Biochar Can Improve Absorption of Nitrogen in Chicken Manure by Black Soldier Fly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chicken Manure and Biochar
2.2. Insect Sourcing
2.3. Experimental Design
2.4. Analytical Methods
2.5. Calculations and Statistical Analysis
3. Results
3.1. Gaseous Nitrogen Emission
3.2. Variations of Residual Nitrogen
3.3. Physicochemical Profile Changes of Substrate
3.4. Bioconversion Performance
3.5. Nitrogen Balance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, X.; Guo, L.; Li, C.; Liu, M.; Wu, G.; Jiang, G. The total biomass nitrogen reservoir and its potential of replacing chemical fertilizers in China. Renew. Sustain. Energy Rev. 2021, 135, 110215. [Google Scholar] [CrossRef]
- Agyarko-Mintah, E.; Cowie, A.; Van Zwieten, L.; Singh, B.P.; Smillie, R.; Harden, S.; Fornasier, F. Biochar lowers ammonia emission and improves nitrogen retention in poultry litter composting. Waste Manag. 2017, 61, 129–137. [Google Scholar] [CrossRef]
- Kacprzak, M.; Malińska, K.; Grosser, A.; Sobik-Szołtysek, J.; Wystalska, K.; Dróżdż, D.; Jasińska, A.; Meers, E. Cycles of carbon, nitrogen and phosphorus in poultry manure management technologies—Environmental aspects. Crit. Rev. Environ. Sci. Technol. 2022, 53, 914–938. [Google Scholar] [CrossRef]
- Kong, Z.; Wang, X.; Liu, Q.; Li, T.; Chen, X.; Chai, L.; Liu, D.; Shen, Q. Evolution of various fractions during the windrow composting of chicken manure with rice chaff. J. Environ. Manag. 2018, 207, 366–377. [Google Scholar] [CrossRef]
- Mazza, L.; Xiao, X.; Rehman, K.U.; Cai, M.; Zhang, D.; Fasulo, S.; Tomberlin, J.K.; Zheng, L.; Soomro, A.A.; Yu, Z.; et al. Management of chicken manure using black soldier fly (Diptera: Stratiomyidae) larvae assisted by companion bacteria. Waste Manag. 2020, 102, 312–318. [Google Scholar] [CrossRef]
- Zhang, J.-B.; Zhang, J.; Li, J.-H.; Tomerlin, J.K.; Xiao, X.-P.; Rehman, K.U.; Cai, M.-M.; Zheng, L.-Y.; Yu, Z.-N. Black soldier fly: A new vista for livestock and poultry manure management. J. Integr. Agric. 2021, 20, 1167–1179. [Google Scholar] [CrossRef]
- Amrul, N.F.; Ahmad, I.K.; Basri, N.E.A.; Suja, F.; Jalil, N.A.A.; Azman, N.A. A Review of Organic Waste Treatment Using Black Soldier Fly (Hermetia illucens). Sustainability 2022, 14, 4565. [Google Scholar] [CrossRef]
- DiGiacomo, K.; Leury, B.J. Review: Insect meal: A future source of protein feed for pigs? Animal 2019, 13, 3022–3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parodi, A.; Gerrits, W.J.; Van Loon, J.J.; De Boer, I.J.; Aarnink, A.J.; Van Zanten, H.H. Black soldier fly reared on pig manure: Bioconversion efficiencies, nutrients in the residual material, greenhouse gas and ammonia emissions. Waste Manag. 2021, 126, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Klammsteiner, T.; Dregulo, A.M.; Kumar, V.; Zhou, Y.; Zhang, Z.; Awasthi, M.K. Black soldier fly larvae for organic manure recycling and its potential for a circular bioeconomy: A review. Sci. Total Environ. 2022, 833, 155122. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Jiang, L.; Yu, X.; Zhu, H.; Zhang, J.; Feng, Z.; Zhang, X.; Chen, G.; Zhang, Z. Black Soldier Fly (Hermetia illucens) Larvae Significantly Change the Microbial Community in Chicken Manure. Curr. Microbiol. 2021, 78, 303–315. [Google Scholar] [CrossRef]
- Zhan, S.; Fang, G.; Cai, M.; Kou, Z.; Xu, J.; Cao, Y.; Bai, L.; Zhang, Y.; Jiang, Y.; Luo, X.; et al. Genomic landscape and genetic manipulation of the black soldier fly Hermetia illucens, a natural waste recycler. Cell Res. 2020, 30, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Liu, T.; Awasthi, S.K.; Duan, Y.; Pandey, A.; Zhang, Z. Manure pretreatments with black soldier fly Hermetia illucens L. (Diptera: Stratiomyidae): A study to reduce pathogen content. Sci. Total Environ. 2020, 737, 139842. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhang, J.; Hou, D.; Li, X.; Jiang, H.; Chen, H.; Yu, Z.; Tomberlin, J.K.; Zhang, Z.; Li, Q. Effects of biochar amendment on bioconversion of soybean dregs by black soldier fly. Sci. Total Environ. 2022, 829, 154605. [Google Scholar] [CrossRef]
- Parodi, A.; Yao, Q.; Gerrits, W.J.; Mishyna, M.; Lakemond, C.M.; Oonincx, D.G.; Van Loon, J.J. Upgrading ammonia-nitrogen from manure into body proteins in black soldier fly larvae. Resour. Conserv. Recycl. 2022, 182, 106343. [Google Scholar] [CrossRef]
- Chen, J.; Hou, D.; Pang, W.; Nowar, E.E.; Tomberlin, J.K.; Hu, R.; Chen, H.; Xie, J.; Zhang, J.; Yu, Z.; et al. Effect of moisture content on greenhouse gas and NH3 emissions from pig manure converted by black soldier fly. Sci. Total Environ. 2019, 697, 133840. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Van Huis, A.; Van Loon, J. Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. J. Insects Food Feed. 2015, 1, 131–139. [Google Scholar] [CrossRef]
- UNFCCC (United Nations Framework Convention on Climate Change). Kyoto Protocol to the United Nations Framework Convention on Climate Change; UNFCCC: Kyoto, Japan, 1997. [Google Scholar]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Raghavan, V. Soil biochar amendment as a climate change mitigation tool: Key parameters and mechanisms involved. J. Environ. Manag. 2016, 181, 484–497. [Google Scholar] [CrossRef]
- Sanchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N.J.B.T. Role of biochar as an additive in organic waste composting. Bioresour. Technol. 2018, 247, 1155–1164. [Google Scholar] [CrossRef]
- Raza, S.; Tang, J.L.; Ali, Z.; Yao, Z.; Bah, H.; Iqbal, H.; Ren, X. Ammonia Volatilization and Greenhouse Gases Emissions during Vermicomposting with Animal Manures and Biochar to Enhance Sustainability. Int. J. Environ. Res. Public Health 2021, 18, 178. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Dong, D.; Deng, H.; Strong, P.J.; Wang, H.; Wu, W. Insight into the Effects of Biochar on Manure Composting: Evidence Supporting the Relationship between N2O Emission and Denitrifying Community. Environ. Sci. Technol. 2013, 47, 7341–7349. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, C.; Tang, J.; Gu, J.; Li, H.; Duan, M.; Wang, X.; Chen, R. Bamboo charcoal enhances cellulase and urease activities during chicken manure composting: Roles of the bacterial community and metabolic functions. J. Environ. Sci. 2021, 108, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Sunyoto, N.M.; Zhu, M.; Zhang, Z.; Zhang, D. Effect of biochar addition on hydrogen and methane production in two-phase anaerobic digestion of aqueous carbohydrates food waste. Bioresour. Technol. 2016, 219, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, F.; Xue, S.; Pan, J.; Khoshnevisan, B.; Yang, Y.; Liu, H.; Qiu, L. Improving anaerobic digestion of chicken manure under optimized biochar supplementation strategies. Bioresour. Technol. 2021, 325, 124697. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Lv, Z.; Sun, H.; Li, R.; Zhai, B.; Wang, Z.; Awasthi, M.K.; Wang, Q.; Zhou, L. Improvement of biochar and bacterial powder addition on gaseous emission and bacterial community in pig manure compost. Bioresour. Technol. 2018, 258, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.; Das, K.; Melear, N.; Lakly, D. Reducing Nitrogen Loss during Poultry Litter Composting Using Biochar. J. Environ. Qual. 2010, 39, 1236–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Dubois, T.; Subramanian, S.; Wangu, M.M.; Ekesi, S.; et al. Biochar and gypsum amendment of agro-industrial waste for enhanced black soldier fly larval biomass and quality frass fertilizer. PLoS ONE 2020, 15, e0238154. [Google Scholar] [CrossRef]
- Pang, W.; Hou, D.; Nowar, E.; Chen, H.; Zhang, J.; Zhang, G.; Li, Q.; Wang, S. The influence on carbon, nitrogen recycling, and greenhouse gas emissions under different C/N ratios by black soldier fly. Environ. Sci. Pollut. Res. 2020, 27, 42767–42777. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, M.; Song, L.; Wang, C.; Yang, S.; Yan, Z.; Wang, Y. Study on nitrogen-retaining microbial agent to reduce nitrogen loss during chicken manure composting and nitrogen transformation mechanism. J. Clean. Prod. 2021, 285, 124813. [Google Scholar] [CrossRef]
- Xie, T.; Reddy, K.R.; Wang, C.W.; Yargicoglu, E.; Spokas, K. Characteristics and Applications of Biochar for Environmental Remediation: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 939–969. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Duan, Y.; Awasthi, S.K.; Liu, T.; Zhang, Z. Influence of bamboo biochar on mitigating greenhouse gas emissions and nitrogen loss during poultry manure composting. Bioresour. Technol. 2020, 303, 122952. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lee, D.-H.; Won, S.; Ahn, H. Evaluation of Optimum Moisture Content for Composting of Beef Manure and Bedding Material Mixtures Using Oxygen Uptake Measurement. Asian-Australas. J. Anim. Sci. 2016, 29, 753–758. [Google Scholar] [CrossRef] [Green Version]
- Rashad, F.M.; Saleh, W.D.; Moselhy, M.A. Bioconversion of rice straw and certain agro-industrial wastes to amendments for organic farming systems: 1. Composting, quality, stability and maturity indices. Bioresour. Technol. 2010, 101, 5952–5960. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Ngo, H.-H.; Lin, C.; Vu, C.-T.; Kaewlaoyoong, A.; Boonsong, T.; Tran, H.-T.; Bui, X.-T.; Vo, T.-D.; Chen, J.-R. Aerobic co-composting degradation of highly PCDD/F-contaminated field soil. A study of bacterial community. Sci. Total Environ. 2018, 660, 595–602. [Google Scholar] [CrossRef]
- Chowdhury, A.; de Neergaard, A.; Jensen, L.S. Potential of aeration flow rate and bio-char addition to reduce greenhouse gas and ammonia emissions during manure composting. Chemosphere 2014, 97, 16–25. [Google Scholar] [CrossRef]
- Tran, H.T.; Lin, C.; Hoang, H.G.; Nguyen, M.T.; Kaewlaoyoong, A.; Cheruiyot, N.K.; Bui, X.-T.; Vu, C.T. Biodegradation of dioxin-contaminated soil via composting: Identification and phylogenetic relationship of bacterial communities. Environ. Technol. Innov. 2020, 19, 101023. [Google Scholar] [CrossRef]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- Wang, Q.; Awasthi, M.K.; Ren, X.; Zhao, J.; Li, R.; Wang, Z.; Chen, H.; Wang, M.; Zhang, Z. Comparison of biochar, zeolite and their mixture amendment for aiding organic matter transformation and nitrogen conservation during pig manure composting. Bioresour. Technol. 2017, 245 Pt A, 300–308. [Google Scholar] [CrossRef]
- Fang, M.; Wong, J. Effects of lime amendment on availability of heavy metals and maturation in sewage sludge composting. Environ. Pollut. 1999, 106, 83–89. [Google Scholar] [CrossRef]
- Lasaridi, K.; Protopapa, I.; Kotsou, M.; Pilidis, G.; Manios, T.; Kyriacou, A. Quality assessment of composts in the Greek market: The need for standards and quality assurance. J. Environ. Manag. 2006, 80, 58–65. [Google Scholar] [CrossRef]
- Dorai, M.; Papadopoulos, A.P. Influence of electric conductivity management on greenhouse tomato yield and fruit quality. Agronomie 2001, 21, 367–383. [Google Scholar] [CrossRef] [Green Version]
- Oonincx, D.G.A.B.; van Broekhoven, S.; van Huis, A.; van Loon, J.J.A. Feed Conversion, Survival and Development, and Composition of Four Insect Species on Diets Composed of Food By-Products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, M.K.; Lin, C.; Hoang, H.G.; Sanderson, P.; Dang, B.T.; Bui, X.T.; Nguyen, N.S.H.; Vo, D.-V.N.; Tran, H.T. Evaluate the role of biochar during the organic waste composting process: A critical review. Chemosphere 2022, 299, 134488. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-X.; Liu, H.-T.; Zhang, J. The role of biochar in organic waste composting and soil improvement: A review. Waste Manag. 2019, 102, 884–899. [Google Scholar] [CrossRef]
- Schmidt, H.-P.; Kammann, C.; Niggli, C.; Evangelou, M.W.; Mackie, K.A.; Abiven, S. Biochar and biochar-compost as soil amendments to a vineyard soil: Influences on plant growth, nutrient uptake, plant health and grape quality. Agric. Ecosyst. Environ. 2014, 191, 117–123. [Google Scholar] [CrossRef]
- Agyarko-Mintah, E.; Cowie, A.; Singh, B.P.; Joseph, S.; Van Zwieten, L.; Cowie, A.; Harden, S.; Smillie, R. Biochar increases nitrogen retention and lowers greenhouse gas emissions when added to composting poultry litter. Waste Manag. 2017, 61, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liao, X.; Wu, Y.; Liang, J.B.; Mi, J.; Huang, J.; Zhang, H.; Wu, Y.; Qiao, Z.; Li, X.; et al. Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manag. 2017, 61, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Artola, A.; Font, X.; Gea, T.; Barrena, R.; Gabriel, D.; Sanchez-Monedero, M.A.; Roig, A.; Cayuela, M.L.; Mondini, C. Greenhouse gas emissions from organic waste composting. Environ. Chem. Lett. 2015, 13, 223–238. [Google Scholar] [CrossRef] [Green Version]
- Ermolaev, E.; Lalander, C.; Vinnerås, B. Greenhouse gas emissions from small-scale fly larvae composting with Hermetia illucens. Waste Manag. 2019, 96, 65–74. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Wang, H.; Zhao, X.; Cui, H.; Wei, Z. Reducing nitrogen loss and phytotoxicity during beer vinasse composting with biochar addition. Waste Manag. 2017, 61, 150–156. [Google Scholar] [CrossRef]
- Xiao, R.; Awasthi, M.K.; Li, R.; Park, J.; Pensky, S.M.; Wang, Q.; Wang, J.J.; Zhang, Z. Recent developments in biochar utilization as an additive in organic solid waste composting: A review. Bioresour. Technol. 2017, 246, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kammann, C.I.; Schmidt, H.-P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.-W.; Conte, P.; Joseph, S. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 11080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagemann, N.; Kammann, C.I.; Schmidt, H.-P.; Kappler, A.; Behrens, S. Nitrate capture and slow release in biochar amended compost and soil. PLoS ONE 2017, 12, e0171214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Qi, H.; Liu, Y.; He, X. Sorption/Desorption Behavior and Mechanism of NH4+ by Biochar as a Nitrogen Fertilizer Sustained-Release Material. J. Agric. Food Chem. 2016, 64, 4958–4964. [Google Scholar] [CrossRef]
- Lawrinenko, M.; Laird, D.A. Anion exchange capacity of biochar. Green Chem. 2015, 17, 4628–4636. [Google Scholar] [CrossRef] [Green Version]
- Harter, J.; Guzman-Bustamante, I.; Kuehfuss, S.; Ruser, R.; Well, R.; Spott, O.; Kappler, A.; Behrens, S. Gas entrapment and microbial N2O reduction reduce N2O emissions from a biochar-amended sandy clay loam soil. Sci. Rep. 2016, 6, 39574. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zheng, H.; Wang, Z.Y. The Formation of Toxic Compounds during Biochar Production. Appl. Mech. Mater. 2013, 361–363, 867–870. [Google Scholar] [CrossRef]
- Cole, D.P.; Smith, E.A.; Lee, Y.J. High-Resolution Mass Spectrometric Characterization of Molecules on Biochar from Pyrolysis and Gasification of Switchgrass. Energy Fuels 2012, 26, 3803–3809. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; Wang, X.; Xu, X.; Cai, R.; Xie, S. Effects of heavy metals on the bioaccumulation, excretion and gut microbiome of black soldier fly larvae (Hermetia illucens). Ecotoxicol. Environ. Saf. 2020, 192, 110323. [Google Scholar] [CrossRef]
- Zhang, H.; Pei, Y.; Zhang, J.; Suo, J.; Deng, X.; Chen, H.; Lin, H.; Yang, S. Effects of cyromazine on the growth of Hermetia illucens (Diptera: Stratiomyidae) in chicken manure and the detoxification of cyromazine by added activated carbon. Acta Entomol. Sin. 2022, 65, 1166–1176. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Sun, H.; Zhou, S.; Zou, G. Straw biochar hastens organic matter degradation and produces nutrient-rich compost. Bioresour. Technol. 2016, 200, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; Lin, C.; Bui, X.-T.; Nguyen, M.K.; Cao, N.D.T.; Mukhtar, H.; Hoang, H.G.; Varjani, S.; Ngo, H.H.; Nghiem, L.D. Phthalates in the environment: Characteristics, fate and transport, and advanced wastewater treatment technologies. Bioresour. Technol. 2022, 344, 126249. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, G.; Hayat, R.; Hussain, Q.; Ahmed, M. Physio-Chemical Characterization of Biochar, Compost and Co-Composted Biochar Derived from Green Waste. Sustainability 2021, 13, 4628. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 2014, 171, 274–284. [Google Scholar] [CrossRef]
- Conrad, R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol. Rev. 1996, 60, 609–640. [Google Scholar] [CrossRef]
- Zhou, Y.; Awasthi, S.K.; Liu, T.; Verma, S.; Zhang, Z.; Pandey, A.; Varjani, S.; Li, R.; Taherzadeh, M.J.; Awasthi, M.K. Patterns of heavy metal resistant bacterial community succession influenced by biochar amendment during poultry manure composting. J. Hazard. Mater. 2021, 420, 126562. [Google Scholar] [CrossRef]
- Cui, E.; Wu, Y.; Zuo, Y.; Chen, H. Effect of different biochars on antibiotic resistance genes and bacterial community during chicken manure composting. Bioresour. Technol. 2016, 203, 11–17. [Google Scholar] [CrossRef]
- Akumah, A.M.; Nartey, E.K.; Ofosu-Budu, G.K.; Ewusie, E.A.; Offei, B.K.; Adamtey, N. Innovations in market crop waste compost production: Use of black soldier fly larvae and biochar. Int. J. Recycl. Org. Waste Agric. 2021, 10, 185–202. [Google Scholar] [CrossRef]
- Ur Rehman, K.; Ur Rehman, R.; Somroo, A.A.; Cai, M.; Zheng, L.; Xiao, X.; Ur Rehman, A.; Rehman, A.; Tomberlin, J.K.; Yu, Z.; et al. Enhanced bioconversion of dairy and chicken manure by the interaction of exogenous bacteria and black soldier fly larvae. J. Environ. Manag. 2019, 237, 75–83. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Chen, Y.; Lu, Q.; Li, M.; Wang, X.; Wei, Y.; Xie, X.; Wei, Z. A regulating method for reducing nitrogen loss based on enriched ammonia-oxidizing bacteria during composting. Bioresour. Technol. 2016, 221, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Q.; Zhang, Z.; Zhang, G.; Li, Z.; Wang, L.; Zheng, J. Nutrient transformation during aerobic composting of pig manure with biochar prepared at different temperatures. Environ. Technol. 2015, 36, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for composting improvement and contaminants reduction. A review. Bioresour. Technol. 2017, 246, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
- Tran, H.T.; Lin, C.; Bui, X.T.; Itayama, T.; Dang, B.T.; Cheruiyot, N.K.; Hoang, H.G.; Vu, C.T. Bacterial community progression during food waste composting containing high dioctyl terephthalate (DOTP) concentration. Chemosphere 2021, 265, 129064. [Google Scholar] [CrossRef]
- Pang, W.; Hou, D.; Chen, J.; Nowar, E.; Li, Z.; Hu, R.; Tomberlin, J.K.; Yu, Z.; Li, Q.; Wang, S. Reducing greenhouse gas emissions and enhancing carbon and nitrogen conversion in food wastes by the black soldier fly. J. Environ. Manag. 2020, 260, 110066. [Google Scholar] [CrossRef]

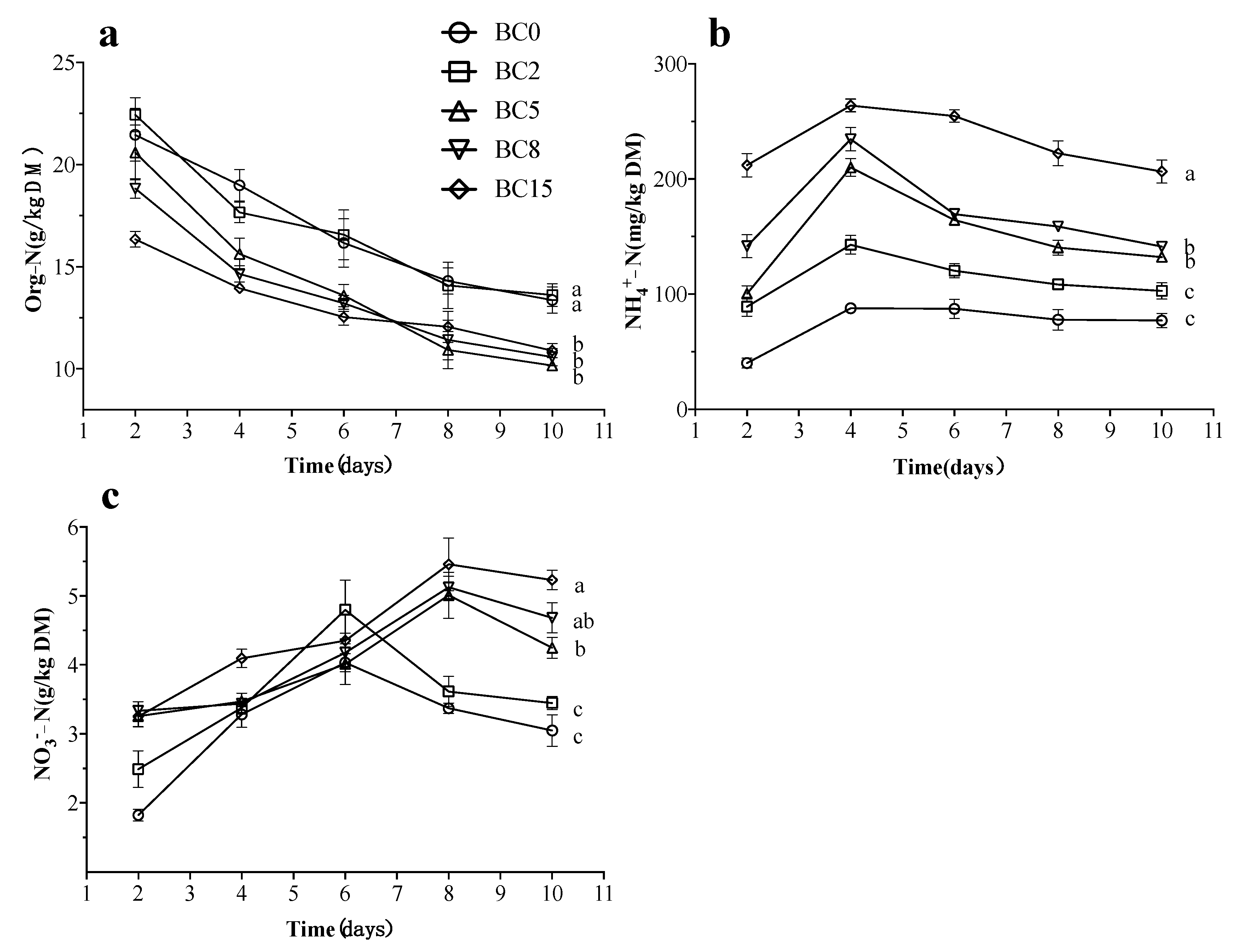

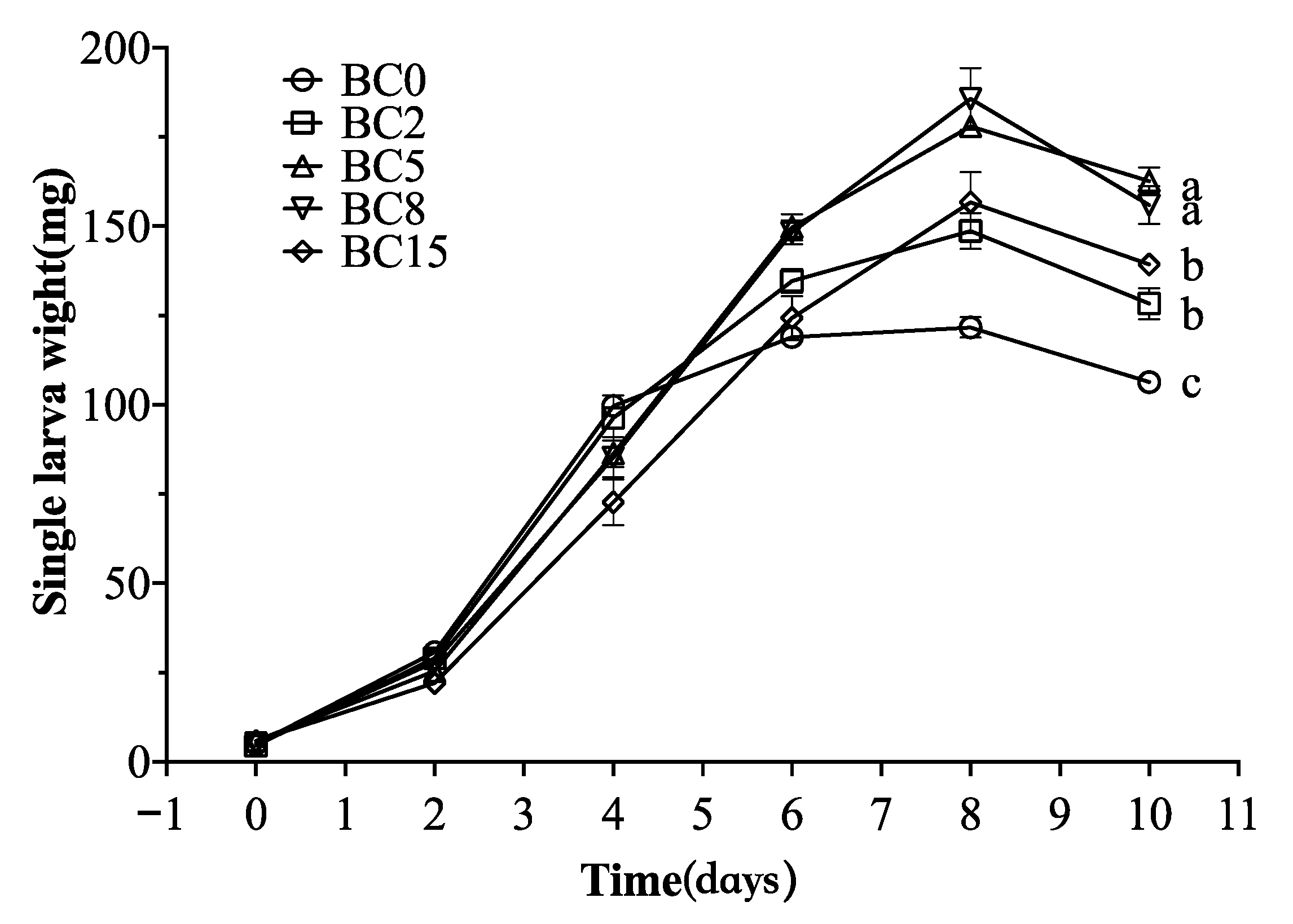
| Treatments | Survival Rate (%) | Fresh Larval Mass (g) | Dry Larval Mass (g) | Nitrogen Accumulated in Larvae (g/kg) |
|---|---|---|---|---|
| BC0 | 91.33 ± 4.62 a | 52.80 ± 2.26 d | 14.57 ± 0.31 c | 68.88 ± 0.64 a |
| BC2 | 93.33 ± 6.11 a | 71.28 ± 1.70 ab | 18.83 ± 0.67 b | 64.48 ± 0.33 b |
| BC5 | 96.67 ± 3.06 a | 77.15 ± 0.58 a | 22.97 ± 0.91 a | 61.48 ± 1.35 c |
| BC8 | 94.67 ± 3.06 a | 69.21 ± 4.53 bc | 20.37 ± 0.67 b | 61.64 ± 0.68 c |
| BC15 | 93.33 ± 1.15 a | 63.88 ± 2.42 c | 19.10 ± 0.75 b | 60.88 ± 2.14 c |
| Treatments | Reduction Rate of CM (%) | Bioconversion Rate (%) | FCR (kg) | ECI (%) |
|---|---|---|---|---|
| BC0 | 43.61 ± 0.64 b | 5.21 ± 0.12 c | 12.79 ± 0.21 a | 11.95 ± 0.41 a |
| BC2 | 50.10 ± 2.33 ab | 7.51 ± 0.48 b | 9.38 ± 0.81 b | 13.66 ± 0.97 a |
| BC5 | 59.75 ± 3.63 a | 8.31 ± 0.34 a | 8.71 ± 0.38 b | 13.96 ± 1.24 a |
| BC8 | 56.93 ± 2.56 a | 7.37 ± 0.25 b | 9.37 ± 0.69 b | 12.98 ± 0.99 a |
| BC15 | 58.04 ± 8.47 a | 6.87 ± 0.30 b | 9.41 ± 0.12 b | 12.18 ± 1.05 a |
| Treatments | Nitrogen Loss (%) | Nitrogen in Residue (%) | Nitrogen in Larvae (%) | |||
|---|---|---|---|---|---|---|
| NH3 | N2O | Org-N | NH4+-N | NO3−-N | ||
| BC0 | 25.90 ± 0.70 a | 0.0012 ± 0.0003 a | 40.95 ± 2.09 a | 0.24 ± 0.03 d | 9.33 ± 0.93 d | 23.58 ± 1.28 c |
| BC2 | 23.50 ± 0.96 ab | 0.0006 ± 0.0001 b | 38.32 ± 1.38 ab | 0.31 ± 0.05 d | 10.33 ± 0.96 d | 27.53 ± 0.35 b |
| BC5 | 21.50 ± 1.46 bc | 0.0004 ± 0.0001 bc | 27.89 ± 0.46 d | 0.45 ± 0.03 c | 14.42 ± 1.08 c | 35.74 ± 0.20 a |
| BC8 | 19.27 ± 0.93 cd | 0.0002 ± 0.0001 c | 32.23 ± 2.22 c | 0.56 ± 0.03 b | 18.55 ± 0.95 b | 29.39 ± 1.36 b |
| BC15 | 16.51 ± 0.92 d | 0.0001 ± 0.0000 c | 34.70 ± 0.76 bc | 0.96 ± 0.05 a | 24.24 ± 0.17 a | 23.59 ± 0.29 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, X.; Chen, M.; Deng, X.; Pei, Y.; Zhang, J.; Chen, H.; Yang, S. Biochar Can Improve Absorption of Nitrogen in Chicken Manure by Black Soldier Fly. Life 2023, 13, 938. https://doi.org/10.3390/life13040938
Zhang H, Zhang X, Chen M, Deng X, Pei Y, Zhang J, Chen H, Yang S. Biochar Can Improve Absorption of Nitrogen in Chicken Manure by Black Soldier Fly. Life. 2023; 13(4):938. https://doi.org/10.3390/life13040938
Chicago/Turabian StyleZhang, Haixu, Xilu Zhang, Mengxiao Chen, Xin Deng, Yaxin Pei, Jiran Zhang, Hongge Chen, and Sen Yang. 2023. "Biochar Can Improve Absorption of Nitrogen in Chicken Manure by Black Soldier Fly" Life 13, no. 4: 938. https://doi.org/10.3390/life13040938





