Screening for Primordial RNA–Peptide Interactions Using High-Density Peptide Arrays
Abstract
1. Introduction
2. Materials and Methods
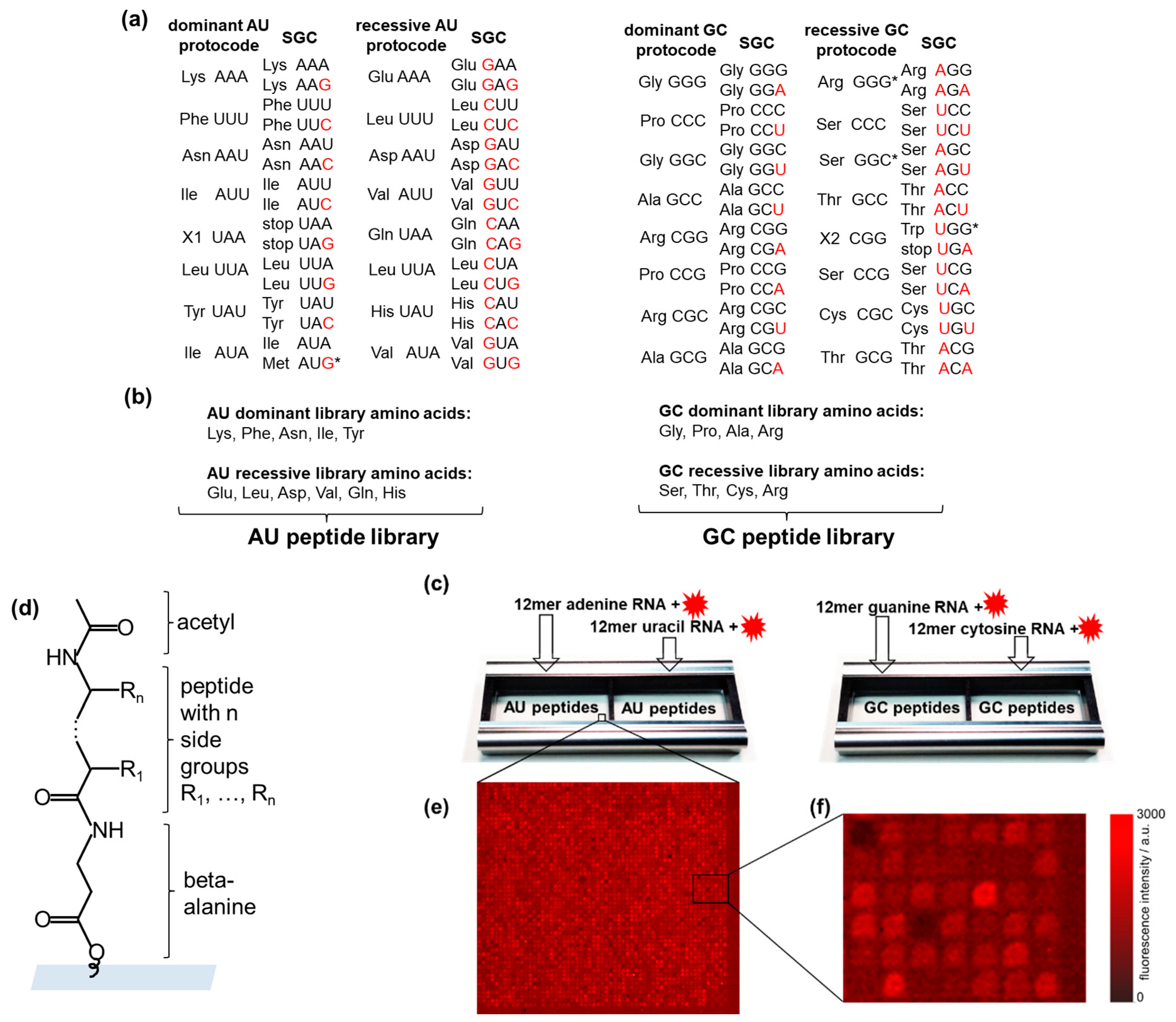
2.1. The Partition of the Standard Genetic Code According to the Combinatorial Fusion of Protocodes
2.2. The Peptide Libraries of the Protocodes
2.3. High-Density Peptide Arrays
2.4. Incubation of the Peptide Chips with RNA
2.5. Confocal Fluorescence Microscopy
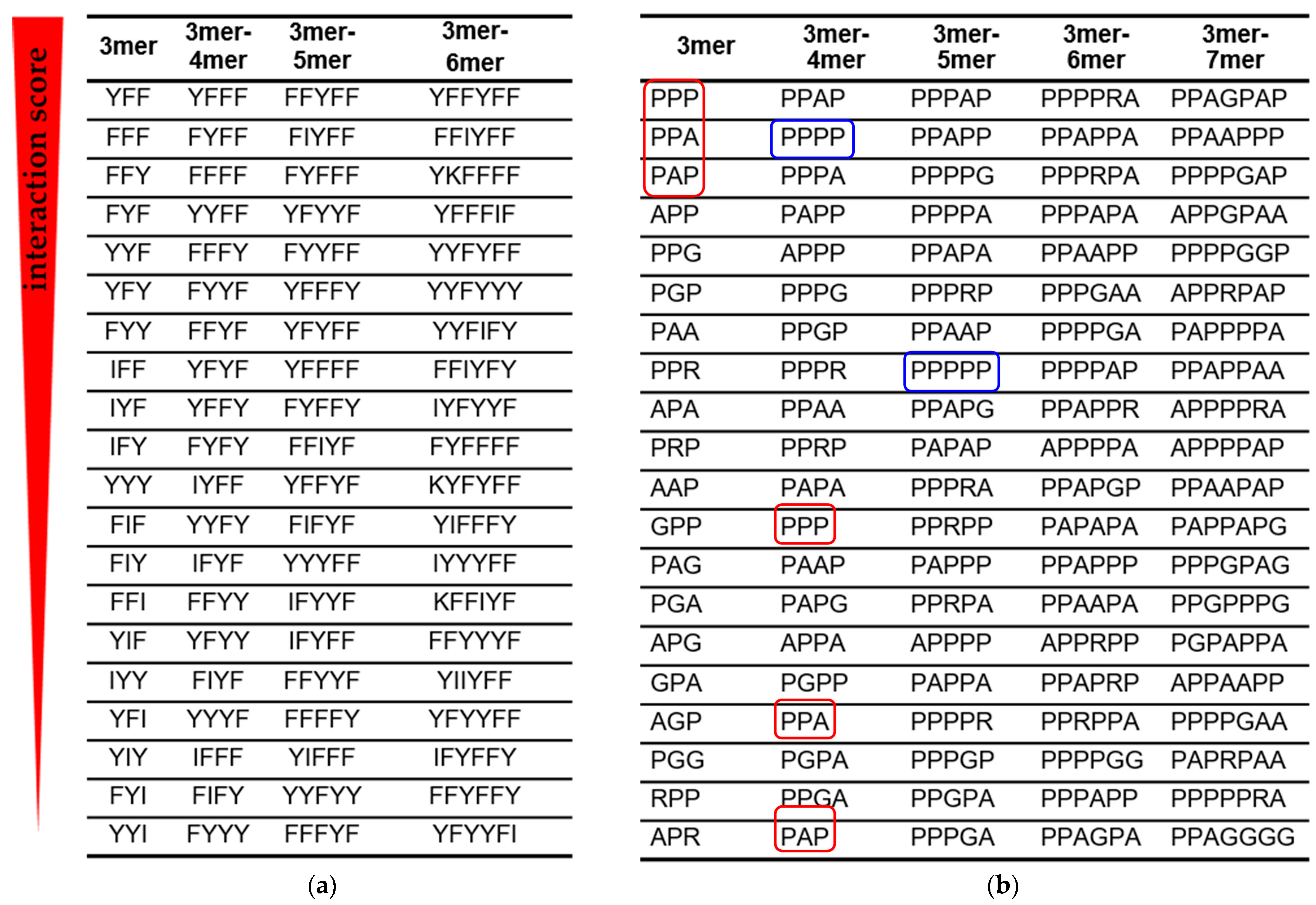
2.6. Ranking of Binding Signatures
3. Results
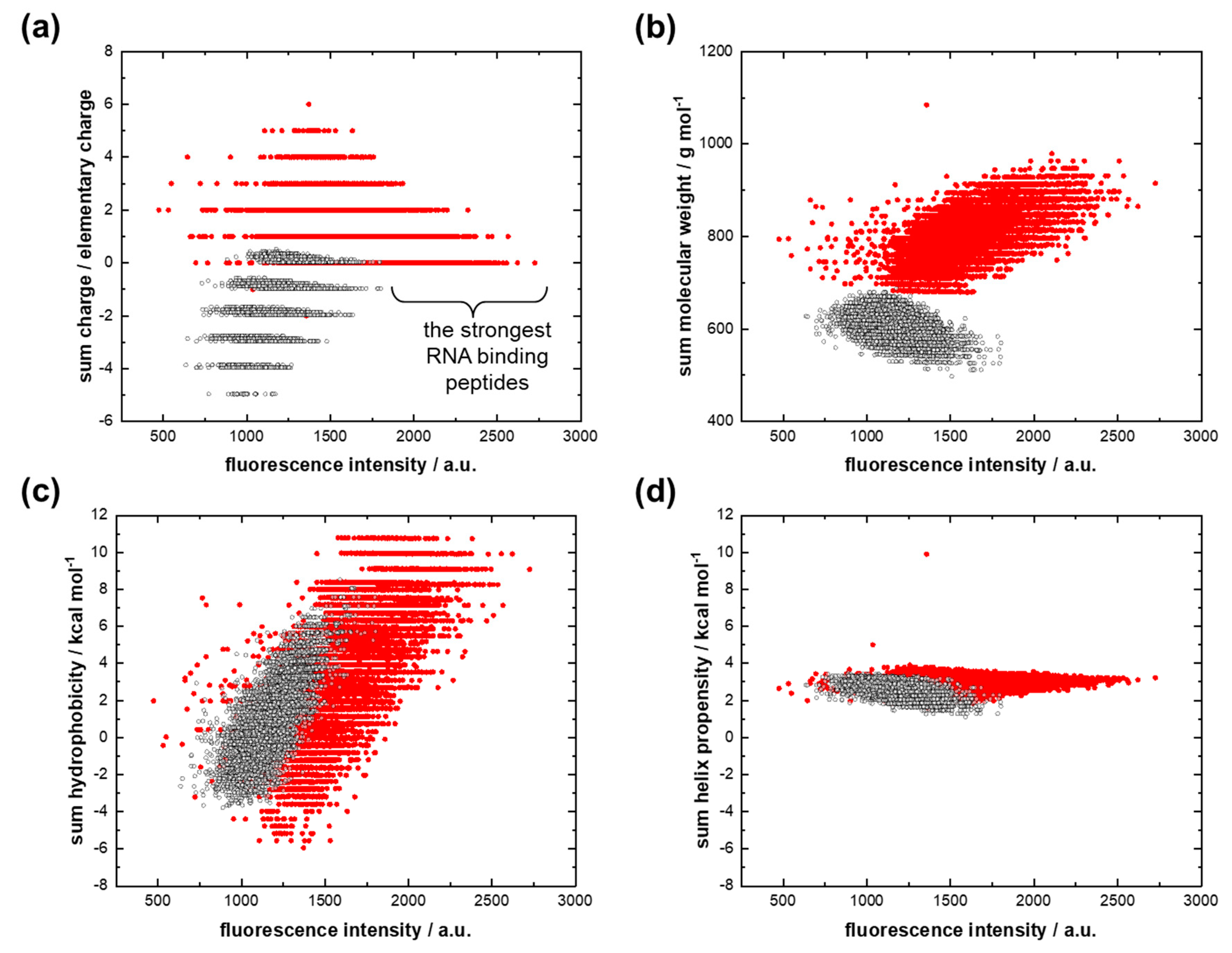
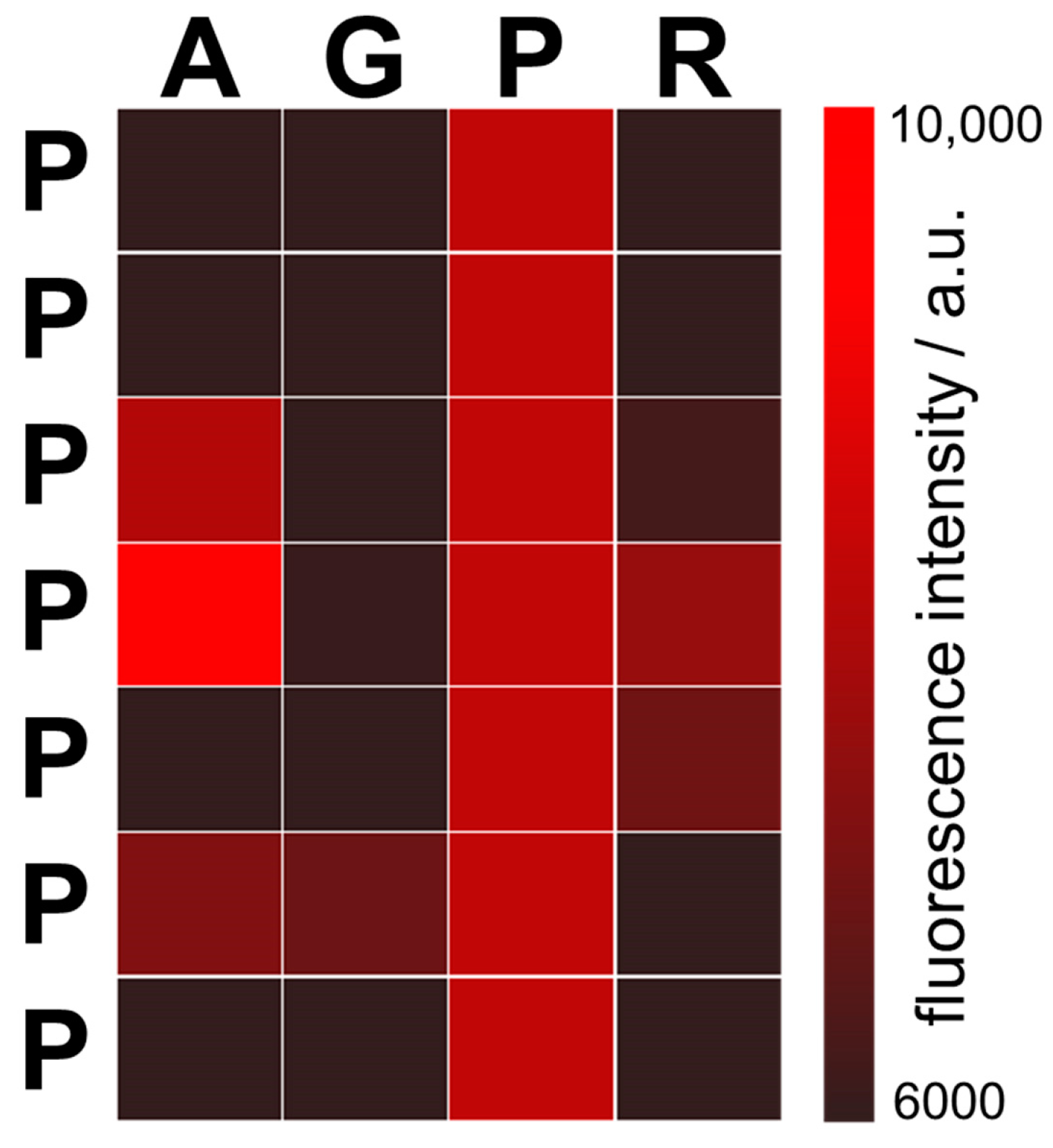
3.1. RNA Interactions with the AU Peptide Library
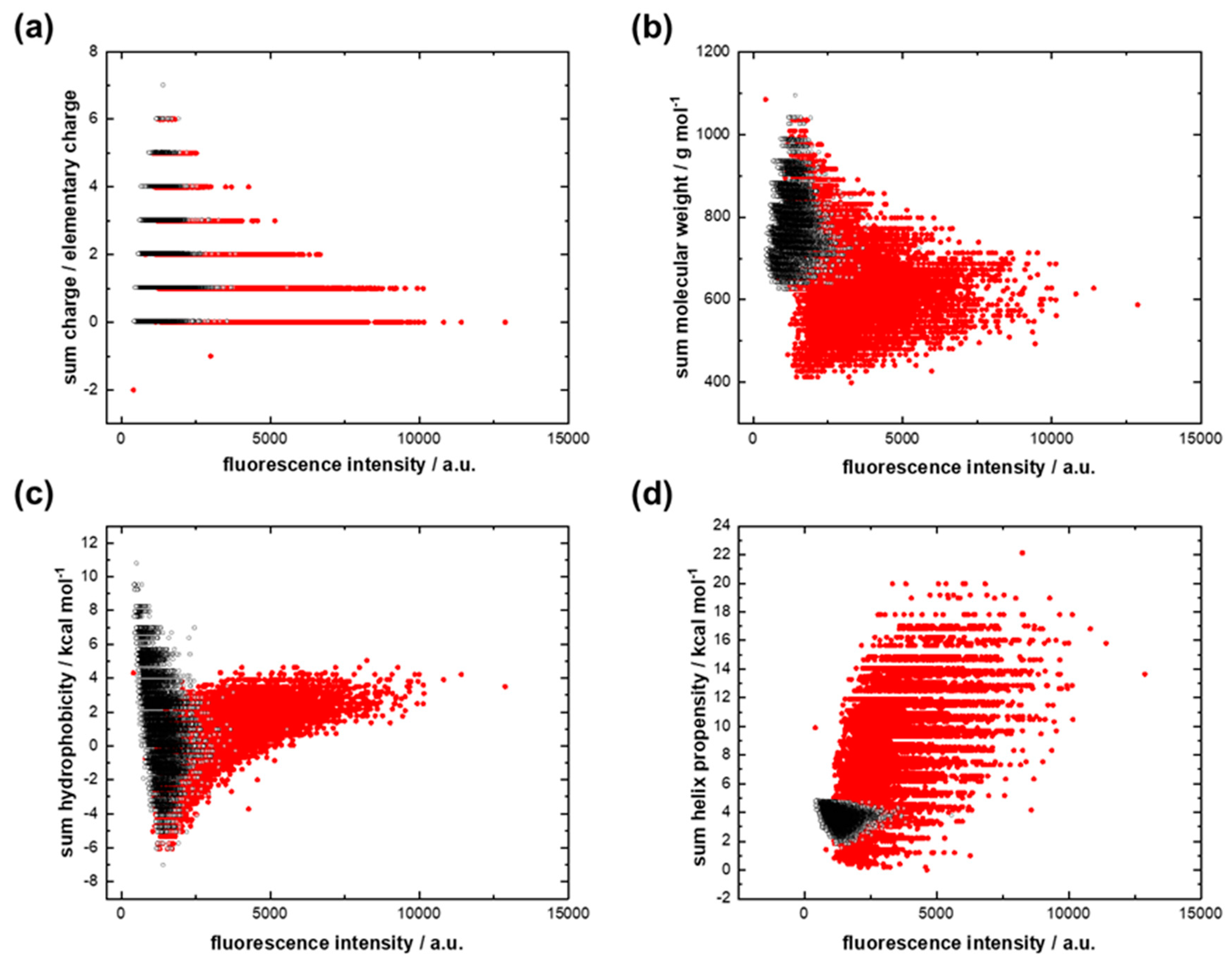
3.2. RNA Interactions with the GC Peptide Library
4. Discussion
4.1. The Combinatorial Fusion Cascade
- Small amphiphilic amino acid–RNA complexes based on complementary RNAs played an important role in primordial translation. Hydrophobicity was provided by hydrophobic amino acids;
- Each hydrophobic amino acid directly determined its hydrophilic partner amino acid.
4.2. The Standard Genetic Code as a Message
4.3. Peptide Arrays for Screening Binders to Ancient Folded RNAs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramakrishnan, V. Ribosome structure and the mechanism of translation. Cell 2002, 108, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Grosjean, H.; Westhof, E. An integrated, structure- and energy-based view of the genetic code. Nucleic Acids Res. 2016, 44, 8020–8040. [Google Scholar] [CrossRef] [PubMed]
- Eigen, M.; Schuster, P. The hypercycle. A principle of natural self-organization. Part A: Emergence of the hypercycle. Naturwissenschaften 1977, 64, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.; Escobar, L.; Xu, F.; Wegrzyn, E.; Nainyte, M.; Amatov, T.; Chan, C.Y.; Pichler, A.; Carell, T. A prebiotically plausible scenario of an RNA-peptide world. Nature 2022, 605, 279–284. [Google Scholar] [CrossRef]
- Di Giulio, M. On the RNA world: Evidence in favor of an early ribonucleopeptide world. J. Mol. Evol. 1997, 45, 571–578. [Google Scholar] [CrossRef]
- Jheeta, S.; Chatzitheodoridis, E.; Devine, K.; Block, J. The Way forward for the Origin of Life: Prions and Prion-Like Molecules First Hypothesis. Life 2021, 11, 872. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. Invitro Selection of Rna Molecules That Bind Specific Ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment—Rna Ligands to Bacteriophage-T4 DNA-Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Pelc, S.R.; Welton, M.G.E. Stereochemical Relationship between Coding Triplets and Amino-Acids. Nature 1966, 209, 868–870. [Google Scholar] [CrossRef]
- Koonin, E.V. Frozen Accident Pushing 50: Stereochemistry, Expansion, and Chance in the Evolution of the Genetic Code. Life 2017, 7, 22. [Google Scholar] [CrossRef]
- Woese, C.R. On the evolution of the genetic code. Proc. Natl. Acad. Sci. USA 1965, 54, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Yarus, M.; Widmann, J.J.; Knight, R. RNA-amino acid binding: A stereochemical era for the genetic code. J. Mol. Evol. 2009, 69, 406–429. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Khrapov, M.; Shaw, C.A. The scene of a frozen accident. RNA 2000, 6, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Yarus, M. RNA-ligand chemistry: A testable source for the genetic code. RNA 2000, 6, 475–484. [Google Scholar] [CrossRef]
- Yarus, M.; Caporaso, J.G.; Knight, R. Origins of the genetic code: The escaped triplet theory. Annu. Rev. Biochem. 2005, 74, 179–198. [Google Scholar] [CrossRef]
- Johnson, D.B.; Wang, L. Imprints of the genetic code in the ribosome. Proc. Natl. Acad. Sci. USA 2010, 107, 8298–8303. [Google Scholar] [CrossRef]
- Gupta, A.; Gribskov, M. The role of RNA sequence and structure in RNA--protein interactions. J. Mol. Biol. 2011, 409, 574–587. [Google Scholar] [CrossRef]
- Blin, K.; Dieterich, C.; Wurmus, R.; Rajewsky, N.; Landthaler, M.; Akalin, A. DoRiNA 2.0--upgrading the doRiNA database of RNA interactions in post-transcriptional regulation. Nucleic Acids Res. 2015, 43, D160–D167. [Google Scholar] [CrossRef]
- Anders, G.; Mackowiak, S.D.; Jens, M.; Maaskola, J.; Kuntzagk, A.; Rajewsky, N.; Landthaler, M.; Dieterich, C. doRiNA: A database of RNA interactions in post-transcriptional regulation. Nucleic Acids Res. 2012, 40, D180–D186. [Google Scholar] [CrossRef]
- Baltz, A.G.; Munschauer, M.; Schwanhausser, B.; Vasile, A.; Murakawa, Y.; Schueler, M.; Youngs, N.; Penfold-Brown, D.; Drew, K.; Milek, M.; et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol. Cell 2012, 46, 674–690. [Google Scholar] [CrossRef]
- Konig, J.; Zarnack, K.; Luscombe, N.M.; Ule, J. Protein-RNA interactions: New genomic technologies and perspectives. Nat. Rev. Genet. 2012, 13, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Polyansky, A.A.; Zagrovic, B. Evidence of direct complementary interactions between messenger RNAs and their cognate proteins. Nucleic Acids Res. 2013, 41, 8434–8443. [Google Scholar] [CrossRef] [PubMed]
- Hlevnjak, M.; Polyansky, A.A.; Zagrovic, B. Sequence signatures of direct complementarity between mRNAs and cognate proteins on multiple levels. Nucleic Acids Res. 2012, 40, 8874–8882. [Google Scholar] [CrossRef] [PubMed]
- Ouzounis, C.A.; Kyrpides, N.C. Reverse interpretation: A hypothetical selection mechanism for adaptive mutagenesis based on autoregulated mRNA stability. J. Theor. Biol. 1994, 167, 373–379. [Google Scholar] [CrossRef]
- Jenne, F.; Biniaminov, S.; Biniaminov, N.; Marquardt, P.; Von Bojnicic-Kninski, C.; Popov, R.; Seckinger, A.; Hose, D.; Nesterov-Mueller, A. Resemblance-Ranking Peptide Library to Screen for Binders to Antibodies on a Peptidomic Scale. Int. J. Mol. Sci. 2022, 23, 3515. [Google Scholar] [CrossRef]
- Fodor, S.P.; Read, J.L.; Pirrung, M.C.; Stryer, L.; Lu, A.T.; Solas, D. Light-directed, spatially addressable parallel chemical synthesis. Science 1991, 251, 767–773. [Google Scholar] [CrossRef]
- Loeffler, F.F.; Foertsch, T.C.; Popov, R.; Mattes, D.S.; Schlageter, M.; Sedlmayr, M.; Ridder, B.; Dang, F.X.; von Bojnicic-Kninski, C.; Weber, L.K.; et al. High-flexibility combinatorial peptide synthesis with laser-based transfer of monomers in solid matrix material. Nat. Commun. 2016, 7, 11844. [Google Scholar] [CrossRef]
- Paris, G.; Heidepriem, J.; Tsouka, A.; Liu, Y.; Mattes, D.S.; Pinzon Martin, S.; Dallabernardina, P.; Mende, M.; Lindner, C.; Wawrzinek, R.; et al. Automated Laser-Transfer Synthesis of High-Density Microarrays for Infectious Disease Screening. Adv. Mater. 2022, 34, e2200359. [Google Scholar] [CrossRef]
- Vetsigian, K.; Woese, C.; Goldenfeld, N. Collective evolution and the genetic code. Proc. Natl. Acad. Sci. USA 2006, 103, 10696–10701. [Google Scholar] [CrossRef]
- Kubyshkin, V.; Acevedo-Rocha, C.G.; Budisa, N. On universal coding events in protein biogenesis. Biosystems 2018, 164, 16–25. [Google Scholar] [CrossRef]
- Ochman, H.; Lawrence, J.G.; Groisman, E.A. Lateral gene transfer and the nature of bacterial innovation. Nature 2000, 405, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Makarova, K.S.; Aravind, L. Horizontal gene transfer in prokaryotes: Quantification and classification. Annu. Rev. Microbiol. 2001, 55, 709–742. [Google Scholar] [CrossRef] [PubMed]
- Trifonov, E.N.; Kirzhner, A.; Kirzhner, V.M.; Berezovsky, I.N. Distinct stages of protein evolution as suggested by protein sequence analysis. J. Mol. Evol. 2001, 53, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Nesterov-Müller, A.; Popov, R. Die Botschaft von LUCA—Der letzte universelle gemeinsame Vorfahre. Biospektrum 2020, 26, 488–489. [Google Scholar] [CrossRef]
- Nesterov-Mueller, A.; Popov, R.; Seligmann, H. Combinatorial Fusion Rules to Describe Codon Assignment in the Standard Genetic Code. Life 2021, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Opron, K.; Burton, Z.F. A tRNA- and Anticodon-Centric View of the Evolution of Aminoacyl-tRNA Synthetases, tRNAomes, and the Genetic Code. Life 2019, 9, 37. [Google Scholar] [CrossRef]
- Trifonov, E.N. Consensus temporal order of amino acids and evolution of the triplet code. Gene 2000, 261, 139–151. [Google Scholar] [CrossRef]
- Gardini, S.; Cheli, S.; Baroni, S.; Di Lascio, G.; Mangiavacchi, G.; Micheletti, N.; Monaco, C.L.; Savini, L.; Alocci, D.; Mangani, S.; et al. On Nature’s Strategy for Assigning Genetic Code Multiplicity. PLoS ONE 2016, 11, e0148174. [Google Scholar] [CrossRef]
- Mukai, T.; Yamaguchi, A.; Ohtake, K.; Takahashi, M.; Hayashi, A.; Iraha, F.; Kira, S.; Yanagisawa, T.; Yokoyama, S.; Hoshi, H.; et al. Reassignment of a rare sense codon to a non-canonical amino acid in Escherichia coli. Nucleic Acids Res. 2015, 43, 8111–8122. [Google Scholar] [CrossRef]
- Ree, R.; Varland, S.; Arnesen, T. Spotlight on protein N-terminal acetylation. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef]
- Biomers.net. 5’ Modification. Available online: https://www.biomers.net/de/Katalog/Modifikationen/Cy5NH/MOD5 (accessed on 24 January 2023).
- Weber, L.K.; Isse, A.; Rentschler, S.; Kneusel, R.E.; Palermo, A.; Hubbuch, J.; Nesterov-Mueller, A.; Breitling, F.; Loeffler, F.F. Antibody fingerprints in lyme disease deciphered with high density peptide arrays. Eng. Life Sci. 2017, 17, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Weber, L.K.; Rentschler, S.; Isse, A.; Sedlmayr, M.; Herbster, K.; List, V.; Hubbuch, J.; Loffler, F.F.; Nesterov-Muller, A.; et al. Identification of a Tetanus Toxin Specific Epitope in Single Amino Acid Resolution. Biotechnol. J. 2017, 12, 1700197. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.S. Amino-Acid and Peptide Net Charges—A Simple Calculational Procedure. Biochem. Educ. 1985, 13, 10–11. [Google Scholar] [CrossRef]
- Fauchere, J.L.; Pliska, V. Hydrophobic Parameters-Pi of Amino-Acid Side-Chains from the Partitioning of N-Acetyl-Amino-Acid Amides. Eur. J. Med. Chem. 1983, 18, 369–375. [Google Scholar]
- Pace, C.N.; Scholtz, J.M. A helix propensity scale based on experimental studies of peptides and proteins. Biophys. J. 1998, 75, 422–427. [Google Scholar] [CrossRef]
- Illangasekare, M.; Yarus, M. Specific, rapid synthesis of Phe-RNA by RNA. Proc. Natl. Acad. Sci. USA 1999, 96, 5470–5475. [Google Scholar] [CrossRef]
- Kubyshkin, V.; Budisa, N. The Alanine World Model for the Development of the Amino Acid Repertoire in Protein Biosynthesis. Int. J. Mol. Sci. 2019, 20, 5507. [Google Scholar] [CrossRef]
- Hartman, H.; Smith, T.F. The evolution of the ribosome and the genetic code. Life 2014, 4, 227–249. [Google Scholar] [CrossRef]
- Carter, C.W.; Wills, P.R. Hierarchical groove discrimination by Class I and II aminoacyl-tRNA synthetases reveals a palimpsest of the operational RNA code in the tRNA acceptor-stem bases. Nucleic Acids Res. 2018, 46, 9667–9683. [Google Scholar] [CrossRef]
- Caetano-Anolles, G.; Wang, M.L.; Caetano-Anolles, D. Structural Phylogenomics Retrodicts the Origin of the Genetic Code and Uncovers the Evolutionary Impact of Protein Flexibility. PLoS ONE 2013, 8, e72225. [Google Scholar] [CrossRef]
- Di Giulio, M. The origin of the genetic code: Theories and their relationships, a review. Biosystems 2005, 80, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Crick, F.H.C. Codon-Anticodon Pairing—Wobble Hypothesis. J. Mol. Biol. 1966, 19, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Copley, S.D.; Smith, E.; Morowitz, H.J. A mechanism for the association of amino acids with their codons and the origin of the genetic code. Proc. Natl. Acad. Sci. USA 2005, 102, 4442–4447. [Google Scholar] [CrossRef] [PubMed]
- Nesterov-Mueller, A.; Popov, R. The Combinatorial Fusion Cascade to Generate the Standard Genetic Code. Life 2021, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- Himbert, S.; Chapman, M.; Deamer, D.W.; Rheinstadter, M.C. Organization of Nucleotides in Different Environments and the Formation of Pre-Polymers. Sci. Rep. 2016, 6, 31285. [Google Scholar] [CrossRef]
- Joyce, G.F.; Szostak, J.W. Protocells and RNA Self-Replication. Csh. Perspect. Biol. 2018, 10, a034801. [Google Scholar] [CrossRef]
- Ikehara, K. Possible steps to the emergence of life: The [GADV]-protein world hypothesis. Chem. Rec. 2005, 5, 107–118. [Google Scholar] [CrossRef]
- Pai, J.; Yoon, T.; Kim, N.D.; Lee, I.S.; Yu, J.; Shin, I. High-Throughput Profiling of Peptide-RNA Interactions Using Peptide Microarrays. J. Am. Chem. Soc. 2012, 134, 19287–19296. [Google Scholar] [CrossRef]
- Burton, Z.F. The 3-Minihelix tRNA Evolution Theorem. J. Mol. Evol. 2020, 88, 234–242. [Google Scholar] [CrossRef]
- Di Giulio, M. The origin of the tRNA molecule: Implications for the origin of protein synthesis. J. Theor. Biol. 2004, 226, 89–93. [Google Scholar] [CrossRef]
- Kanai, A. Disrupted tRNA Genes and tRNA Fragments: A Perspective on tRNA Gene Evolution. Life 2015, 5, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Frankel, A.D. Sequence and structure space of RNA-binding peptides. Biopolymers 2003, 70, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hyun, S.; Kim, H.J.; Yu, J. Amphiphilic helical peptides containing two acridine moieties display picomolar affinity toward HIV-1 RRE and TAR. Angew. Chem. Int. Edit. 2008, 47, 134–137. [Google Scholar] [CrossRef]
- Zhou, Q.A.; Sharp, P.A. Tat-SF1: Cofactor for stimulation of transcriptional elongation by HIV-1 Tat. Science 1996, 274, 605–610. [Google Scholar] [CrossRef] [PubMed]
| Protocode RNA | AU Dominant 12-mer A | AU Recessive 12-mer A | AU Dominant 12-mer U | AU Recessive 12-mer U |
|---|---|---|---|---|
| Mean | 1569 | 1191 | 56 | 61 |
| Stand. deviation | 228 | 148 | 8 | 14 |
| Protocode RNA | GC Dominant 12-mer G | GC Recessive 12-mer G | GC Dominant 12-mer C | GC Recessive 12-mer C |
|---|---|---|---|---|
| Mean | 684 | 559 | 3117 | 1248 |
| Stand. deviation | 251 | 196 | 1299 | 433 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenne, F.; Berezkin, I.; Tempel, F.; Schmidt, D.; Popov, R.; Nesterov-Mueller, A. Screening for Primordial RNA–Peptide Interactions Using High-Density Peptide Arrays. Life 2023, 13, 796. https://doi.org/10.3390/life13030796
Jenne F, Berezkin I, Tempel F, Schmidt D, Popov R, Nesterov-Mueller A. Screening for Primordial RNA–Peptide Interactions Using High-Density Peptide Arrays. Life. 2023; 13(3):796. https://doi.org/10.3390/life13030796
Chicago/Turabian StyleJenne, Felix, Ivan Berezkin, Frank Tempel, Dimitry Schmidt, Roman Popov, and Alexander Nesterov-Mueller. 2023. "Screening for Primordial RNA–Peptide Interactions Using High-Density Peptide Arrays" Life 13, no. 3: 796. https://doi.org/10.3390/life13030796
APA StyleJenne, F., Berezkin, I., Tempel, F., Schmidt, D., Popov, R., & Nesterov-Mueller, A. (2023). Screening for Primordial RNA–Peptide Interactions Using High-Density Peptide Arrays. Life, 13(3), 796. https://doi.org/10.3390/life13030796






