The Bacterial Nucleoid: From Electron Microscopy to Polymer Physics—A Personal Recollection
Abstract
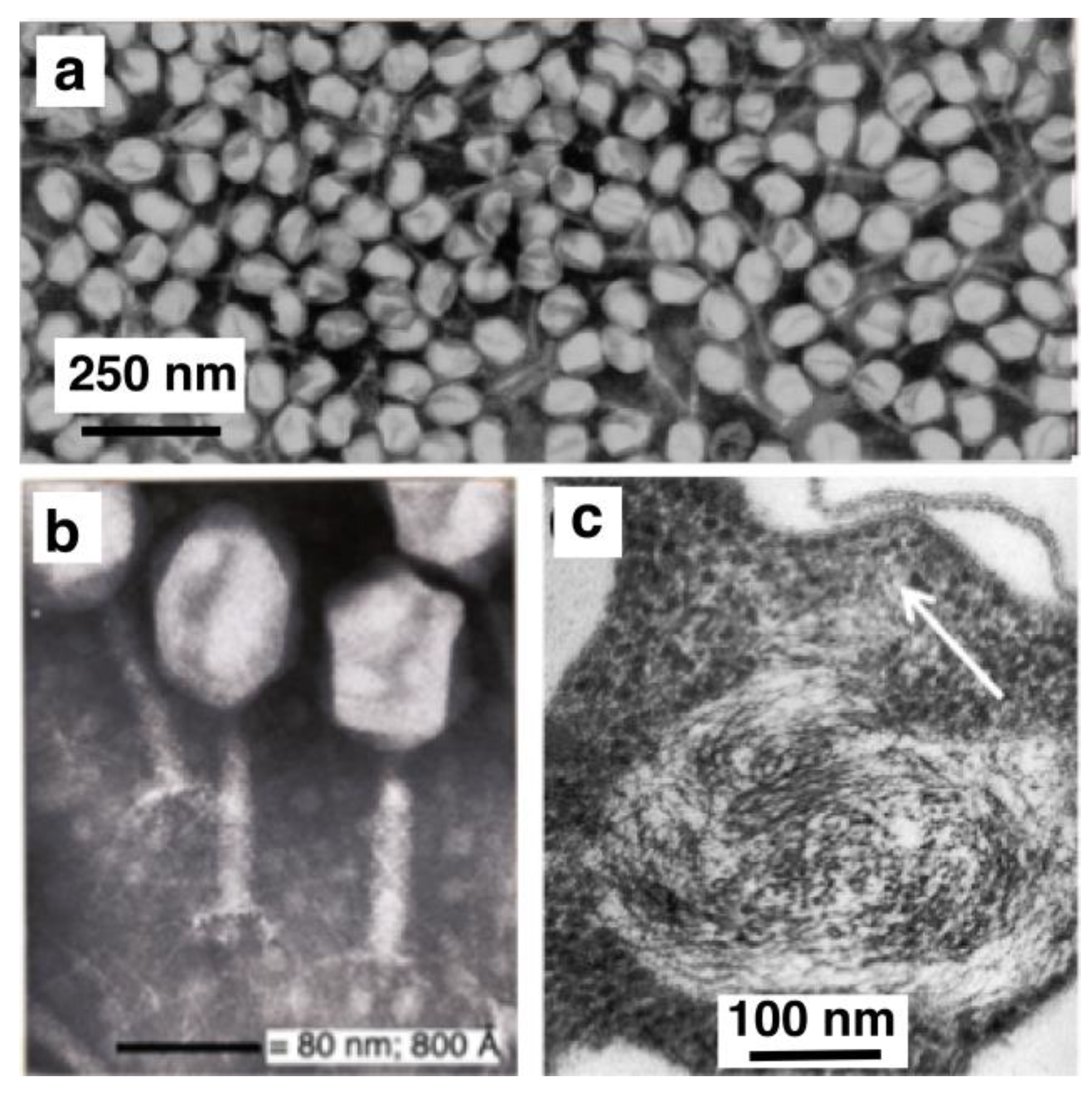
:1. Electron and Light Microscopy
2. Cell Size, Shape and Growth Models
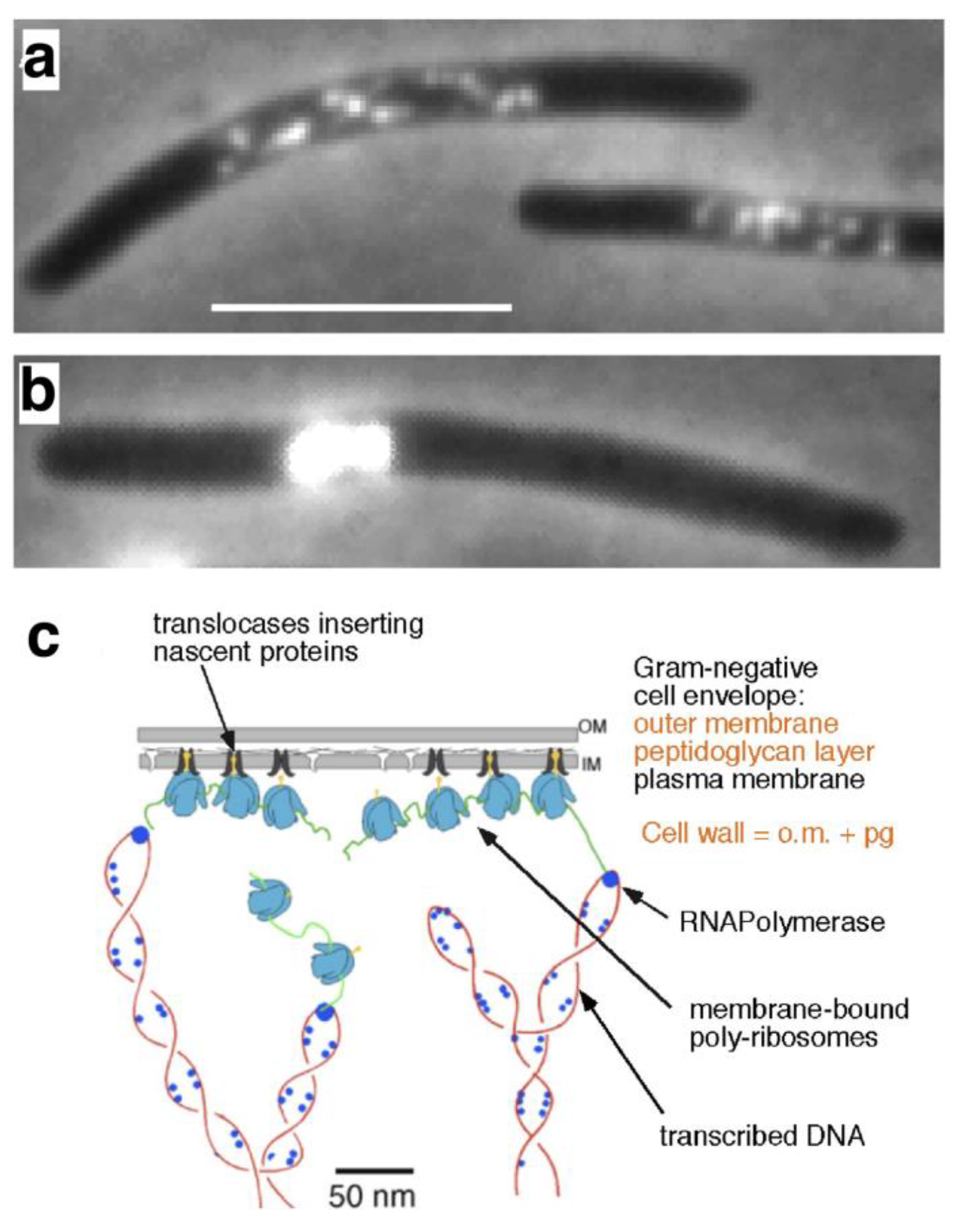
3. Nucleoid Occlusion and Transertion
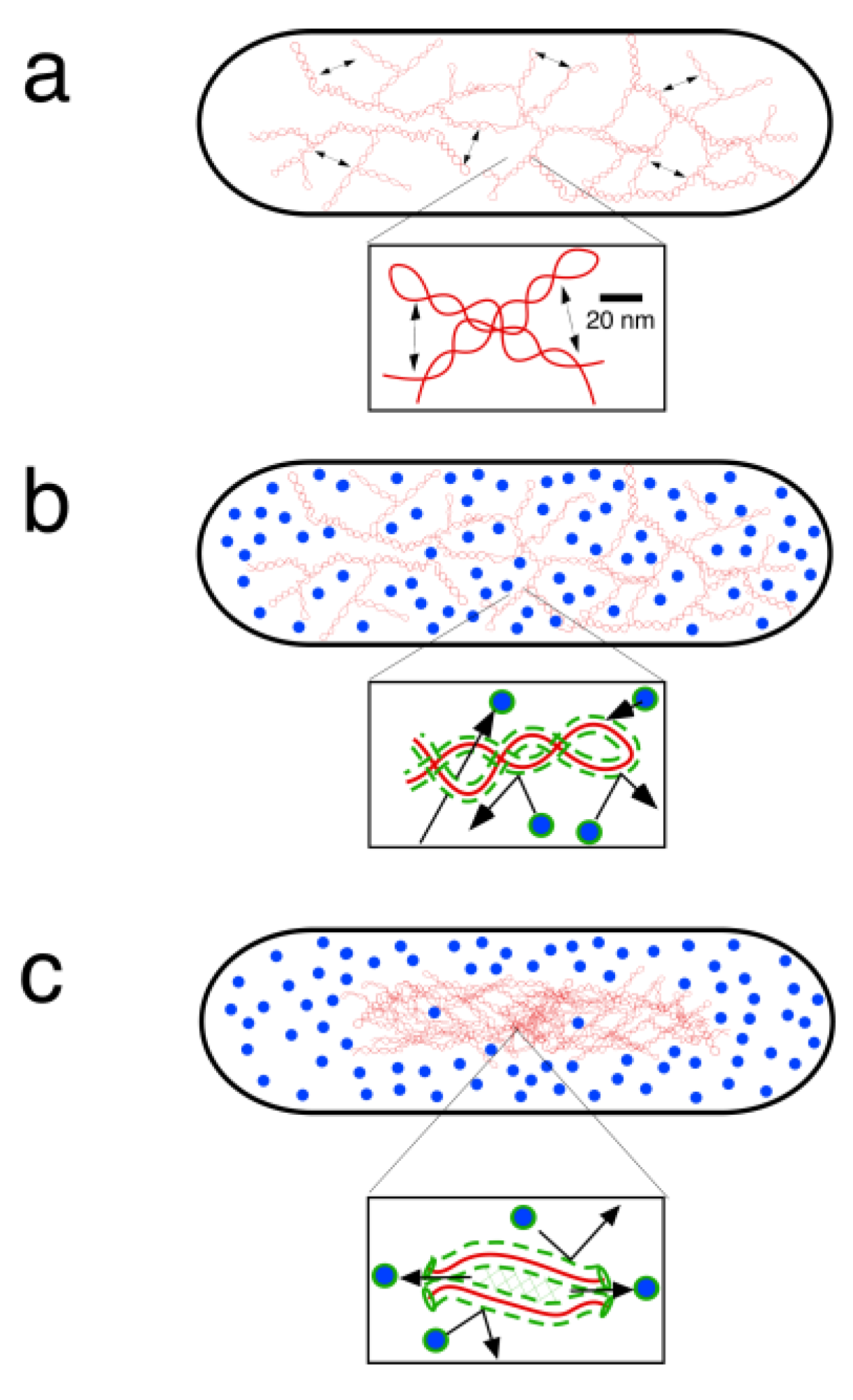
4. Physical DNA Model
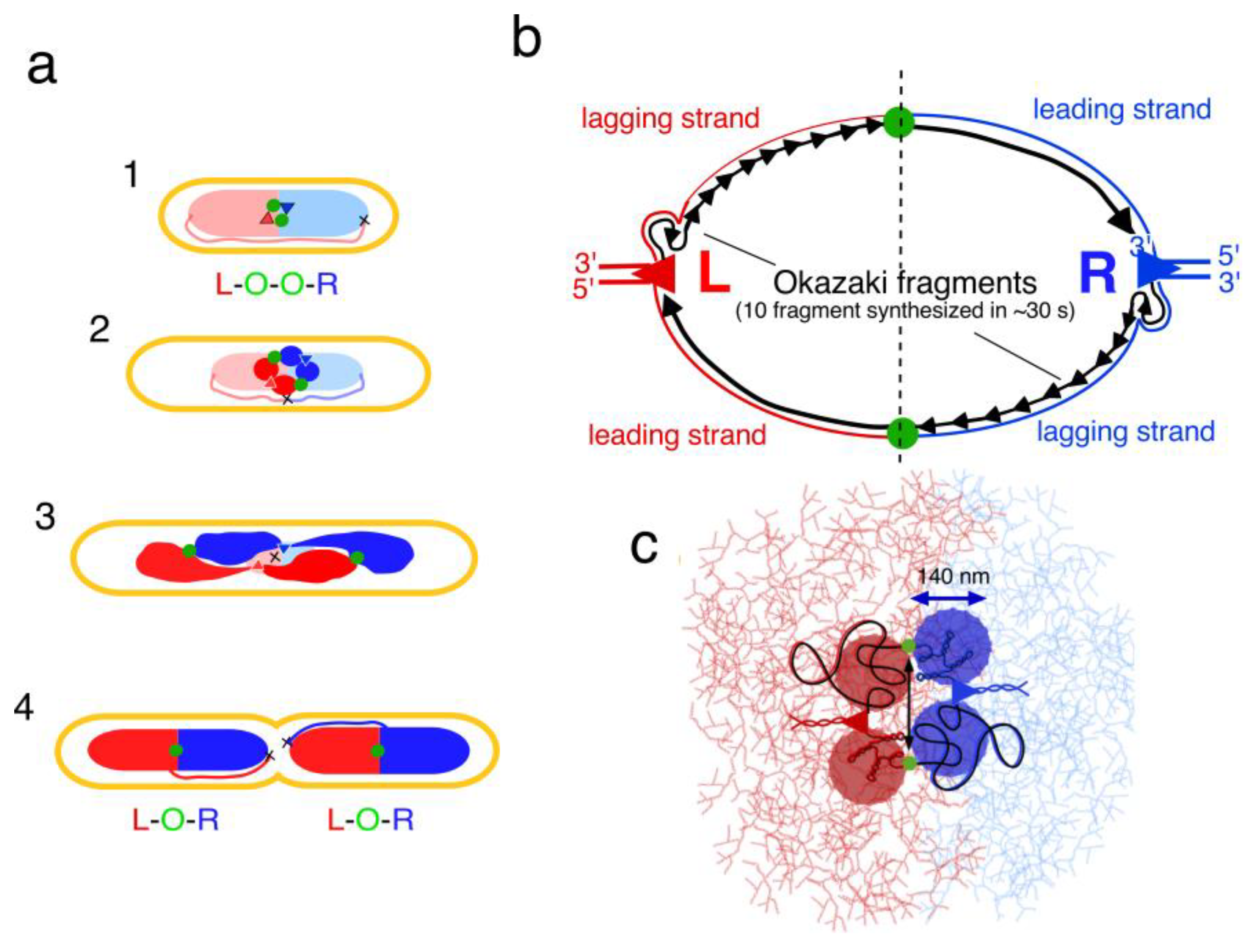
5. Segregation of Chromosome Arms
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryter, A.; Kellenberger, E.; Birch-Andersen, A.; Maaløe, O. Etude au microscope électronique de plasmas contenantde l’acide déoxyribonucléique. I. Les nucléoides des bactéries en croissance active. Z. Naturf. 1958, 13, 597–605. [Google Scholar] [CrossRef]
- Jacob, F.; Brenner, S.; Cuzin, F. On the regulation of DNA replication in bacteria. Cold Spring Harb. Symp. Quant. Biol. 1963, 28, 329–348. [Google Scholar] [CrossRef]
- van Iterson, W. Symposium on the fine structure of bacteria and their parts. II. Bacterial cytoplasm. Bacteriol. Rev. 1965, 29, 290–325. [Google Scholar] [CrossRef] [PubMed]
- Woldringh, C.L.; Nanninga, N. Structure of nucleoid and cytoplasm in the intact cell. In Molecular Cytology of Escherichia coli; Nanninga, N., Ed.; Academic Press: New York, NY, USA, 1985; pp. 161–197. [Google Scholar]
- Brakenhoff, G.J.; Blom, P.; Barends, P.J. Confocal scanning light microscopy with high aperture lenses. J. Microsc. 1979, 117, 219. [Google Scholar] [CrossRef]
- Valkenburg, J.A.C.; Woldringh, C.L.; Brakenhoff, G.J.; van der Voort, H.T.M.; Nanninga, N. Confocal scanning light microscopy of the Escherichia coli nucleoid: Comparison with phase-contrast and electron microscope images. J. Bacteriol. 1985, 161, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Nanninga, N. Het Vertekende Beeld; Amsterdam University Press: Amsterdam, The Netherlands, 2007; pp. 123–132. [Google Scholar]
- Maaløe, O.; Kjeldgaard, N.O. The Control of Macromolecular Synthesis; W.A. Benjamin Inc.: New York, NY, USA, 1966. [Google Scholar]
- Helmstetter, H.E.; Cooper, S.; Pierucci, O.; Revelas, E. On the bacterial life sequence. Cold Spring Harb. Symp. Quant. Biol. 1968, 33, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Woldringh, C.L. Morphological analysis of nuclear separation and cell division during the life cycle of Escherichia coli. J. Bacteriol. 1976, 125, 248–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijsman, H.J.W. A genetic map of several mutations affecting the mucopeptide layer of Escherichia coli. Genet. Res. 1972, 20, 65–74. [Google Scholar] [CrossRef]
- Kellenberger, E.; Arber, W. Electron microscopical studies of phage multiplication: I. A method for quantitative analysis of particle suspensions. Virology 1957, 3, 245–255. [Google Scholar] [CrossRef]
- Woldringh, C.L.; de Jong, M.A.; van den Berg, W.; Koppes, L. Morphological analysis of the division cycle of two Escherichia coli substrains during slow growth. J. Bacteriol. 1977, 131, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Zaritsky, A.; Pritchard, R.H. Changes in cell size and shape associated with changes in the replication time of the chromosome of Escherichia coli. J. Bacteriol. 1973, 114, 824–837. [Google Scholar] [CrossRef] [Green Version]
- Zaritsky, A. On dimensional determination of rod-shaped bacteria. J. Theor. Biol. 1975, 54, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Zaritsky, A.; Woldringh, C.L. Chromosome replication, cell growth, division and shape: A personal perspective. Front. Microbiol. 2015, 6, 756. [Google Scholar] [CrossRef] [Green Version]
- Zaritsky, A.; Woldringh, C.L.; Einav, M.; Alexeeva, S. Use of thymine limitation and thymine starvation to study bacterial physiology and cytology. J. Bacteriol. 2006, 188, 1667–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voorn, W.J.; Koppes, L.J.H.; Grover, N.B. Mathematics of cell division in Escherichia coli: Comparison between sloppy-size and incremental-size kinetics. Curr. Top. Mol. Gen. 1993, 1, 187–194. [Google Scholar]
- Koppes, L.J.H.; Woldringh, C.L.; Nanninga, N. Size variations and correlation of different cell cycle events in slow-growing Escherichia coli. J. Bacteriol. 1978, 134, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Koppes, L.J.H.; Nanninga, N. Positive correlation between size at initiation of chromosome replication in Escherichia coli and and size at initiation of cell constriction. J. Bacteriol. 1980, 143, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amir, A. Cell size regulation in bacteria. Phys. Rev. Lett. 2014, 112, 208102. [Google Scholar] [CrossRef] [Green Version]
- Jun, S.; Taheri-Araghi, S. Cell-size maintenance: Universal strategy revealed. Trends Microbiol. 2014, 23, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Taheri-Araghi, S.; Bradde, S.; Sauls, J.T.; Hill, N.S.; Levin, P.A.; Paulsson, J.; Vergassola, M.; Jun, S. Cell-size control and homeostasis. Curr. Biol. 2015, 25, 385–391. [Google Scholar]
- Huls, P.G.; Vischer, N.O.E.; Woldringh, C.L. Different amounts of DNA in newborn cells of Escherichia coli preclude a role for the chromosome in size control according to the “adder” model. Front. Microbiol. 2018, 9, 664. [Google Scholar] [CrossRef] [PubMed]
- Hansen, F.G.; Atlung, T. The DnaA tale. Front. Microbiol. 2018, 9, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiruvadi-Krishnan, S.; Männik, J.; Kar, P.; Lin, J.; Amir, A.; Männik, J. Coupling between DNA replication, segregation, and the onset of constriction in Escherichia coli. Cell Rep. 2022, 38, 110539. [Google Scholar] [CrossRef]
- Vischer, N.O.E.; Huls, P.G.; Woldringh, C.L. Object-image: An interactive image analysis program using structured point collection. Binary 1994, 6, 160–166. [Google Scholar]
- Zaritsky, A.; Wang, P.; Vischer, N.O.E. Instructive simulation of the bacterial division cycle. Microbiology 2011, 157, 1876–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmstetter, C.E.; Cooper, S. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/R. J. Mol. Biol. 1968, 31, 507–518. [Google Scholar]
- Mulder, E.; Woldringh, C.L. Actively replicating nucleoids influence the positioning of division sites in DNA-less cell forming filaments of Escherichia coli. J. Bacteriol. 1989, 171, 4303–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woldringh, C.L.; Mulder, E.; Valkenburg, J.A.C.; Wientjes, F.B.; Zaritsky, A.; Nanninga, N. Role of nucleoid in toporegulation of division. Res. Microbiol. 1990, 141, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Norris, V.; Madsen, M.S. Autocatalytic gene expression occurs via transertion and membrane domain formation and underlies differentiation in bacteria: A model. J. Mol. Biol. 1995, 253, 739–748. [Google Scholar] [CrossRef]
- Woldringh, C.L.; Zaritsky, A.; Grover, N.B. Nucleoid partitioning and the division plane in Escherichia coli. J. Bacteriol. 1994, 176, 6030–6038. [Google Scholar] [CrossRef] [Green Version]
- van Helvoort, J.M.L.M.; Kool, J.; Woldringh, C.L. Chloramphenicol causes fusion of separated nucleoids in Escherichia coli K-12 cells and filaments. J. Bacteriol. 1996, 178, 4289–4293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Helvoort, J.M.L.M.; Huls, P.G.; Vischer, N.O.E.; Woldringh, C.L. Fused nucleoids resegregate faster than cell elongation in Escherichia coli pbpB(Ts) filaments after release from chloramphenicol inhibition. Microbiology 1998, 144, 1309–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woldringh, C.L.; Hansen, F.G.; Vischer, N.O.E.; Atlung, T. Segregation of chromosome arms in growing and non-growing cells. Front. Microbiol. 2015, 6, 448. [Google Scholar] [CrossRef]
- Woldringh, C.L. The role of co-transcriptional translation and protein translocation (transertion) in bacterial chromosome segregation. Mol. Microbiol. 2002, 45, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, J.A.C.; Woldringh, C.L. Phase separation between nucleoid and cytoplasm in Escherichia coli as defined by immersive refractometry. J. Bacteriol. 1984, 160, 1151–1157. [Google Scholar] [CrossRef] [Green Version]
- Bremer, H.; Dennis, P.D. Modulation of chemical composition and other parameters of the cell at different exponential growth rates. In EcoSal Plus; Slauch, J.M., Ed.; ASM Press: Washington, DC, USA, 2008. [Google Scholar] [CrossRef]
- Odijk, T. Osmotic compaction of supercoiled DNA into a bacterial nucleoid. Biophys. Chem. 1998, 73, 23–30. [Google Scholar] [CrossRef]
- Woldringh, C.L.; Odijk, T. Structure of DNA within the bacterial cell: Physics and physiology. In Organization of the Prokaryotic Genome; Charlebois, R.L., Ed.; American Society for Microbiology: Washington, DC, USA, 1999; Chapter 10; pp. 171–187. [Google Scholar]
- Mason, D.J.; Powelson, D.M. Nuclear division as observed in live bacteria by a new technique. J. Bacteriol. 1956, 71, 474–479. [Google Scholar] [CrossRef] [Green Version]
- Meijer, M.; de Jong, M.A.; Woldringh, C.L.; Nanninga, N. Factors affecting the release of folded chromosomes from Escherichia coli. Eur. J. Biochem. 1976, 63, 469–475. [Google Scholar]
- Sloof, P.; Maagdelijn, A.; Boswinkel, E. Folding of prokaryotic DNA: Isolation and characterization of nucleoids from Bacillus licheniformis. J. Mol. Biol. 1983, 163, 277–297. [Google Scholar] [CrossRef]
- Pelletier, J.; Halvorsen, K.; Ha, B.-Y.; Paparcone, R.; Sandler, S.J.; Woldringh, C.L.; Wong, W.P.; Jun, S. Physical manipulation of the Escherichia coli chromosome reveals its soft nature. Proc. Natl. Acad. Sci. USA 2012, 109, E2649–E2656. [Google Scholar] [CrossRef] [Green Version]
- Cunha, S.; Woldringh, C.L.; Odijk, T. Polymer-mediated compaction and internal dynamics of isolated Escherichia coli nucleoids. J. Struct. Biol. 2001, 136, 53–66. [Google Scholar] [CrossRef]
- Cunha, S.; Woldringh, C.L.; Odijk, T. Restricted diffusion of DNA segments within the isolated Escherichia coli nucleoid. J. Struct. Biol. 2005, 150, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S.; Müller, M.; Vischer, N.O.E.; Odijk, T.; Woldringh, C.L. Single-particle tracking of oriC-GFP fluorescent spots during chromosome segregation in Escherichia coli. J. Struct. Biol. 2005, 151, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Wegner, A.S.; Alexeeva, S.; Odijk, T.; Woldringh, C.L. Characterization of Escherichia coli nucleoids released by osmotic shock. J. Struct. Biol. 2012, 178, 260–269. [Google Scholar] [CrossRef]
- Nielsen, H.J.; Ottesen, J.R.; Youngren, B.; Austin, S.J.; Hansen, F.G. The Escherichia coli chromosome is organized with the left and right chromosome arms in separate cell halves. Mol. Microbiol. 2006, 62, 331–338. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Possoz, C.; Sherratt, D.J. The two Escherichia coli chromosome arms locate to separate cell halves. Gen. Dev. 2006, 20, 1727–1731. [Google Scholar] [CrossRef] [Green Version]
- Norris, V.; Kayser, C.; Muskhelishvili, G.; Konto-Ghiorghi, Y. The roles of nucleoid-associated proteins and topoisomerases in chromosome structure, strand segregation, and the generation of phenotypic heterogeneity in bacteria. FEMS Microbiol. Rev. 2022, fuac049. [Google Scholar] [CrossRef]
- Reyes-Lamothe, R.; Wang, X.; Sherratt, D. Escherichia coli and its chromosome. Trends Microbiol. 2008, 16, 238–245. [Google Scholar] [CrossRef]
- Wang, X.; Rudner, D.Z. Spatial organization of bacterial chromosomes. Curr. Opin. Microbiol. 2014, 22, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Leonard, A.C.; Grimwade, J.E. The orisome: Structure and function. Front. Microbiol. 2015, 6, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, D.; Kleckner, N. Chromosome and replisome dynamics in E. coli: Loss of sister cohesion triggers global chromosome movement and mediates chromosome segregation. Cell 2005, 121, 899–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, M.C.; Magnan, D.; Montminy, T.P.; Lies, M.; Stepankiw, N.; Bates, D. Regulation of sister chromosome cohesion by the replication fork tracking protein SeqA. PLoS Genet. 2013, 9, e1003673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Reyes-Lamothe, R.; Sherratt, D.J. Modulation of Escherichia coli sister chromosome cohesion by topoisomerase IV. Genes Dev. 2008, 22, 2426–2433. [Google Scholar] [CrossRef] [Green Version]
- Jun, S.; Mulder, B. Entropy-driven spatial organization of highly confined polymers: Lessons for the bacterial chromosome. Proc. Natl. Acad. Sci. USA 2006, 103, 12388–12393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, S. Chromosome, cell cycle and entropy. Biophys. J. 2015, 108, 785–786. [Google Scholar] [CrossRef] [Green Version]
- Lemon, K.P.; Grossman, A.D. The extrusion-capture model for chromosome partitioning in bacteria. Genes Develop. 2001, 15, 2031–2041. [Google Scholar] [CrossRef] [Green Version]
- Gogou, C.H.; Japaridze, A.; Dekker, C. Mechanisms for chromosome segregation in bacteris. Front. Microbiol. 2021, 12, 685687. [Google Scholar] [CrossRef]
- Hirano, T. Condensin-based chromosome organization from bacteria to vertebrates. Cell 2016, 164, 847–857. [Google Scholar] [CrossRef] [Green Version]
- Mir, M.; Babacan, S.D.; Bednarz, M.; Do, M.N.; Golding, I.; Popescu, G. Visualizing Escherichia coli Sub-Cellular Structure Using Sparse Deconvolution Spatial Light Interference Tomography. PLoS ONE 2012, 7, e39816. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Sung Ryu, J.; Lee, M.; Jung, J.; Han, S.Y.; Jung Chung, H.; Park, Y. Three-dimsional label-free observation of individual bacteria upon antibiotic treatment using optical diffraction tomography. Biomed. Opt. Express 2020, 11, 1257–1267. [Google Scholar] [CrossRef]
- Spahn, C.H.; Glaesmann, M.; Grimm, J.B.; Ayala, A.X.; Lavis, L.D.; Heilemann, M. A toolbox for multiplexed super-resolution imaging of the E. coli nucleoid and membrane using novel PAINT labels. Sci. Rep. 2018, 8, 14768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woldringh, C.L. The Bacterial Nucleoid: From Electron Microscopy to Polymer Physics—A Personal Recollection. Life 2023, 13, 895. https://doi.org/10.3390/life13040895
Woldringh CL. The Bacterial Nucleoid: From Electron Microscopy to Polymer Physics—A Personal Recollection. Life. 2023; 13(4):895. https://doi.org/10.3390/life13040895
Chicago/Turabian StyleWoldringh, Conrad L. 2023. "The Bacterial Nucleoid: From Electron Microscopy to Polymer Physics—A Personal Recollection" Life 13, no. 4: 895. https://doi.org/10.3390/life13040895
APA StyleWoldringh, C. L. (2023). The Bacterial Nucleoid: From Electron Microscopy to Polymer Physics—A Personal Recollection. Life, 13(4), 895. https://doi.org/10.3390/life13040895





