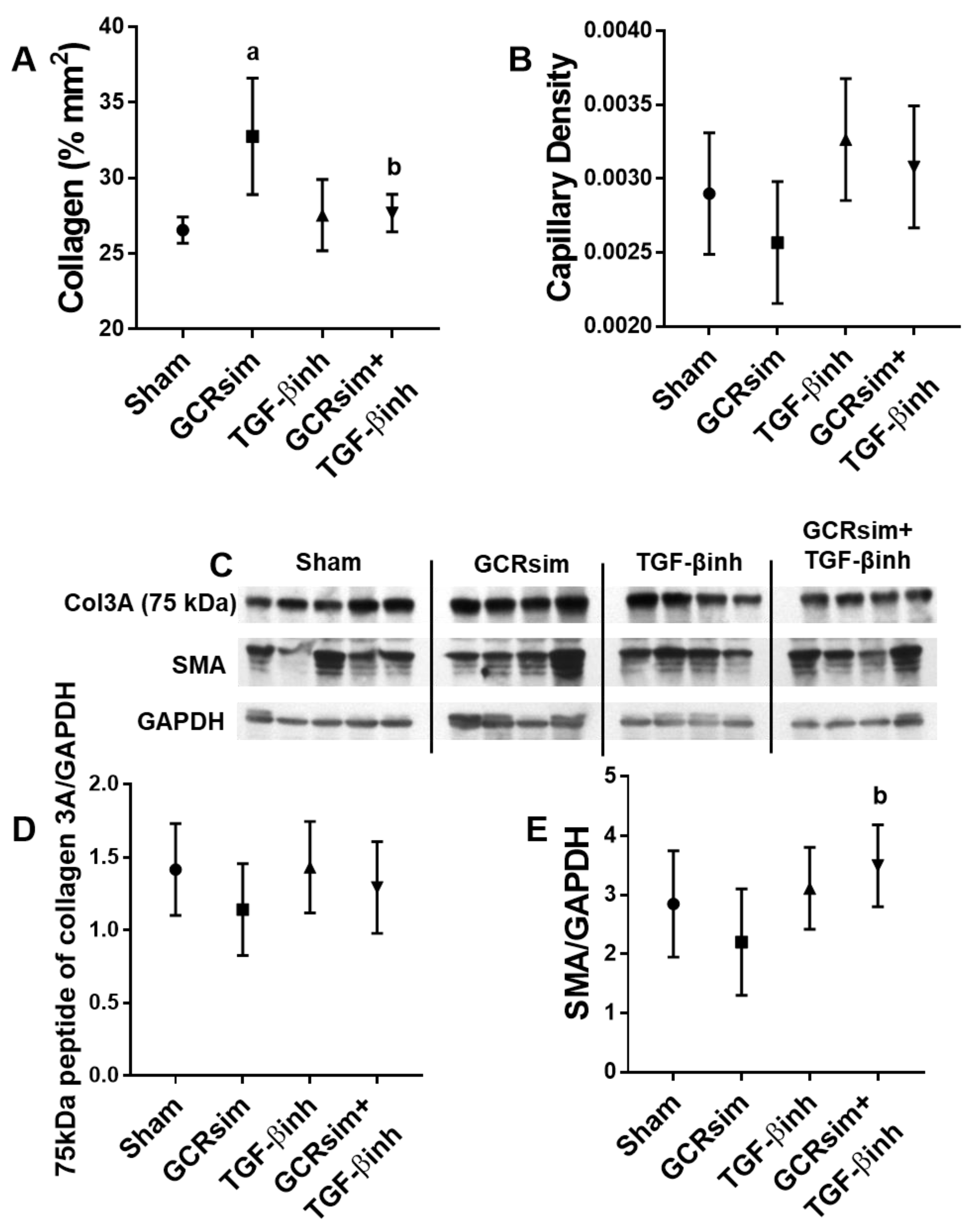
3.1. BALB/c Mice
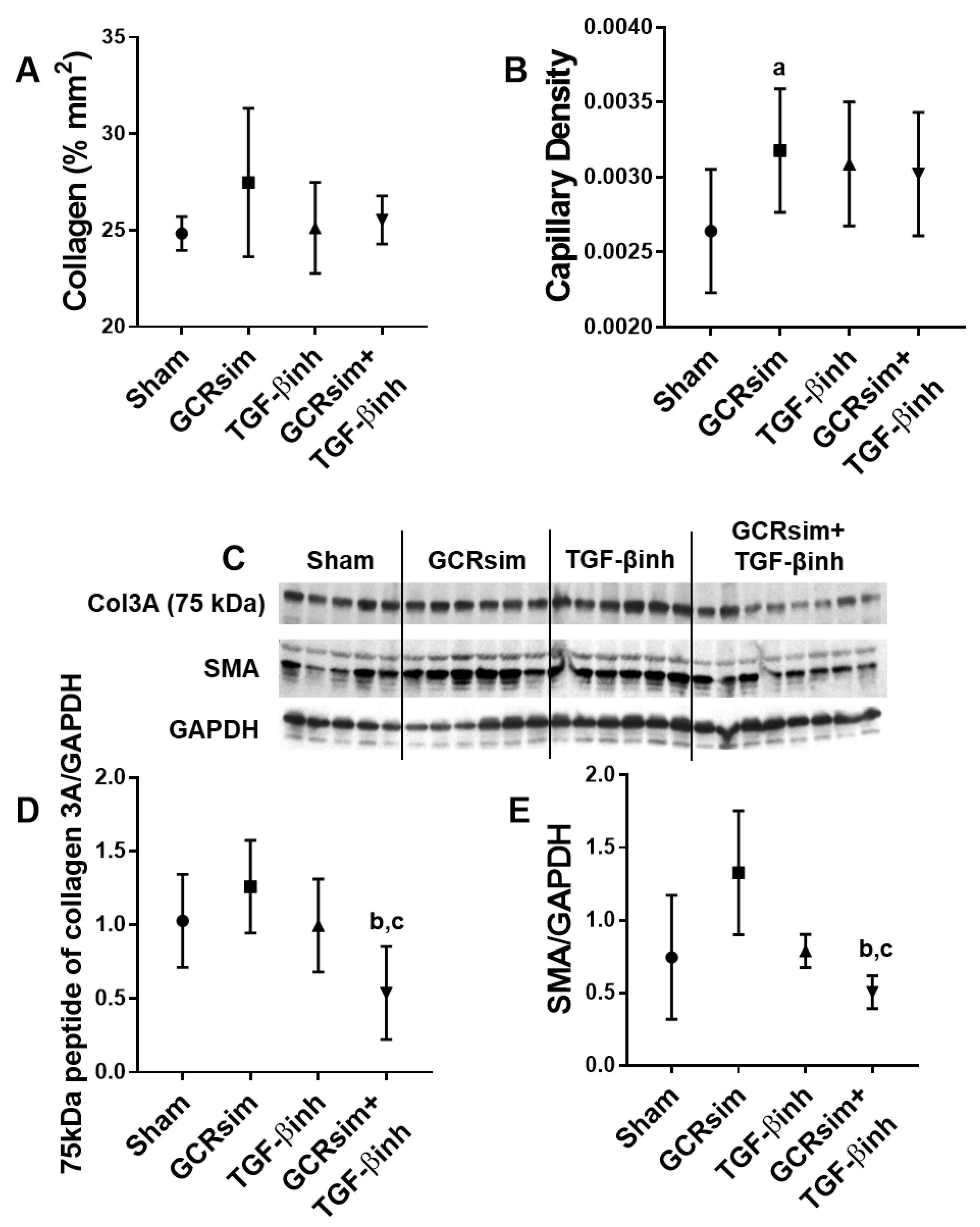
3.2. CD1 Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nemec-Bakk, A.S.; Sridharan, V.; Landes, R.D.; Singh, P.; Cao, M.; Seawright, J.W.; Liu, X.; Zheng, G.; Dominic, P.; Pathak, R.; et al. Mitigation of late cardiovascular effects of oxygen ion radiation by γ-tocotrienol in a mouse model. Life Sci. Space Res. 2021, 31, 43–50. [Google Scholar] [CrossRef]
- Seawright, J.W.; Sridharan, V.; Landes, R.D.; Cao, M.; Singh, P.; Koturbash, I.; Mao, X.W.; Miousse, I.R.; Singh, S.P.; Nelson, G.A.; et al. Effects of low-dose oxygen ions and protons on cardiac function and structure in male C57BL/6J mice. Life Sci. Space Res. 2019, 20, 72–84. [Google Scholar] [CrossRef]
- Sasi, S.P.; Yan, X.; Zuriaga-Herrero, M.; Gee, H.; Lee, J.; Mehrzad, R.; Song, J.; Onufrak, J.; Morgan, J.; Enderling, H.; et al. Different Sequences of Fractionated Low-Dose Proton and Single Iron-Radiation-Induced Divergent Biological Responses in the Heart. Radiat. Res. 2017, 188, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Tungjai, M.; Whorton, E.B.; Rithidech, K.N. Persistence of apoptosis and inflammatory responses in the heart and bone marrow of mice following whole-body exposure to ²⁸Silicon (²⁸Si) ions. Radiat. Environ. Biophys. 2013, 52, 339–350. [Google Scholar] [CrossRef]
- Hoving, S.; Heeneman, S.; Gijbels, M.J.; te Poele, J.A.; Russell, N.S.; Daemen, M.J.; Stewart, F.A. Single-dose and fractionated irradiation promote initiation and progression of atherosclerosis and induce an inflammatory plaque phenotype in ApoE(-/-) mice. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Nemec-Bakk, A.; Sridharan, V.; Landes, R.D.; Singh, P.; Cao, M.; Dominic, P.; Seawright, J.W.; Chancellor, J.C.; Boerma, M. Effects of low-dose oxygen ions on cardiac function and structure in female C57BL/6J mice. Life Sci. Space Res. 2022, 32, 105–112. [Google Scholar] [CrossRef]
- Yan, X.; Sasi, S.P.; Gee, H.; Lee, J.; Yang, Y.; Mehrzad, R.; Onufrak, J.; Song, J.; Enderling, H.; Agarwal, A.; et al. Cardiovascular risks associated with low dose ionizing particle radiation. PLoS ONE 2014, 9, e110269. [Google Scholar] [CrossRef] [PubMed]
- Soucy, K.G.; Lim, H.K.; Kim, J.H.; Oh, Y.; Attarzadeh, D.O.; Sevinc, B.; Kuo, M.M.; Shoukas, A.A.; Vazquez, M.E.; Berkowitz, D.E. HZE 56Fe-ion irradiation induces endothelial dysfunction in rat aorta: Role of xanthine oxidase. Radiat. Res. 2011, 176, 474–485. [Google Scholar] [CrossRef]
- Coleman, M.A.; Sasi, S.P.; Onufrak, J.; Natarajan, M.; Manickam, K.; Schwab, J.; Muralidharan, S.; Peterson, L.E.; Alekseyev, Y.O.; Yan, X.; et al. Low-dose radiation affects cardiac physiology: Gene networks and molecular signaling in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1947–H1963. [Google Scholar] [CrossRef]
- Sasi, S.P.; Yan, X.; Lee, J.; Sisakyan, H.; Carrozza, J.; Goukassian, D.A. Radiation-associated degenerative cardiovascular risks during normal aging and after adverse CV event 10 months post-initial exposure. J. Radiat. Res. 2014, 55, i111–i112. [Google Scholar] [CrossRef]
- Norbury, J.W.; Schimmerling, W.; Slaba, T.C.; Azzam, E.I.; Badavi, F.F.; Baiocco, G.; Benton, E.; Bindi, V.; Blakely, E.A.; Blattnig, S.R.; et al. Galactic cosmic ray simulation at the NASA Space Radiation Laboratory. Life Sci. Space Res. 2016, 8, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.C.; Slaba, T.C.; Guida, P.; Rusek, A. NASA’s first ground-based Galactic Cosmic Ray Simulator: Enabling a new era in space radiobiology research. PLoS Biol. 2020, 18, e3000669. [Google Scholar] [CrossRef] [PubMed]
- Van der Worp, H.B.; Howells, D.W.; Sena, E.S.; Porritt, M.J.; Rewell, S.; O'Collins, V.; Macleod, M.R. Can animal models of disease reliably inform human studies? PLoS Med. 2010, 7, e1000245. [Google Scholar] [CrossRef]
- Rogers, A.B. Stress of Strains: Inbred Mice in Liver Research. Gene Expr. 2018, 19, 61–67. [Google Scholar] [CrossRef]
- Patterson, A.M.; Plett, P.A.; Chua, H.L.; Sampson, C.H.; Fisher, A.; Feng, H.; Unthank, J.L.; Miller, S.J.; Katz, B.P.; MacVittie, T.J.; et al. Development of a Model of the Acute and Delayed Effects of High Dose Radiation Exposure in Jackson Diversity Outbred Mice; Comparison to Inbred C57BL/6 Mice. Health Phys. 2020, 119, 633–646. [Google Scholar] [CrossRef]
- Boerma, M.; Nelson, G.A.; Sridharan, V.; Mao, X.W.; Koturbash, I.; Hauer-Jensen, M. Space radiation and cardiovascular disease risk. World J. Cardiol. 2015, 7, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Hardenbergh, P.H.; Constine, L.S.; Lipshultz, S.E. Radiation-associated cardiovascular disease. Crit. Rev. Oncol./Hematol. 2003, 45, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.C.; Convertino, V.A.; Fanton, J.W.; Reister, C.A.; Gaffney, F.A.; Ludwig, D.A.; Krotov, V.P.; Trambovetsky, E.V.; Latham, R.D. Evidence for increased cardiac compliance during exposure to simulated microgravity. Am. J. Physiol. 1998, 275, R1343–R1352. [Google Scholar] [CrossRef]
- Vernice, N.A.; Meydan, C.; Afshinnekoo, E.; Mason, C.E. Long-term spaceflight and the cardiovascular system. Precis. Clin. Med. 2020, 3, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.R.; Todd, P. Biological countermeasures in space radiation health. Gravit. Space Biol. Bull. 2003, 16, 37–44. [Google Scholar]
- Kennedy, A.R. Biological Effects of Space Radiation and Development of Effective Countermeasures. Life Sci. Space Res. 2014, 1, 10–43. [Google Scholar] [CrossRef]
- Suematsu, Y.; Miura, S.i.; Goto, M.; Matsuo, Y.; Arimura, T.; Kuwano, T.; Imaizumi, S.; Iwata, A.; Yahiro, E.; Saku, K. LCZ696, an angiotensin receptor–neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur. J. Heart Fail. 2016, 18, 386–393. [Google Scholar] [CrossRef]
- Abed, H.S.; Samuel, C.S.; Lau, D.H.; Kelly, D.J.; Royce, S.G.; Alasady, M.; Mahajan, R.; Kuklik, P.; Zhang, Y.; Brooks, A.G. Obesity results in progressive atrial structural and electrical remodeling: Implications for atrial fibrillation. Heart Rhythm 2013, 10, 90–100. [Google Scholar] [CrossRef]
- Chen, P.Y.; Qin, L.; Li, G.; Wang, Z.; Dahlman, J.E.; Malagon-Lopez, J.; Gujja, S.; Cilfone, N.A.; Kauffman, K.J.; Sun, L.; et al. Endothelial TGF-β signalling drives vascular inflammation and atherosclerosis. Nat. Metab. 2019, 1, 912–926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Alexander, P.B.; Wang, X.F. TGF-β Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb. Perspect. Biol. 2017, 9, a022145. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, S.; Flesch, M.; Amann, K.; Haeuseler, C.; Kilter, H.; Seeland, U.; Schlüter, K.-D.; Böhm, M. Alterations of β-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-β1. Am. J. Physiol.-Heart Circ. Physiol. 2002, 283, H1253–H1262. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Qin, Q.; Yao, J.; Sun, L.; Qin, X. Induction of LOX by TGF-β1/Smad/AP-1 signaling aggravates rat myocardial fibrosis and heart failure. IUBMB Life 2019, 71, 1729–1739. [Google Scholar] [CrossRef]
- Boerma, M.; Wang, J.; Sridharan, V.; Herbert, J.M.; Hauer-Jensen, M. Pharmacological induction of transforming growth factor-beta1 in rat models enhances radiation injury in the intestine and the heart. PLoS ONE 2013, 8, e70479. [Google Scholar] [CrossRef]
- Subramanian, V.; Seemann, I.; Merl-Pham, J.; Hauck, S.M.; Stewart, F.A.; Atkinson, M.J.; Tapio, S.; Azimzadeh, O. Role of TGF Beta and PPAR Alpha Signaling Pathways in Radiation Response of Locally Exposed Heart: Integrated Global Transcriptomics and Proteomics Analysis. J. Proteome Res. 2017, 16, 307–318. [Google Scholar] [CrossRef]
- Fish, B.L.; Hart, B.; Gasperetti, T.; Narayanan, J.; Gao, F.; Veley, D.; Pierce, L.; Himburg, H.A.; MacVittie, T.; Medhora, M. IPW-5371 mitigates the delayed effects of acute radiation exposure in WAG/RijCmcr rats when started 15 days after PBI with bone marrow sparing. Int. J. Radiat. Biol. 2023, 15, 1–11. [Google Scholar] [CrossRef]
- Rabender, C.; Mezzaroma, E.; Mauro, A.G.; Mullangi, R.; Abbate, A.; Anscher, M.; Hart, B.; Mikkelsen, R. IPW-5371 Proves Effective as a Radiation Countermeasure by Mitigating Radiation-Induced Late Effects. Radiat. Res. 2016, 186, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Bu, L.; Gong, H.; Jiang, G.; Li, L.; Ma, H.; Zhou, N.; Lin, L.; Chen, Z.; Ye, Y.; et al. Effects of heart rate and anesthetic timing on high-resolution echocardiographic assessment under isoflurane anesthesia in mice. J. Ultrasound Med. 2010, 29, 1771–1778. [Google Scholar] [CrossRef]
- Kenward, M.G.; Roger, J.H. An improved approximation to the precision of fixed effects from restricted maximum likelihood. Comput. Stat. Data Anal. 2009, 53, 2583–2595. [Google Scholar] [CrossRef]
- Ramadan, S.S.; Sridharan, V.; Koturbash, I.; Miousse, I.R.; Hauer-Jensen, M.; Nelson, G.A.; Boerma, M. A priming dose of protons alters the early cardiac cellular and molecular response to (56)Fe irradiation. Life Sci. Space Res. 2016, 8, 8–13. [Google Scholar] [CrossRef]
- Boerma, M.; Zurcher, C.; Esveldt, I.; Schutte-Bart, C.I.; Wondergem, J. Histopathology of ventricles, coronary arteries and mast cell accumulation in transverse and longitudinal sections of the rat heart after irradiation. Oncol. Rep. 2004, 12, 213–219. [Google Scholar] [CrossRef]
- Wang, X.; Guo, D.; Li, W.; Zhang, Q.; Jiang, Y.; Wang, Q.; Li, C.; Qiu, Q.; Wang, Y. Danshen (Salvia miltiorrhiza) restricts MD2/TLR4-MyD88 complex formation and signalling in acute myocardial infarction-induced heart failure. J. Cell. Mol. Med. 2020, 24, 10677–10692. [Google Scholar] [CrossRef]
- Sumneang, N.; Apaijai, N.; Chattipakorn, S.C.; Chattipakorn, N. Myeloid differentiation factor 2 in the heart: Bench to bedside evidence for potential clinical benefits? Pharmacol. Res. 2021, 163, 105239. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, K.; Grue, K.; Frias, E.S.; Pietrykowski, J.; Jones, T.; Nelson, G.; Rosi, S. Female mice are protected from space radiation-induced maladaptive responses. Brain Behav. Immun. 2018, 74, 106–120. [Google Scholar] [CrossRef]
- Parihar, V.K.; Angulo, M.C.; Allen, B.D.; Syage, A.; Usmani, M.T.; Passerat de la Chapelle, E.; Amin, A.N.; Flores, L.; Lin, X.; Giedzinski, E.; et al. Sex-Specific Cognitive Deficits Following Space Radiation Exposure. Front. Behav. Neurosci. 2020, 14, 535885. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxid. Med. Cell. Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef]
- Lagranha, C.J.; Silva, T.L.A.; Silva, S.C.A.; Braz, G.R.F.; da Silva, A.I.; Fernandes, M.P.; Sellitti, D.F. Protective effects of estrogen against cardiovascular disease mediated via oxidative stress in the brain. Life Sci. 2018, 192, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Bishawi, M.; Lee, F.H.; Abraham, D.M.; Glass, C.; Blocker, S.J.; Cox, D.J.; Brown, Z.D.; Rockman, H.A.; Mao, L.; Slaba, T.C.; et al. Late onset cardiovascular dysfunction in adult mice resulting from galactic cosmic ray exposure. iScience 2022, 25, 104086. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Janicki, J.S.; Brower, G.L.; Levick, S.P. The emerging prominence of the cardiac mast cell as a potent mediator of adverse myocardial remodeling. Methods Mol. Biol. 2015, 1220, 121–139. [Google Scholar] [CrossRef]
- Mortaz, E.; Givi, M.E.; Da Silva, C.A.; Folkerts, G.; Redegeld, F.A. A relation between TGF-β and mast cell tryptase in experimental emphysema models. Biochim. Biophys. Acta 2012, 1822, 1154–1160. [Google Scholar] [CrossRef]
- Funaba, M.; Ikeda, T.; Murakami, M.; Ogawa, K.; Abe, M. Up-regulation of mouse mast cell protease-6 gene by transforming growth factor-beta and activin in mast cell progenitors. Cell. Signal. 2005, 17, 121–128. [Google Scholar] [CrossRef]
- Rödel, F.; Frey, B.; Capalbo, G.; Gaipl, U.; Keilholz, L.; Voll, R.; Hildebrandt, G.; Rödel, C. Discontinuous induction of X-linked inhibitor of apoptosis in EA.hy.926 endothelial cells is linked to NF-κB activation and mediates the anti-inflammatory properties of low-dose ionising-radiation. Radiother. Oncol. 2010, 97, 346–351. [Google Scholar] [CrossRef]
- Roedel, F.; Kley, N.; Beuscher, H.U.; Hildebrandt, G.; Keilholz, L.; Kern, P.; Voll, R.; Herrmann, M.; Sauer, R. Anti-inflammatory effect of low-dose X-irradiation and the involvement of a TGF-beta1-induced down-regulation of leukocyte/endothelial cell adhesion. Int. J. Radiat. Biol. 2002, 78, 711–719. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Durante, M. Cancer risk from exposure to galactic cosmic rays: Implications for space exploration by human beings. Lancet Oncol. 2006, 7, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, J.C.; Blue, R.S.; Cengel, K.A.; Auñón-Chancellor, S.M.; Rubins, K.H.; Katzgraber, H.G.; Kennedy, A.R. Limitations in predicting the space radiation health risk for exploration astronauts. NPJ Microgravity 2018, 4, 8. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemec-Bakk, A.S.; Sridharan, V.; Desai, P.; Landes, R.D.; Hart, B.; Allen, A.R.; Boerma, M. Effects of Simulated 5-Ion Galactic Cosmic Radiation on Function and Structure of the Mouse Heart. Life 2023, 13, 795. https://doi.org/10.3390/life13030795
Nemec-Bakk AS, Sridharan V, Desai P, Landes RD, Hart B, Allen AR, Boerma M. Effects of Simulated 5-Ion Galactic Cosmic Radiation on Function and Structure of the Mouse Heart. Life. 2023; 13(3):795. https://doi.org/10.3390/life13030795
Chicago/Turabian StyleNemec-Bakk, Ashley S., Vijayalakshmi Sridharan, Parth Desai, Reid D. Landes, Barry Hart, Antiño R. Allen, and Marjan Boerma. 2023. "Effects of Simulated 5-Ion Galactic Cosmic Radiation on Function and Structure of the Mouse Heart" Life 13, no. 3: 795. https://doi.org/10.3390/life13030795
APA StyleNemec-Bakk, A. S., Sridharan, V., Desai, P., Landes, R. D., Hart, B., Allen, A. R., & Boerma, M. (2023). Effects of Simulated 5-Ion Galactic Cosmic Radiation on Function and Structure of the Mouse Heart. Life, 13(3), 795. https://doi.org/10.3390/life13030795








