Efficacy of Deep Dry Needling versus Percutaneous Electrolysis in Ultrasound-Guided Treatment of Active Myofascial Trigger Points of the Levator Scapulae in Short-Term: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Material and Methods
2.1. Design
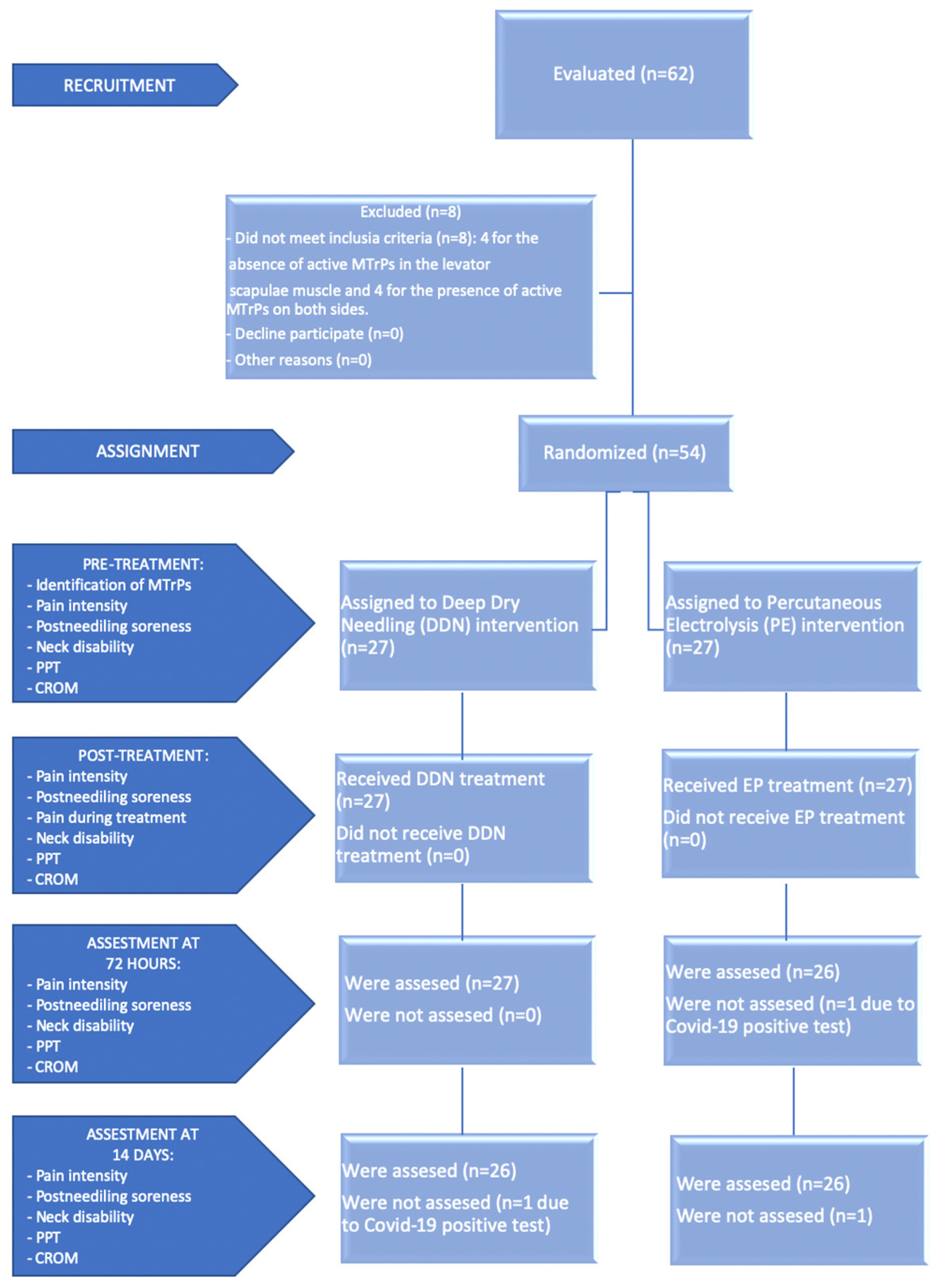
2.2. Participants
2.3. Simple Size Calculation
2.4. Study Variables
2.4.1. Pain Intensity
2.4.2. Neck Disability
2.4.3. Pressure Pain Threshold
2.4.4. Cervical Range of Motion
2.5. Treatment Allocation
2.6. Intervention Group: Deep Dry Needling (DDN)
2.7. Experimental Group: Percutaneous Electrolysis (PE)
2.8. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristic by Treatment Groups
3.2. Pain Intensity
3.3. Post-Needling Soreness
3.4. Pain Intensity during Treatment
3.5. Neck Disability
3.6. Pain Pressure Threshold
3.7. Cervical Range of Motion
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borghouts, J.A.J.; Koes, B.W.; Bouter, L.M. The clinical course and prognostic factors of non-specific neck pain: A systematic review. Pain 1998, 77, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schellingerhout, J.M.; Verhagen, A.P.; Heymans, M.W.; Pool, J.J.M.; Vonk, F.; Koes, B.W.; de Vet, H.C.W. Which subgroups of patients with non-specific neck pain are more likely to benefit from spinal manipulation therapy, physiotherapy, or usual care? Pain 2008, 139, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Vonk, F.; Verhagen, A.P.; Twisk, J.W.; Köke, A.J.A.; Luiten, M.W.C.T.; Koes, B.W. Effectiveness of a behaviour graded activity program versus conventional exercise for chronic neck pain patients. Eur. J. Pain 2009, 13, 533–541. [Google Scholar] [CrossRef]
- Cohen, S.P. Epidemiology, diagnosis, and treatment of neck pain. Mayo Clin. Proc. 2015, 90, 284–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Instituto Nacional de Estadística (INE); Ministerio de Sanidad (MS). Encuesta Europea de Salud en España (EESE 2020). 2020. Available online: https://www.ine.es/dynt3/inebase/es/index.htm?type=pcaxis&path=/t15/p420/a2019/p01/&file=pcaxis (accessed on 1 January 2020).
- Cerezo-Téllez, E.; Torres-Lacomba, M.; Mayoral-del Moral, O.; Sánchez-Sánchez, B.; Dommerholt, J.; Gutiérrez-Ortega, C. Prevalence of myofascial pain syndrome in chronic non-specific neck pain: A population- based cross-sectional descriptive study. Pain Med. 2016, 17, 2369–2377. [Google Scholar] [CrossRef] [Green Version]
- Gallego-Sendarrubias, G.M.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; Martín, J.L. Efficacy of dry needling as an adjunct to manual therapy for patients with chronic mechanical neck pain: A randomised clinical trial. Acupunct. Med. 2020, 38, 244–254. [Google Scholar] [CrossRef]
- Chiarotto, A.; Clijsen, R.; Fernandez-De-Las-Penas, C.; Barbero, M. Prevalence of Myofascial Trigger Points in Spinal Disorders: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2016, 97, 316–337. [Google Scholar] [CrossRef]
- Fernaández-de-las-Peñas, C.; Cleland, J.; Huijbregts, P. Neck and Arm Pain Syndromes: Evidence-Informed Screening, Diagnosis and Management; Elsevier Inc.: Maryland Heights, MO, USA, 2011; Available online: http://lib.ugent.be/catalog/ebk01:2670000000092961 (accessed on 1 January 2020).
- Hong, C.Z. Lidocaine injection versus dry needling to myofascial trigger point: The importance of the local twitch response. Am. J. Phys. Med. Rehabil. 1994, 73, 256–263. [Google Scholar] [CrossRef]
- Abat, F.; Diesel, W.; Gelber, P.; Polidori, F.; Monllau, J. Effectiveness of the Intratissue Percutaneous Electrolysis (EPI®) technique and isoinertial eccentric exercise in the treatment of patellar tendinopathy at two years follow-up. Muscle Ligaments Tendons J. 2014, 4, 188–193. [Google Scholar] [CrossRef]
- Abat, F.; Gelber, P.E.; Polidori, F.; Monllau, J.C.; Sanchez-Ibañez, J.M. Clinical results after ultrasound-guided intratissue percutaneous electrolysis (EPI®) and eccentric exercise in the treatment of patellar tendinopathy. Knee Surgery, Sport. Traumatol. Arthrosc. 2015, 23, 1046–1052. [Google Scholar] [CrossRef]
- Gattie, E.; Cleland, J.A.; Snodgrass, S. The effectiveness of trigger point dry needling for musculoskeletal conditions by physical therapists: A systematic review and meta-analysis. J. Orthop. Sport. Phys. Ther. 2017, 47, 133–149. [Google Scholar] [CrossRef]
- Sánchez-Infante, J.; Navarro-Santana, M.J.; Bravo-Sanchez, A.; Jimenez-Diaz, F.; Abian-Vicen, J. Is dry needling applied by physical therapists effective for pain in musculoskeletal conditions? A systematic review and meta-analysis. Phys. Ther. 2021, 101, pzab070. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Plaza-Manzano, G.; Sanchez-Infante, J.; Gómez-Chiguano, G.F.; Cleland, J.A.; Arias-Buría, J.L.; Navarro-Santana, M.J. The importance of the local twitch response during needling interventions in spinal pain associated with myofascial trigger points: A systematic review and meta-analysis. Acupunct. Med. 2022, 40, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Valera, F.; Minaya, M. Fisioterapia Invasiva, 2nd ed.; Elsevier: Barcelona, Spain, 2017. [Google Scholar]
- Lopez-Martos, R.; Gonzalez-Perez, L.M.; Ruiz-Canela-Mendez, P.; Urresti-Lopez, F.J.; Gutierrez-Perez, J.L.; Infante-Cossio, P. Randomized, double-blind study comparing percutaneous electrolysis and dry needling for the management of temporomandibular myofascial pain. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e454–e462. [Google Scholar] [CrossRef] [PubMed]
- Al-Boloushi, Z.; Gómez-Trullén, E.M.; Arian, M.; Fernández, D.; Herrero, P.; Bellosta-López, P. Comparing two dry needling interventions for plantar heel pain: A randomised controlled trial. BMJ Open 2020, 10, e038033. [Google Scholar] [CrossRef]
- García Naranjo, J.; Barroso Rosa, S.; Loro Ferrer, J.F.; Limiñana Cañal, J.M.; Suarez Hernández, E. A novel approach in the treatment of acute whiplash syndrome: Ultrasound-guided needle percutaneous electrolysis. A randomized controlled trial. Orthop. Traumatol. Surg. Res. 2017, 103, 1229–1234. [Google Scholar] [CrossRef]
- Simons Travell, J.G.; Simons, L.S.; Travell, J.G.; David, G. Travell & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual; Williams & Wilkins: Baltimore, MD, USA, 1999. [Google Scholar]
- Fernández-de-las-Peñas, C.; Alonso-Blanco, C.; Miangolarra, J.C. Myofascial trigger points in subjects presenting with mechanical neck pain: A blinded, controlled study. Man. Ther. 2007, 12, 29–33. [Google Scholar] [CrossRef]
- Muñoz-Muñoz, S.; Muñoz-García, M.T.; Alburquerque-Sendín, F.; Arroyo-Morales, M.; Fernández-De-Las-Peñas, C. Myofascial trigger points, pain, disability, and sleep quality in individuals with mechanical neck pain. J. Manip. Physiol. Ther. 2012, 35, 608–613. [Google Scholar] [CrossRef]
- Travell, J.; Simons, D. Myofascial Pain and Dysfunction: The Trigger Point Manual; Williams & Wilkins: Baltimore, MD, USA, 1983. [Google Scholar]
- Fernández-de-las-Peñas, C.; Dommerholt, J. International consensus on diagnostic criteria and clinical considerations of myofascial trigger points: A delphi study. Pain Med. 2018, 19, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Myburgh, C.; Larsen, A.H.; Hartvigsen, J. A Systematic, Critical Review of Manual Palpation for Identifying Myofascial Trigger Points: Evidence and Clinical Significance. Arch. Phys. Med. Rehabil. 2008, 89, 1169–1176. [Google Scholar] [CrossRef]
- González, V.M.; Stewart, A.; Ritter, P.L.; Lorig, K. Translation and validation of arthritis outcome measures into spanish. Arthritis Rheum. 1995, 38, 1429–1446. [Google Scholar] [CrossRef]
- Price, D.D.; McGrath, P.A.; Rafii, A.B.B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983, 17, 45–56. [Google Scholar] [CrossRef]
- Gattie, E.R.; Cleland, J.A.; Snodgrass, S.J. Dry Needling for Patients with Neck Pain: Protocol of a Randomized Clinical Trial. JMIR Res. Protoc. 2017, 6, e227. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.J.; Kim, W.H.; Kim, S.G. Correlations among visual analogue scale, neck disability index, shoulder joint range of motion, and muscle strength in young women with forward head posture. J. Exerc. Rehabil. 2017, 13, 413–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleland, J.A.; Childs, J.D.; Whitman, J.M. Psychometric Properties of the Neck Disability Index and Numeric Pain Rating Scale in Patients with Mechanical Neck Pain. Arch. Phys. Med. Rehabil. 2008, 89, 69–74. [Google Scholar] [CrossRef] [PubMed]
- León-Hernández, J.; Martín-Pintado-Zugasti, A.; Frutos, L.; Alguacil-Diego, I.; de la Llave-Rincón, A.; Fernandez-Carnero, J. Immediate and short-term effects of the combination of dry needling and percutaneous TENS on post-needling soreness in patients with chronic myofascial neck pain. Braz. J. Phys. Ther. 2016, 20, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, T.; Balsa, A.; de Murieta, J.S.; Zamorano, E.; González, I.; Martin-Mola, E. Spanish version of the Northwick Park neck pain questionnaire: Reliability and validity. Clin. Exp. Rheumatol. 2001, 19, 41–46. [Google Scholar]
- Leak, A.M.; Frank, A.O. The northwick park neck pain questionnaire, devised to measure neck pain and disability. Rheumatology 1994, 33, 1204. [Google Scholar] [CrossRef]
- Sim, J.; Jordan, K.; Lewis, M.; Hill, J.; Hay, E.M.; Dziedzic, K. Sensitivity to change and internal consistency of the Northwick Park neck pain questionnaire and derivation of a minimal clinically important difference. Clin. J. Pain 2006, 22, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Voogt, L.; de Vries, J.; Meeus, M.; Struyf, F.; Meuffels, D.; Nijs, J. Analgesic effects of manual therapy in patients with musculoskeletal pain: A systematic review. Man. Ther. 2015, 20, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinser, A.M.; Sands, W.A.; Stone, M.H. Reliability and validity of a pressure algometer. J. Strength Cond. Res. 2009, 23, 312–314. [Google Scholar] [CrossRef] [PubMed]
- List, T.; Helkimo, M.; Falk, G. Reliability and validity of a pressure threshold meter in recording tenderness in the masseter muscle and the anterior temporalis muscle. Cranio-J. Craniomandib. Pract. 1989, 7, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.A. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain 1987, 30, 115–126. [Google Scholar] [CrossRef]
- Park, G.; Kim, C.W.; Park, S.B.; Kim, M.J.; Jang, S.H. Reliability and Usefulness of the Pressure Pain Threshold Measurement in Patients with Myofascial Pain. Ann. Rehabil. Med. 2011, 35, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.C.; Um, B.Y.; Kim, H.S. Evaluation of pressure pain threshold in head and neck muscles by electronic algometer: Intrarater and interrater reliability. Cranio 1992, 10, 28–34. [Google Scholar] [CrossRef]
- Walton, D.; Macdermid, J.; Nielson, W.; Teasell, R.; Chiasson, M.; Brown, L. Reliability, standard error, and minimum detectable change of clinical pressure pain threshold testing in people with and without acute neck pain. J. Orthop. Sports Phys. Ther. 2011, 41, 644–650. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, J.P.; Bandy, W.D. Intrarater Reliability of CROM Measurement of Cervical Spine Active Range of Motion in Persons with and without Neck Pain. J. Orthop. Sport. Phys. Ther. 2008, 38, 640–645. Available online: http://www.jospt.org/doi/10.2519/jospt.2008.2680 (accessed on 1 January 2020). [CrossRef] [PubMed]
- Farooq, M.N.; Mohseni Bandpei, M.A.; Ali, M.; Khan, G.A. Reliability of the universal goniometer for assessing active cervical range of motion in asymptomatic healthy persons. Pak. J. Med. Sci. 2016, 32, 457–461. [Google Scholar] [CrossRef] [Green Version]
- Shamsi, M.B.; Mirzaei, M.; Khabiri, S.S. Universal goniometer and electrogoniometer intra-examiner reliability in measuring the knee range of motion during active knee extension test in patients with chronic low back pain with short hamstring muscle. BMC Sport. Sci. Med. Rehabil. 2019, 11, 4. [Google Scholar] [CrossRef]
- Fernández-Carnero, J.; Gilarranz-de-Frutos, L.; León-Hernández, J.V.; Pecos-Martin, D.; Alguacil-Diego, I.; Gallego-Izquierdo, T.; Martín-Pintado-Zugasti, A. Effectiveness of Different Deep Dry Needling Dosages in the Treatment of Patients with Cervical Myofascial Pain: A pilot RCT. Am. J. Phys. Med. Rehabil. 2017, 96, 726–733. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Valera-Calero, J.A.; Sánchez-Mayoral-Martín, A.; Varol, U. Short-term effectiveness of high- and low-intensity percutaneous electrolysis in patients with patellofemoral pain syndrome: A pilot study. World J. Orthop. 2021, 12, 781–790. [Google Scholar] [CrossRef] [PubMed]
| Total Group N = 52 | Dry Needling Group n = 26 | Percutaneus Electrolysis Group n = 26 | ||
|---|---|---|---|---|
| Descriptive Data | Mean ± SD (95% CI) | Mean ± SD (95% CI) | Mean ± SD (95% CI) | p Value |
| Age (years) | 38.77 ± 9.39 (36.15–41.38) | 39.35 ± 9.85 (35.37–43.33) | 38,19 ± 9.06 (34.53–41.85) | 0.700 * |
| Weight (kg) | 69.55 ± 14.37 (65.55–73.55) | 69.63 ± 15.48 (63.38–75.89) | 69.47 ± 13.47 (64.03–74.90) | 0.805 * |
| Height (m) | 1.67 ± 0.09 (1.65–1.70) | 1.66 ± 0.10 (1.62–1.70) | 1.68 ± 0.09 (1.65–1.72) | 0.196 * |
| BMI (Kg/m2) | 24.79 ± 4.37 (23.58–26.01) | 25.08 ± 4.62 (23.22–26.95) | 24.50 ± 4.17 (22.82–26.19) | 0.891 * |
| Dry Needling Group N = 26 | Percutaneus Electrolysis Group N = 26 | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Mean ± SD (95% CI) | Median (IR) | p Value (Kolmogorov–Smirnov) | Mean ± SD (95% CI) | Median (IR) | p Value (Kolmogorov–Smirnov) | p Value |
| VNPS neck pain intensity before treatment | 6.80 ± 1.13 (6.35–7.26) | 6.50 (6.00–7.00) | 0.001 | 6.77 ± 1.03 (6.35–7.19) | 7.00 (6.00–7.00) | 0.014 | 0.907 ** |
| VNPS neck pain intensity after treatment | 5.23 ± 1.97 (4.44–6.02) | 5.00 (4.55–6.00) | 0.250 | 5.04 ± 1.59 (4.40–5.68) | 5.00 (4.00–6.00) | 0.196 | 0.772 * |
| VNPS neck pain intensity 72 h after treatment | 3.27 ± 1.87 (2.52–4.02) | 3.00 (2.55–4.00) | 0.355 | 3.35 ± 2.10 (2.50–4.19) | 3.00 (2.00–4.00) | 0.198 | 0.940 * |
| VNPS neck pain intensity 14 days after treatment | 3.00 ± 2.04 (2.18–3.82) | 3.00 (2.00–4.00) | 0.164 | 2.77 ± 2.29 (1.85–3.69) | 2.50 (1.00–4.00) | 0.042 | 0.650 ** |
| VNPS post-needling soreness before treatment | 0.00 ± 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | NA | 0.00 ± 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | NA | NA |
| VNPS post-needling soreness after treatment | 4.31 ± 2.20 (3.42–5.20) | 4.00 (3.00–6.00) | 0.178 | 4.42 ± 2.77 (3.30–5.54) | 4.50 (2.00–7.00) | 0.009 | 0.868 ** |
| VNPS post-needling soreness 72 h after treatment | 0.88 ± 1.24 (0.38–1.39) | 0.00 (0.00–2.00) | <0.0001 | 0.42 ± 1.27 (−0.09–0.94) | 0.00 (0.00–0.00) | <0.0001 | 0.061 ** |
| VNPS post-needling soreness 14 days after treatment | 0.00 ± 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | NA | 0.00 ± 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | NA | NA |
| VNPS pain during treatment | 4.54 ± 2.21 (3.64–5.43) | 4.00 (3.00–6.00) | 0.3883 | 6.54 ± 2.27 (5.62–7.45) | 7.00 (5.00–8.00) | 0.1691 | 0.002 * |
| NPQ before treatment | 25.9 ± 8 (22.6–29.2) | 25.0 (22.2–27.8) | 0.1014 | 31.8 ± 11 (27.5–36.1) | 32.3 (23.7–37.4) | 0.1126 | 0.059 * |
| NPQ after treatment | 23.9 ± 8 (20.6–27.2) | 23.6 (19.4–26.3) | 0.6283 | 30 ± 11 (25.6–34.5) | 29.4 (22.3–36.1) | 0.1988 | 0.047 * |
| NPQ 72 h after treatment | 17.1 ± 9 (13.3–20.9) | 16.7 (11.1–22.2) | 0.5637 | 17.5 ± 12 (12.7–22.2) | 15.3 (11.9–23.3) | 0.3994 | 0.978 * |
| NPQ 14 days after treatment | 14 ± 11 (9.6–18.4) | 13.9 (5.6–16.7) | 0.1400 | 15.7 ± 12 (11.1–20.4) | 13.9 (8.3–22.2) | 0.1947 | 0.639 * |
| Dry Needling Group N = 26 | Percutaneus Electrolysis Group N = 26 | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Mean ± SD (95% CI) | Median (IR) | p Value (Kolmogorov–Smirnov) | Mean ± SD (95% CI) | Median (IR) | p Value (Kolmogorov–Smirnov) | p Value |
| PPT on the central MTrP before treatment | 2.52 ± 0.70 (2.24–2.80) | 2.53 (2.15–3.02) | 0.198 | 2.56 ± 0.75 (2.25–2.86) | 2.52 (1.99–2.97) | 0.129 | 0.934 * |
| PPT on the central MTrP after treatment | 2.50 ± 0.71 (2.21–2.78) | 2.48 (2–3.02) | 0.257 | 2.56 ± 0.77 (2.25–2.87) | 2.52 (2.07–3.05) | 0.898 | 0.700 * |
| PPT on the central MTrP 72 h after treatment | 2.66 ± 0.8 (2.34–2.99) | 2.58 (2.09–3.02) | 0.130 | 2.72 ± 0.84 (2.38–3.06) | 2.60 (2.23–3.01) | 0.683 | 0.762 * |
| PPT on the central MTrP 14 days after treatment | 2.74 ± 0.81 (2.41–3.06) | 2.82 (2.19–3.25) | 0.261 | 2.72 ± 0.77 (2.41–3.03) | 2.78 (2.15–3.14) | 0.758 | 0.941 * |
| PPT on the insertional MTrP before treatment | 2.44 ± 0.73 (2.14–2.73) | 2.33 (1.89–2.98) | 0.074 | 2.59 ± 0.69 (2.31–2.87) | 2.58 (2.12–2.86) | 0.292 | 0.341 * |
| PPT on the insertional MTrP after treatment | 2.36 ± 0.64 (2.11–2.62) | 2.42 (1.82–2.82) | 0.387 | 2.44 ± 0.76 (2.13–2.74) | 2.38 (2.11–2.58) | 0.082 | 0.978 * |
| PPT on the insertional MTrP 72 h after treatment | 2.51 ± 0.66 (2.25–2.78) | 2.43 (2.13–2.68) | 0.153 | 2.60 ± 0.81 (2.27–2.92) | 2.52 (2.09–2.80) | 0.039 | 0.790 ** |
| PPT on the insertional MTrP 14 days after treatment | 2.48 ± 0.69 (2.20–2.76) | 2.38 (2.20–2.65) | 0.461 | 2.56 ± 0.60 (2.32–2.80) | 2.58 (2.25–2.95) | 0.632 | 0.533 * |
| CROM of ipsilateral rotation before treatment | 68.79 ± 8.50 (65.36–72.23) | 71 (66.07–73.63) | 0.002 | 72.06 ± 4.04 (70.43–73.70) | 72.33 (70.37–72.97) | 0.402 | 0.368 ** |
| CROM of ipsilateral rotation after treatment | 73.90 ± 7.35 (70.93–76.86) | 76 (74–78) | 0.000 | 77.88 ± 2.89 (76.72–79.05) | 78 (76–79.48) | 0.683 | 0.043 ** |
| CROM of ipsilateral rotation 72 h after treatment | 74.54 ± 6 (72.12–76.96) | 74.67 (72.67–77.63) | 0.183 | 77.49 ± 3.58 (76.04–78.93) | 78.67 (75.33–79.33) | 0.181 | 0.045 * |
| CROM of ipsilateral rotation 14 days after treatment | 74.13 ± 4.97 (72.12–76.14) | 74.67 (72.37–76.60) | 0.071 | 75.13 ± 3.84 (73.58–76.68) | 75 (73.03–77.27) | 0.708 | 0.653 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benito-de-Pedro, A.I.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; Benito-de-Pedro, M. Efficacy of Deep Dry Needling versus Percutaneous Electrolysis in Ultrasound-Guided Treatment of Active Myofascial Trigger Points of the Levator Scapulae in Short-Term: A Randomized Controlled Trial. Life 2023, 13, 939. https://doi.org/10.3390/life13040939
Benito-de-Pedro AI, Becerro-de-Bengoa-Vallejo R, Losa-Iglesias ME, Rodríguez-Sanz D, Calvo-Lobo C, Benito-de-Pedro M. Efficacy of Deep Dry Needling versus Percutaneous Electrolysis in Ultrasound-Guided Treatment of Active Myofascial Trigger Points of the Levator Scapulae in Short-Term: A Randomized Controlled Trial. Life. 2023; 13(4):939. https://doi.org/10.3390/life13040939
Chicago/Turabian StyleBenito-de-Pedro, Ana Isabel, Ricardo Becerro-de-Bengoa-Vallejo, Marta Elena Losa-Iglesias, David Rodríguez-Sanz, César Calvo-Lobo, and María Benito-de-Pedro. 2023. "Efficacy of Deep Dry Needling versus Percutaneous Electrolysis in Ultrasound-Guided Treatment of Active Myofascial Trigger Points of the Levator Scapulae in Short-Term: A Randomized Controlled Trial" Life 13, no. 4: 939. https://doi.org/10.3390/life13040939
APA StyleBenito-de-Pedro, A. I., Becerro-de-Bengoa-Vallejo, R., Losa-Iglesias, M. E., Rodríguez-Sanz, D., Calvo-Lobo, C., & Benito-de-Pedro, M. (2023). Efficacy of Deep Dry Needling versus Percutaneous Electrolysis in Ultrasound-Guided Treatment of Active Myofascial Trigger Points of the Levator Scapulae in Short-Term: A Randomized Controlled Trial. Life, 13(4), 939. https://doi.org/10.3390/life13040939









