The Role of Seaweed Polysaccharides in Gastrointestinal Health: Protective Effect against Inflammatory Bowel Disease
Abstract
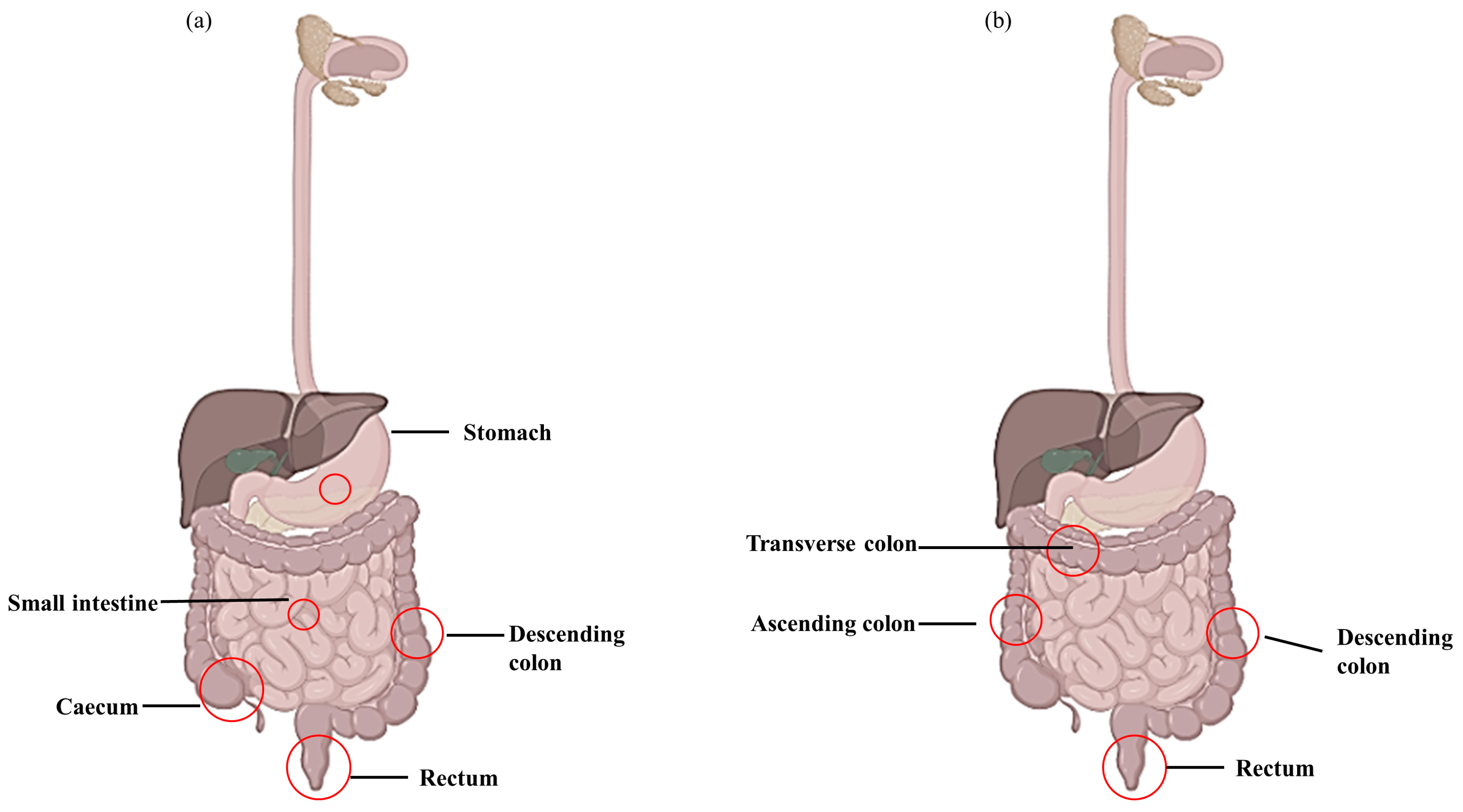
:1. Introduction
2. Methodology
3. IBD Mechanism
3.1. Intestinal Epithelium
3.2. Intestinal Microflora
3.3. Immune System
4. Marine Polysaccharides
4.1. Brown Seaweeds
4.2. Green Algal Polysaccharides
4.3. Red Algal Polysaccharides
5. Therapeutic Targets of Algal Polysaccharides against IBD
5.1. Intracellular Adhesion Molecules
5.2. Intestinal Epithelial Cells
5.3. Pro-Inflammatory Cytokines
5.4. Intestinal Microbiome
6. Algal Polysaccharides in Drug Delivery Systems
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izcue, A.; Coombes, J.L.; Powrie, F. Regulatory Lymphocytes and Intestinal Inflammation. Annu. Rev. Immunol. 2009, 27, 313–338. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.J.; Blumberg, R.S. The Immunology of Mucosal Models of Inflammation. Annu. Rev. Immunol. 2002, 20, 495–549. [Google Scholar] [CrossRef] [PubMed]
- Buret, A.G.; Motta, J.-P.; Allain, T.; Ferraz, J.; Wallace, J.L. Pathobiont release from dysbiotic gut microbiota biofilms in intestinal inflammatory diseases: A role for iron? J. Biomed. Sci. 2019, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- World Gastroenterology Organisation. Global Guardian of Digestive Health. Serving the World. 2022. [Google Scholar]
- Li, C.; Ai, G.; Wang, Y.; Lu, Q.; Luo, C.; Tan, L.; Lin, G.; Liu, Y.; Li, Y.; Zeng, H.; et al. Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: Impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-κB pathway. Pharmacol. Res. 2020, 152, 104603. [Google Scholar] [CrossRef]
- Lee, S.H.; eun Kwon, J.; Cho, M.-L. Immunological pathogenesis of inflammatory bowel disease. Intestig. Res. 2018, 16, 26. [Google Scholar] [CrossRef]
- Elia, P.P.; Tolentino, Y.F.M.; Bernardazzi, C.; de Souza, H.S.P. The Role of Innate Immunity Receptors in the Pathogenesis of Inflammatory Bowel Disease. Mediat. Inflamm. 2015, 2015, 936193. [Google Scholar] [CrossRef]
- Duff, W.; Haskey, N.; Potter, G.; Alcorn, J.; Hunter, P.; Fowler, S. Non-pharmacological therapies for inflammatory bowel disease: Recommendations for self-care and physician guidance. World J. Gastroenterol. 2018, 24, 3055. [Google Scholar] [CrossRef]
- Nielsen, O.H. New Strategies for Treatment of Inflammatory Bowel Disease. Front. Med. 2014, 1, 3. [Google Scholar] [CrossRef]
- Ren, Y.; Geng, Y.; Du, Y.; Li, W.; Lu, Z.-M.; Xu, H.-Y.; Xu, G.-H.; Shi, J.-S.; Xu, Z.-H. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018, 57, 67–76. [Google Scholar] [CrossRef]
- Catalan-Serra, I.; Brenna, Ø. Immunotherapy in inflammatory bowel disease: Novel and emerging treatments. Hum. Vaccines Immunother. 2018, 14, 2597–2611. [Google Scholar] [CrossRef]
- Dulai, P.S.; Siegel, C.A. The risk of malignancy associated with the use of biological agents in patients with inflammatory bowel disease. Gastroenterol. Clin. 2014, 43, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Nagahawatta, D.P.; Liyanage, N.M.; Je, J.-G.; Jayawardhana, H.H.A.C.K.; Jayawardena, T.U.; Jeong, S.-H.; Kwon, H.-J.; Choi, C.S.; Jeon, Y.-J. Polyphenolic Compounds Isolated from Marine Algae Attenuate the Replication of SARS-CoV-2 in the Host Cell through a Multi-Target Approach of 3CLpro and PLpro. Mar. Drugs 2022, 20, 786. [Google Scholar] [PubMed]
- Liyanage, N.M.; Nagahawatta, D.P.; Jayawardena, T.U.; Jayawardhana, H.H.A.C.K.; Lee, H.-G.; Kim, Y.-S.; Jeon, Y.-J. Clionasterol-Rich Fraction of Caulerpa racemosa against Particulate Matter-Induced Skin Damage via Inhibition of Oxidative Stress and Apoptosis-Related Signaling Pathway. Antioxidants 2022, 11, 1941. [Google Scholar] [CrossRef] [PubMed]
- Nagahawatta, D.P.; Liyanage, N.M.; Jayawardhana, H.H.A.C.K.; Jayawardena, T.U.; Lee, H.-G.; Heo, M.-S.; Jeon, Y.-J. Eckmaxol Isolated from Ecklonia maxima Attenuates Particulate-Matter-Induced Inflammation in MH-S Lung Macrophage. Mar. Drugs 2022, 20, 766. [Google Scholar]
- Nagahawatta, D.P.; Liyanage, N.M.; Jayawardhana, H.H.A.C.K.; Lee, H.-G.; Jayawardena, T.U.; Jeon, Y.-J. Anti-Fine Dust Effect of Fucoidan Extracted from Ecklonia maxima Leaves in Macrophages via Inhibiting Inflammatory Signaling Pathways. Mar. Drugs 2022, 20, 413. [Google Scholar] [PubMed]
- Liyanage, N.M.; Lee, H.-G.; Nagahawatta, D.P.; Jayawardhana, H.H.A.C.K.; Ryu, B.; Jeon, Y.-J. Characterization and therapeutic effect of Sargassum coreanum fucoidan that inhibits lipopolysaccharide-induced inflammation in RAW 264.7 macrophages by blocking NF-κB signaling. Int. J. Biol. Macromol. 2022, 223, 500–510. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar]
- Choi, J.-I.; Raghavendran, H.R.B.; Sung, N.-Y.; Kim, J.-H.; Chun, B.S.; Ahn, D.H.; Choi, H.-S.; Kang, K.-W.; Lee, J.-W. Effect of fucoidan on aspirin-induced stomach ulceration in rats. Chem. Biol. Interact. 2010, 183, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Lajili, S.; Ammar, H.H.; Mzoughi, Z.; Amor, H.B.H.; Muller, C.D.; Majdoub, H.; Bouraoui, A. Characterization of sulfated polysaccharide from Laurencia obtusa and its apoptotic, gastroprotective and antioxidant activities. Int. J. Biol. Macromol. 2019, 126, 326–336. [Google Scholar] [CrossRef]
- Okolie, C.L.; CK Rajendran, S.R.; Udenigwe, C.C.; Aryee, A.N.; Mason, B. Prospects of brown seaweed polysaccharides (BSP) as prebiotics and potential immunomodulators. J. Food Biochem. 2017, 41, e12392. [Google Scholar] [CrossRef]
- Xie, S.-Z.; Liu, B.; Ye, H.-Y.; Li, Q.-M.; Pan, L.-H.; Zha, X.-Q.; Liu, J.; Duan, J.; Luo, J.-P. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr. Polym. 2019, 206, 149–162. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, P.; Li, X.; Guo, L.; Gao, W. The potential roles of natural plant polysaccharides in inflammatory bowel disease: A review. Carbohydr. Polym. 2022, 277, 118821. [Google Scholar] [CrossRef]
- Ho Do, M.; Seo, Y.S.; Park, H.-Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. 2021, 61, 1212–1224. [Google Scholar] [CrossRef]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences: The Epithelial Barrier and its Relationship With Mucosal Immunity in Inflammatory Bowel Disease. CMGH 2017, 4, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Ghyselinck, J.; Verstrepen, L.; Moens, F.; Van den Abbeele, P.; Said, J.; Smith, B.; Bjarnason, I.; Basit, A.W.; Gaisford, S. A 4-strain probiotic supplement influences gut microbiota composition and gut wall function in patients with ulcerative colitis. Int. J. Pharm. 2020, 587, 119648. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.-Y.; Xiang, X.-W.; Du, M.; Zhang, L.-F.; Cheng, N.-X.; Liu, X.-L.; Zheng, B.; Wen, Z.-S. Protective effect of polysaccharides of sea cucumber Acaudina leucoprocta on hydrogen peroxide-induced oxidative injury in RAW264.7 cells. Int. J. Biol. Macromol. 2019, 139, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Liu, X.; Luo, Y.; Yu, R.; Chen, S.; Zhang, J.; Xu, Y.; Meng, Z.; Huang, Y.; Ren, Z. Characterization of polysaccharides isolated from Hericium erinaceus and their protective effects on the DON-induced oxidative stress. Int. J. Biol. Macromol. 2020, 152, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cheng, L.; Zeng, X.; Zhang, X.; Wu, Z.; Weng, P. The modulatory effect of plant polysaccharides on gut flora and the implication for neurodegenerative diseases from the perspective of the microbiota-gut-brain axis. Int. J. Biol. Macromol. 2020, 164, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Wu, W.; Lepp, D.; Wellings, H.R.; Hutchinson, A.L.; Liddle, D.M.; Graf, D.; Pauls, K.P.; Robinson, L.E.; Power, K.A. Navy bean supplemented high-fat diet improves intestinal health, epithelial barrier integrity and critical aspects of the obese inflammatory phenotype. J. Nutr. Biochem. 2019, 70, 91–104. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Wang, M.; Wei, J.; Yang, L.; Wu, G. Perioperative alterations in the intestinal microbiota and functional changes mediate innate immune activation after small bowel transplantation. Life Sci. 2021, 277, 119468. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shen, F.; Wang, W.; Qi, C.; Wang, C.; Shang, A.; Xuan, S. The effect of multispecies probiotics on cognitive reactivity to sad mood in patients with Crohn’s disease. J. Funct. Foods 2021, 82, 104431. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, R.; Cheng, L.; Yang, J.; Zhu, L. Peroxisome proliferator-activated receptor gamma activation promotes intestinal barrier function by improving mucus and tight junctions in a mouse colitis model. JGLD 2018, 50, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, Y.; Zhang, S.; Zhang, X.; Du, Z.; Li, M.; Ding, K. Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and SCFAs metabolism. Int. J. Biol. Macromol. 2021, 181, 357–368. [Google Scholar] [CrossRef]
- Ueno, A.; Jeffery, L.; Kobayashi, T.; Hibi, T.; Ghosh, S.; Jijon, H. Th17 plasticity and its relevance to inflammatory bowel disease. J. Autoimmun. 2018, 87, 38–49. [Google Scholar] [CrossRef]
- Huang, C.; Yao, R.; Zhu, Z.; Pang, D.; Cao, X.; Feng, B.; Paulsen, B.S.; Li, L.; Yin, Z.; Chen, X. A pectic polysaccharide from water decoction of Xinjiang Lycium barbarum fruit protects against intestinal endoplasmic reticulum stress. Int. J. Biol. Macromol. 2019, 130, 508–514. [Google Scholar] [CrossRef]
- Okada, S.; Puel, A.; Casanova, J.L.; Kobayashi, M. Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. CTI 2016, 5, e114. [Google Scholar] [CrossRef]
- Belkaid, Y.; Rouse, B.T. Natural regulatory T cells in infectious disease. Nat. Immunol. 2005, 6, 353–360. [Google Scholar] [CrossRef]
- Niu, W.; Chen, X.; Xu, R.; Dong, H.; Yang, F.; Wang, Y.; Zhang, Z.; Ju, J. Polysaccharides from natural resources exhibit great potential in the treatment of ulcerative colitis: A review. Carbohydr. Polym. 2021, 254, 117189. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, S.; Ye, J.; Wang, X.; Zhang, X.; Shen, J.; Yuan, M.; Liao, W. Polysaccharide from flammuliana velutipes improves colitis via regulation of colonic microbial dysbiosis and inflammatory responses. Int. J. Biol. Macromol. 2020, 149, 1252–1261. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Conlon, M.A.; Franco, C.M.M.; Zhang, W. The development of seaweed-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends Food Sci. Technol. 2017, 70, 20–33. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Miranda, J.M.; Mondragon, A.D.; Lamas, A.; Cardelle-Cobas, A.; Franco, C.M.; Cepeda, A. Potential Use of Marine Seaweeds as Prebiotics: A Review. Molecules 2020, 25, 1004. [Google Scholar] [CrossRef] [PubMed]
- Gurpilhares, D.D.B.; Cinelli, L.P.; Simas, N.K.; Pessoa, A., Jr.; Sette, L.D. Marine prebiotics: Polysaccharides and oligosaccharides obtained by using microbial enzymes. Food Chem. 2019, 280, 175–186. [Google Scholar] [CrossRef]
- Hwang, J.; Yadav, D.; Lee, P.C.; Jin, J.O. Immunomodulatory effects of polysaccharides from marine algae for treating cancer, infectious disease, and inflammation. Phytother. Res. 2022, 36, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-O.; Chauhan, P.S.; Arukha, A.P.; Chavda, V.; Dubey, A.; Yadav, D. The Therapeutic Potential of the Anticancer Activity of Fucoidan: Current Advances and Hurdles. Mar. Drugs 2021, 19, 265. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, F.; Zhang, J.; Wang, W.; Li, L.; Yan, J. Modulatory effects of polysaccharides from plants, marine algae and edible mushrooms on gut microbiota and related health benefits: A review. Int. J. Biol. Macromol. 2022, 204, 169–192. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Karuppusamy, S.; Rajauria, G.; Fitzpatrick, S.; Lyons, H.; McMahon, H.; Curtin, J.; Tiwari, B.K.; O’Donnell, C. Biological Properties and Health-Promoting Functions of Laminarin: A Comprehensive Review of Preclinical and Clinical Studies. Mar. Drugs 2022, 20, 772. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, H.; Mao, X.; Ci, F. Laminarin and Laminarin Oligosaccharides Originating from Brown Algae: Preparation, Biological Activities, and Potential Applications. J. Ocean Univ. 2021, 20, 641–653. [Google Scholar] [CrossRef]
- Cui, D.; Ma, J.; Liang, T.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Selenium nanoparticles fabricated in laminarin polysaccharides solutions exert their cytotoxicities in HepG2 cells by inhibiting autophagy and promoting apoptosis. Int. J. Biol. Macromol. 2019, 137, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Graves, B.; Child, R.; Rice, P.J.; Ma, Z.; Lowman, D.W.; Ensley, H.E.; Ryter, K.T.; Evans, J.T.; Williams, D.L. Immunoregulatory Activity of the Natural Product Laminarin Varies Widely as a Result of Its Physical Properties. J. Immun. 2018, 200, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Corino, C.; Di Giancamillo, A.; Modina, S.C.; Rossi, R. Prebiotic Effects of Seaweed Polysaccharides in Pigs. Animals 2021, 11, 1573. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Nagahawatta, D.P.; Fernando, I.P.S.; Kim, Y.-T.; Kim, J.-S.; Kim, W.-S.; Lee, J.S.; Jeon, Y.-J. A Review on Fucoidan Structure, Extraction Techniques, and Its Role as an Immunomodulatory Agent. Mar. Drugs 2022, 20, 755. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Brunel, M.; Bonnin, E.J.C.R. Fine chemical structure analysis of oligosaccharides produced by an ulvan-lyase degradation of the water-soluble cell-wall polysaccharides from Ulva sp. (Ulvales, Chlorophyta). Carbohydr. Res. 1997, 304, 325–333. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Kesavan, S.; Meena, K.S.; Sharmili, S.A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Alobaidi, A.S.; Alanzi, K.F.; Vaseeharan, B. Ulvan loaded graphene oxide nanoparticle fabricated with chitosan and d-mannose for targeted anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2021, 65, 102760. [Google Scholar] [CrossRef]
- Hung, Y.-H.R.; Chen, G.-W.; Pan, C.-L.; Lin, H.-T.V. Production of Ulvan Oligosaccharides with Antioxidant and Angiotensin-Converting Enzyme-Inhibitory Activities by Microbial Enzymatic Hydrolysis. Fermentation 2021, 7, 160. [Google Scholar] [CrossRef]
- Liu, X.; Yu, K.; Cheng, S.; Ren, T.; Maitusong, M.; Liu, F.; Chen, J.; Qian, Y.; Xu, D.; Zhu, G.; et al. Ulvan mediated VE cadherin antibody and REDV peptide co-modification to improve endothelialization potential of bioprosthetic heart valves. Mater. Sci. Eng. C 2021, 128, 112337. [Google Scholar] [CrossRef]
- Li, B.; Xu, H.; Wang, X.; Wan, Y.; Jiang, N.; Qi, H.; Liu, X. Antioxidant and antihyperlipidemic activities of high sulfate content purified polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 146, 756–762. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral potential of algae polysaccharides isolated from marine sources: A review. Biomed Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef]
- Kalitnik, A.A.; Byankina Barabanova, A.O.; Nagorskaya, V.P.; Reunov, A.V.; Glazunov, V.P.; Solov’eva, T.F.; Yermak, I.M. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2013, 25, 65–72. [Google Scholar] [CrossRef]
- Lahaye, M. Developments on gelling algal galactans, their structure and physico-chemistry. J. Appl. Phycol. 2001, 13, 173–184. [Google Scholar] [CrossRef]
- Abu-Khudir, R.; Ismail, G.A.; Diab, T. Antimicrobial, antioxidant, and anti-tumor activities of Sargassum linearifolium and Cystoseira crinita from Egyptian Mediterranean Coast. Nutr. Cancer 2021, 73, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Oliyaei, N.; Moosavi-Nasab, M.; Mazloomi, S.M. Therapeutic activity of fucoidan and carrageenan as marine algal polysaccharides against viruses. 3 Biotech 2022, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Al-Bawardy, B.; Shivashankar, R.; Proctor, D.D. Novel and emerging therapies for inflammatory bowel disease. Front. Pharmacol. 2021, 12, 651415. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.V.; Mazurov, A.V.; Preobrazhenskaia, M.E.; Ushakova, N.A.; Mikhaĭlov, V.I.; Berman, A.E.; Usov, A.I.; Nifant’ev, N.E.; Bovin, N.V. Sulfated polysaccharides as inhibitors of receptor activity of P-selectin and P-selectin-dependent inflammation. Vopr. Med. Khimii 1998, 44, 135–144. [Google Scholar] [PubMed]
- Nuñez-Andrade, N.; Lamana, A.; Sancho, D.; Gisbert, J.P.; Gonzalez-Amaro, R.; Sanchez-Madrid, F.; Urzainqui, A. P-selectin glycoprotein ligand-1 modulates immune inflammatory responses in the enteric lamina propria. J. Pathol. 2011, 224, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonell, R.; Yao, S.-J.; Das, S.; Guma, M. Dysregulation of Intestinal Epithelial Cell RIPK Pathways Promotes Chronic Inflammation in the IBD Gut. Front. Immunol. 2019, 10, 94. [Google Scholar] [CrossRef]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The Antioxidant Activity of Polysaccharides Derived from Marine Organisms: An Overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef]
- Iraha, A.; Chinen, H.; Hokama, A.; Yonashiro, T.; Kinjo, T.; Kishimoto, K.; Nakamoto, M.; Hirata, T.; Kinjo, N.; Higa, F. Fucoidan enhances intestinal barrier function by upregulating the expression of claudin-1. World J. Gastroenterol. 2013, 19, 5500. [Google Scholar] [CrossRef]
- Yang, H.-S.; Haj, F.G.; Lee, M.; Kang, I.; Zhang, G.; Lee, Y. Laminaria japonica Extract Enhances Intestinal Barrier Function by Altering Inflammatory Response and Tight Junction-Related Protein in Lipopolysaccharide-Stimulated Caco-2 Cells. Nutrients 2019, 11, 1001. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-J.; Don, T.-M.; Lin, C.-W.; Mi, F.-L. Delivery of Berberine Using Chitosan/Fucoidan-Taurine Conjugate Nanoparticles for Treatment of Defective Intestinal Epithelial Tight Junction Barrier. Mar. Drugs 2014, 12, 5677–5697. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zheng, W.; Zhang, C.; Zhang, P.; Guo, X.; Song, S.; Ai, C. Fucoidan from Scytosiphon lomentaria protects against destruction of intestinal barrier, inflammation and lipid abnormality by modulating the gut microbiota in dietary fibers-deficient mice. Int. J. Biol. Macromol. 2023, 224, 556–567. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Wang, X.; Yu, Y.; Xie, J. Natural Food Polysaccharides Ameliorate Inflammatory Bowel Disease and Its Mechanisms. Foods 2021, 10, 1288. [Google Scholar] [CrossRef]
- Rana, S.V.; Sharma, S.; Kaur, J.; Prasad, K.K.; Sinha, S.K.; Kochhar, R.; Malik, A.; Morya, R.K. Relationship of cytokines, oxidative stress and GI motility with bacterial overgrowth in ulcerative colitis patients. J. Crohns Colitis 2014, 8, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, S.; Nikfar, S.; Abdolghaffari, A.H.; Abdollahi, M. New biologic therapeutics for ulcerative colitis and Crohn’s disease. Expert Opin. Biol. Ther. 2014, 14, 583–600. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Ruder, B.; Atreya, R.; Becker, C. Tumour Necrosis Factor Alpha in Intestinal Homeostasis and Gut Related Diseases. Int. J. Mol. Sci. 2019, 20, 1887. [Google Scholar] [CrossRef]
- Pereira, L. Biological and therapeutic properties of the seaweed polysaccharides. Int. Biol. Rev. 2018, 2. [Google Scholar] [CrossRef]
- O’Shea, C.J.; O’Doherty, J.V.; Callanan, J.J.; Doyle, D.; Thornton, K.; Sweeney, T. The effect of algal polysaccharides laminarin and fucoidan on colonic pathology, cytokine gene expression and Enterobacteriaceae in a dextran sodium sulfate-challenged porcine model. J. Nutr. Sci. 2016, 5, e15. [Google Scholar] [CrossRef]
- Sudirman, S.; Hsu, Y.-H.; He, J.-L.; Kong, Z.-L. Dietary polysaccharide-rich extract from Eucheuma cottonii modulates the inflammatory response and suppresses colonic injury on dextran sulfate sodium-induced colitis in mice. PLoS ONE 2018, 13, e0205252. [Google Scholar] [CrossRef]
- Ryan, M.T.; O’Shea, C.J.; Collins, C.B.; O’Doherty, J.V.; Sweeney, T. Effects of dietary supplementation with Laminaria hyperborea, Laminaria digitata, and Saccharomyces cerevisiae on the IL-17 pathway in the porcine colon. J. Anim. Sci. 2012, 90 (Suppl. 4), 263–265. [Google Scholar] [CrossRef]
- Lean, Q.Y.; Eri, R.D.; Fitton, J.H.; Patel, R.P.; Gueven, N. Fucoidan Extracts Ameliorate Acute Colitis. PLoS ONE 2015, 10, e0128453. [Google Scholar] [CrossRef] [PubMed]
- Kiron, V.; Hayes, M.; Avni, D. Inflammatory bowel disease—A peek into the bacterial community shift and algae-based ‘biotic’ approach to combat the disease. Trends Food Sci. Technol. 2022, 129, 210–220. [Google Scholar] [CrossRef]
- Pan, X.; Yin, M.; Guo, M.; Niu, X.; Han, L. The latest progress of natural food polysaccharides preventing ulcerative colitis by regulating intestinal microbiota. J. Funct. Foods 2022, 96, 105201. [Google Scholar] [CrossRef]
- Zuo, T.; Ng, S.C. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar] [CrossRef]
- Al Mijan, M.; Lim, B.O. Diets, functional foods, and nutraceuticals as alternative therapies for inflammatory bowel disease: Present status and future trends. World J. Gastroenterol. 2018, 24, 2673. [Google Scholar] [CrossRef]
- Liyanage, N.M.; Kim, Y.-S.; Nagahawatta, D.P.; Jin, H.; Yang, H.-W.; Jayawardhana, H.H.A.C.K.; Jayawardena, T.U.; Jeon, Y.-J. Sargassum horneri as a Prebiotic Dietary Supplement for Immunity Development in Streptococcus parauberis Infected Zebrafish Model. Front. Mar. Sci. 2022, 9, 1676. [Google Scholar] [CrossRef]
- Huang, W.; Tan, H.; Nie, S. Beneficial effects of seaweed-derived dietary fiber: Highlights of the sulfated polysaccharides. Food Chem. 2022, 373, 131608. [Google Scholar] [CrossRef]
- Nie, Y.; Lin, Q.; Luo, F. Effects of Non-Starch Polysaccharides on Inflammatory Bowel Disease. Int. J. Mol. Sci. 2017, 18, 1372. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Goulart, R.d.A.; Aranão, A.L.d.C.; de Oliveira, P.G.C. Inflammatory bowel diseases and fermentable oligosaccharides, disaccharides, monosaccharides, and polyols: An overview. J. Med. Food 2018, 21, 633–640. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The Potential of Seaweeds as a Source of Functional Ingredients of Prebiotic and Antioxidant Value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; De Morais, A.M.; De Morais, R.M. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef]
- Cheng, L.; Kong, L.; Xia, C.; Zeng, X.; Wu, Z.; Guo, Y.; Pan, D. Sources, Processing-Related Transformation, and Gut Axis Regulation of Conventional and Potential Prebiotics. J. Agric. Food Chem. 2022, 70, 4509–4521. [Google Scholar] [CrossRef] [PubMed]
- Segarra, S.; Martínez-Subiela, S.; Cerdà-Cuéllar, M.; Martínez-Puig, D.; Muñoz-Prieto, A.; Rodríguez-Franco, F.; Rodríguez-Bertos, A.; Allenspach, K.; Velasco, A.; Cerón, J. Oral chondroitin sulfate and prebiotics for the treatment of canine Inflammatory Bowel Disease: A randomized, controlled clinical trial. BMC Vet. Res. 2016, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shang, Q.; Li, G.; Wang, X.; Yu, G. Degradation of Marine Algae-Derived Carbohydrates by Bacteroidetes Isolated from Human Gut Microbiota. Mar. Drugs 2017, 15, 92. [Google Scholar] [CrossRef]
- Martínez-Abad, B.; Garrote, J.A.; Bernardo, D.; Montalvillo, E.; Escudero-Hernández, C.; Vázquez, E.; Rueda, R.; Arranz, E. Differential immunomodulatory effects of Lactobacillus rhamnosus DR20, Lactobacillus fermentum CECT 5716 and Bifidobacterium animalis subsp. lactis on monocyte-derived dendritic cells. J. Funct. Foods 2016, 22, 300–312. [Google Scholar] [CrossRef]
- Stagg, A.J.; Hart, A.L.; Knight, S.C.; Kamm, M.A. The dendritic cell: Its role in intestinal inflammation and relationship with gut bacteria. Gut 2003, 52, 1522. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.L.; Lammers, K.; Brigidi, P.; Vitali, B.; Rizzello, F.; Gionchetti, P.; Campieri, M.; Kamm, M.A.; Knight, S.C.; Stagg, A.J. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut 2004, 53, 1602. [Google Scholar] [CrossRef]
- Kusnetsova, T.; Zaporozhets, T.; Makarenkova, I.; Besednova, N.; Timchenko, N.; Zvyagintseva, T.; Shevchenko, N.; Mandrakova, N.; Melnikov, V. The prebiotic potential of polysaccharides from the brown alga Fucus evanescens and significance for the clinical use. Pac. Med. J. 2012, 1, 37–40. [Google Scholar]
- Zaporozhets, T.S.; Besednova, N.N.; Kuznetsova, T.A.; Zvyagintseva, T.N.; Makarenkova, I.D.; Kryzhanovsky, S.P.; Melnikov, V.G. The prebiotic potential of polysaccharides and extracts of seaweeds. Russ. J. Mar. Biol. 2014, 40, 1–9. [Google Scholar] [CrossRef]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- Kong, Q.; Dong, S.; Gao, J.; Jiang, C. In vitro fermentation of sulfated polysaccharides from E. prolifera and L. japonica by human fecal microbiota. Int. J. Biol. Macromol. 2016, 91, 867–871. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, R.; You, L.; Ma, Y.; Liao, L.; Pedisić, S. In vitro fermentation characteristics of polysaccharide from Sargassum fusiforme and its modulation effects on gut microbiota. FCT 2021, 151, 112145. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chang, Y.; Gao, Y.; Wang, X.; Chen, X.; Wang, Y.; Xue, C.; Tang, Q. Dietary fucoidan of Acaudina molpadioides alters gut microbiota and mitigates intestinal mucosal injury induced by cyclophosphamide. Food Funct. 2017, 8, 3383–3393. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, W.; Sun, X.; Jiang, G.; Wu, S.; Xu, Y.; Song, S.; Ai, C. Sulfated polysaccharides from Undaria pinnatifida improved high fat diet-induced metabolic syndrome, gut microbiota dysbiosis and inflammation in BALB/c mice. Int. J. Biol. Macromol. 2021, 167, 1587–1597. [Google Scholar] [CrossRef]
- Fu, X.; Cao, C.; Ren, B.; Zhang, B.; Huang, Q.; Li, C. Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota. Carbohydr. Polym. 2018, 183, 230–239. [Google Scholar] [CrossRef]
- Ikeda-Ohtsubo, W.; López Nadal, A.; Zaccaria, E.; Iha, M.; Kitazawa, H.; Kleerebezem, M.; Brugman, S. Intestinal Microbiota and Immune Modulation in Zebrafish by Fucoidan from Okinawa Mozuku (Cladosiphon okamuranus). Front. Nutr. 2020, 7, 67. [Google Scholar] [CrossRef]
- Brito, T.V.; Neto, J.P.R.P.; Prudêncio, R.S.; Batista, J.A.; Júnior, J.S.C.; Silva, R.O.; Franco, Á.X.; Aragão, K.S.; Soares, P.M.G.; Souza, M.H.L.P.; et al. Sulfated-polysaccharide fraction extracted from red algae Gracilaria birdiae ameliorates trinitrobenzenesulfonic acid-induced colitis in rats. J. Pharm. Pharmacol. 2014, 66, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Dutra, N.L.S.; de Brito, T.V.; Magalhães, D.d.A.; Sousa, S.G.; Batista, J.A.; Pereira, C.M.C.; Ferreira, J.d.S.; Rodrigues, L.d.R.; Lima, J.V.d.N.; de Albuquerque, I.F.; et al. Sulfated polysaccharide extracted from seaweed Gracilaria caudata attenuates acetic acid-induced ulcerative colitis. Food Hydrocoll. 2021, 111, 106221. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ko, C.-I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.-S.; Jeon, Y.-J. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Brito, T.V.; Barros, F.C.N.; Silva, R.O.; Dias Júnior, G.J.; Júnior, J.S.C.; Franco, Á.X.; Soares, P.M.G.; Chaves, L.S.; Abreu, C.M.W.S.; de Paula, R.C.M.; et al. Sulfated polysaccharide from the marine algae Hypnea musciformis inhibits TNBS-induced intestinal damage in rats. Carbohydr. Polym. 2016, 151, 957–964. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Liu, Y.; Tang, S.; Zhang, W.; Yu, Q.; Shi, C.; Cheong, K.-L. Gracilaria lemaneiformis polysaccharides alleviate colitis by modulating the gut microbiota and intestinal barrier in mice. Food Chem. X 2022, 13, 100197. [Google Scholar] [CrossRef]
- Song, W.; Li, Y.; Zhang, X.; Wang, Z. Potent anti-inflammatory activity of polysaccharides extracted from Blidingia minima and their effect in a mouse model of inflammatory bowel disease. J. Funct. Foods 2019, 61, 103494. [Google Scholar] [CrossRef]
- Li, Y.; Ye, H.; Wang, T.; Wang, P.; Liu, R.; Li, Y.; Tian, Y.; Zhang, J. Characterization of Low Molecular Weight Sulfate Ulva Polysaccharide and its Protective Effect against IBD in Mice. Mar. Drugs 2020, 18, 499. [Google Scholar] [CrossRef]
- Misra, A.; Shahiwala, A. Novel Drug Delivery Technologies; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Zhang, M.; Merlin, D. Nanoparticle-Based Oral Drug Delivery Systems Targeting the Colon for Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, T.M.; Fong, P.M.; Goyal, A.; Saltzman, W.M. Targeted for drug delivery. Mater. Today 2005, 8 (Suppl. 8), 18–26. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Huang, Y.-C. Soluble eggshell membrane protein-loaded chitosan/fucoidan nanoparticles for treatment of defective intestinal epithelial cells. Int. J. Biol. Macromol. 2019, 131, 949–958. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.C.R.P.; Todaro, V.; do Carmo, F.A.; Frattani, F.S.; de Sousa, V.P.; Rodrigues, C.R.; Sathler, P.C.; Cabral, L.M. A promising oral fucoidan-based antithrombotic nanosystem: Development, activity and safety. Nanotechnology 2018, 29, 165102. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, S.; Song, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Chen, T.; Zheng, W.; Huang, Z. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-κB mediated hyper inflammation. J. Nanobiotechnol. 2017, 15, 20. [Google Scholar] [CrossRef] [PubMed]

| Source | Polysaccharide Type | Inducer | Model | Function | Reference |
|---|---|---|---|---|---|
| Laminaria japonica | Fucoidan | Cefoperazone | Mice | Reduces H2O2-induced paracellular permeability. Up-regulates the expression of claudin-1, claudin-2, and occludin. | [76] |
| Sargassum fusiforme | Crude sulfated polysaccharide | - | Bacteria | Increases the availability of Faecalibacterium, Phascolarctobacterium, Ruminococcaeceae, and Lactobacillus. | [110] |
| Laminaria japonica | fucoidan | - | Mice | Increases Ruminococcaceae and Lactobacillus. | [111] |
| Undaria pinnatifida | Fucoidan | Hight-fat diet | Mice | Reduces weight gain and fat accumulation, as wel as restores the normal level of Firmicutes and Bacteriodetes in mice fed high-fat diets. | [112] |
| Acaudina molpadioides | Fucoidan | Cyclophosphamide | Mice | Alleviates inflammation, increases protein expression in tight junctions and the abundance of Coprococcus, Rikenella, and Butyricicoccus bacteria. | [111] |
| Sargassum thungbergii | Crude polysaccharide | - | - | Increases intestinal beneficial bacteria. | [113] |
| Cladosiphon okamuranusv | Fucoidan | LPS | Zebrafish | Down-regulates IL-1β pro-inflammatory gene expression. | [114] |
| Gracilaria biridae | Sulfated polysaccharide | Trinitrobenzenesulfonic acid (TNBS) | Rat | Reduces TNBS-induced intestinal damage. | [115] |
| c | Sulfated polysaccharide | Acetic acid | Mice | Inhibits colonic inflammation by reducing the wet weight of colon, reduces MPO enzyme activity, GSH consumption, and pro-inflammatory cytokine production. | [116] |
| Ecklonia cava | Fucoidan | LPS | zebrafish | Inhibits ROS and NO production. | [117] |
| Hypnea musciformis | Sulfated polysaccharides | TNBS | Rats | Reduces inflammatory responses by reducing neutrophil migration, decreases cytokine levels, and downregulates oxidative stress and intestinal damage | [118] |
| Gracilaria lemaneiformis | polysaccharides | Dextran sulfate sodium (DSS) | Mice | Improves DSS-induced colitis symptoms, alters intestinal microbes, increases SCFA production, and downregulates CCL25/CCR9,TGF-β1 levels. | [119] |
| Blidingia minima | Polysaccharides | DSS | Mice | Repairs colon dysfunction and colonic morphology, improves the expression of tight junction proteins, and decreases pro-inflammatory cytokine levels. | [120] |
| Eucheuma cottonii | Polysaccharide | DSS | Mice | Protects against weight loss, decreases the colon weight per length ratio, decreases pro-inflammatory cytokine levels, increass IL-10 production, and reduces colonic damage. | [87] |
| Ulva pertusa | Sulfate ulva polysaccharide | DSS | Mice | Decreases colon shortening and colonic tissue damage. | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liyanage, N.M.; Nagahawatta, D.P.; Jayawardena, T.U.; Jeon, Y.-J. The Role of Seaweed Polysaccharides in Gastrointestinal Health: Protective Effect against Inflammatory Bowel Disease. Life 2023, 13, 1026. https://doi.org/10.3390/life13041026
Liyanage NM, Nagahawatta DP, Jayawardena TU, Jeon Y-J. The Role of Seaweed Polysaccharides in Gastrointestinal Health: Protective Effect against Inflammatory Bowel Disease. Life. 2023; 13(4):1026. https://doi.org/10.3390/life13041026
Chicago/Turabian StyleLiyanage, N. M., D. P. Nagahawatta, Thilina U. Jayawardena, and You-Jin Jeon. 2023. "The Role of Seaweed Polysaccharides in Gastrointestinal Health: Protective Effect against Inflammatory Bowel Disease" Life 13, no. 4: 1026. https://doi.org/10.3390/life13041026








