Brain Structure and Function: Insights from Chemical Neuroanatomy
Abstract
1. General Premises
2. From the Contributions of Golgi and Cajal to Modern Chemical Neuroanatomy: A Brief Survey
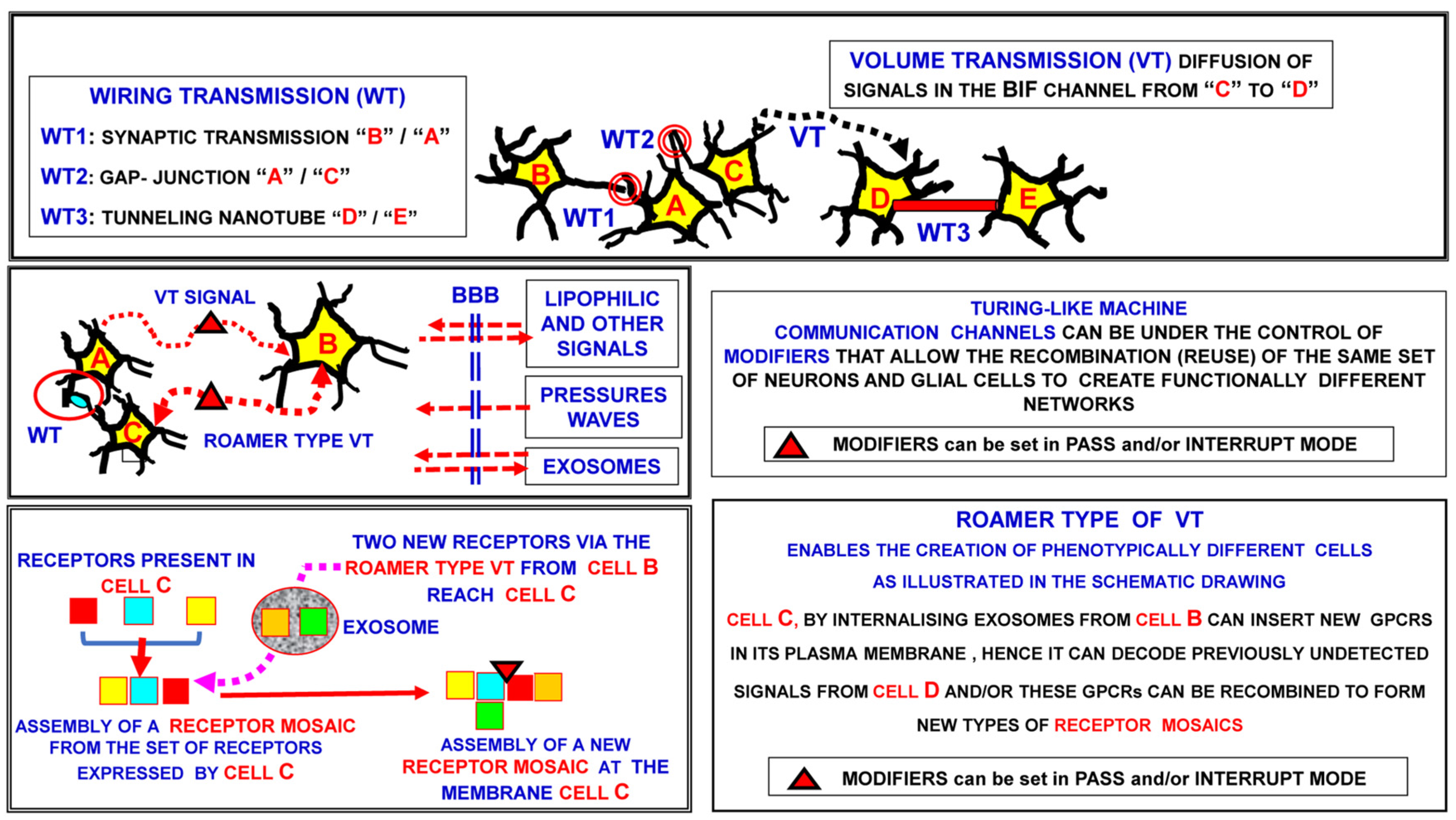
- Private channel: physically delimited pathway between two nodes of the network
- Diffuse channel: the whole available space between the network nodes is potentially used to exchange signals
- Reserved signal: Signal needing a specific “decoder” in order to be decrypted. Neurotransmitters and, more generally, signals using specific receptor systems are of this type
- Broadcast signal: “Public” signal, i.e., interpreted by all the elements that it can reach. Physical processes (e.g., pressure waves) or membrane permeable molecules (e.g., oxygen) are of this type
- −
- Macro-scale: brain areas and, in greater detail, functional modules (see [73] for a definition of functional modules)
- −
- Meso-scale: local circuits formed by the assembly of portions of brain cells, which can work as independent integrative units; a special role is played by synaptic clusters
- −
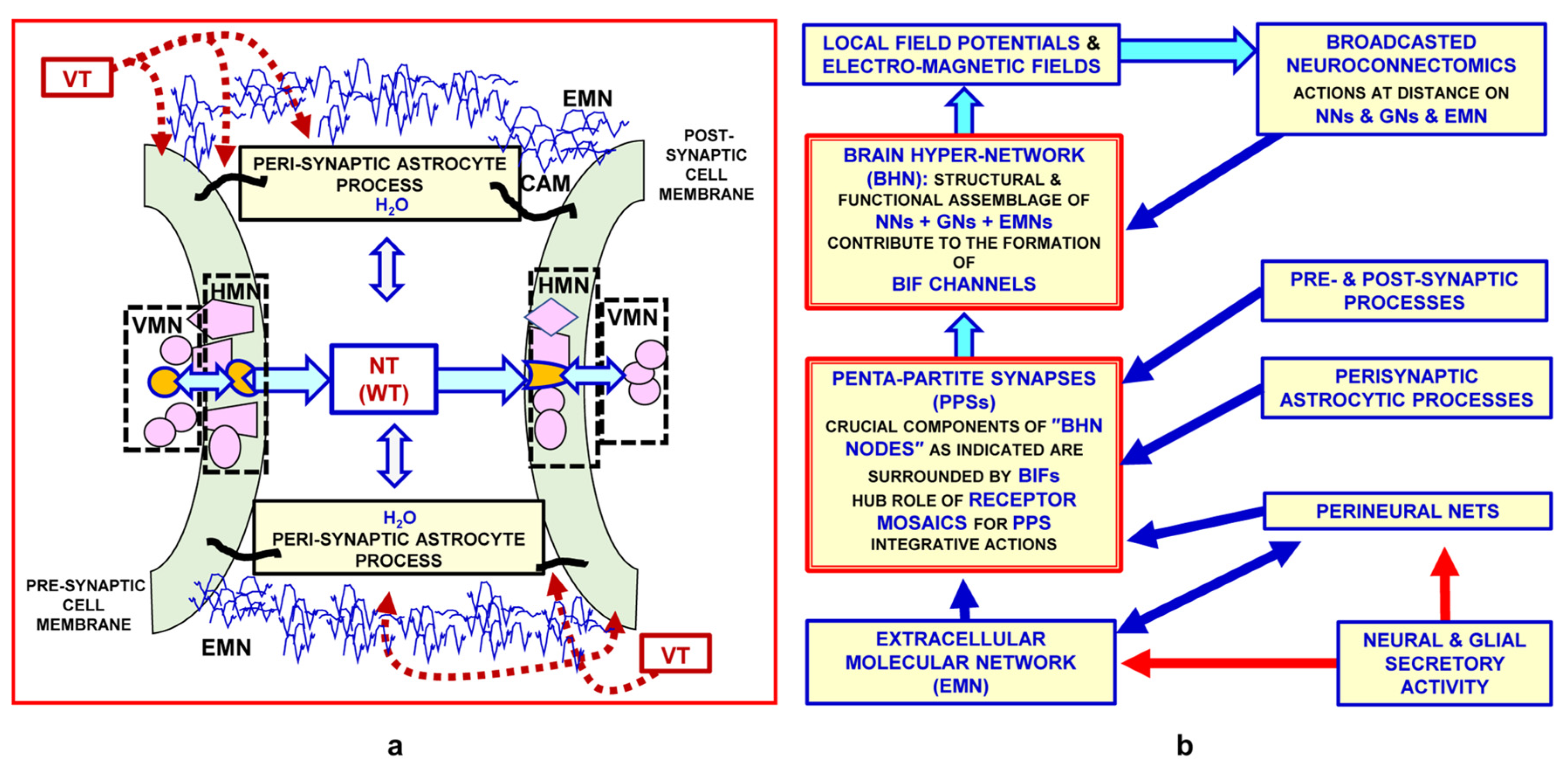
- Micro-scale: in particular, penta-partite synapses formed by pre- and post-synaptic membranes, extracellular molecules and astrocytic processes
- −
- Electrical signals from the pre-synaptic side can affect the post-synaptic side by means of induction;
- Electrical signals can be conducted by the extracellular fluid (electrotonic currents);
- A chemical mediator (neurotransmitter) can cross the synaptic cleft;
- Transient connection can take place between the pre-synaptic and post-synaptic neuron and also via the extracellular matrix surrounding the synaptic contact; the matrix is part of the extracellular molecular network, and affects pre- and post-synaptic morpho-functional aspects of some synaptic contacts [94].
3. Experimental Contributions to Investigations of the Morpho-Functional Organization of Brain Networks at Different Levels of Miniaturization
- −
- Visualization of brain structures at different levels of miniaturization—from the cell networks to the molecular levels—by means of chemical neuroanatomical methods;
- −
- Computer-assisted image analysis of the structures visualized.
3.1. The “Mismatch” in Several Histochemical Images between the Nerve Terminals, and Hence the Neurotransmitter Stores, and Their Respective Decoding Receptors: A Basic Datum in the Proposal of Non-Synaptic Transmission, i.e., Volume Transmission
3.1.1. Types of VT Signals
3.1.2. Pathways of VT-Signal Migration
3.1.3. Energy Gradients for VT-Signal Migration
- Concentration Gradients (ref. [104] and References Therein)
- Gradients of Electrical Potentials (for Charged Signals) (ref. [104] and References Therein)
- Pressure Gradients (ref. [104] and References Therein)
3.1.4. Decoding Systems for VT-Signals
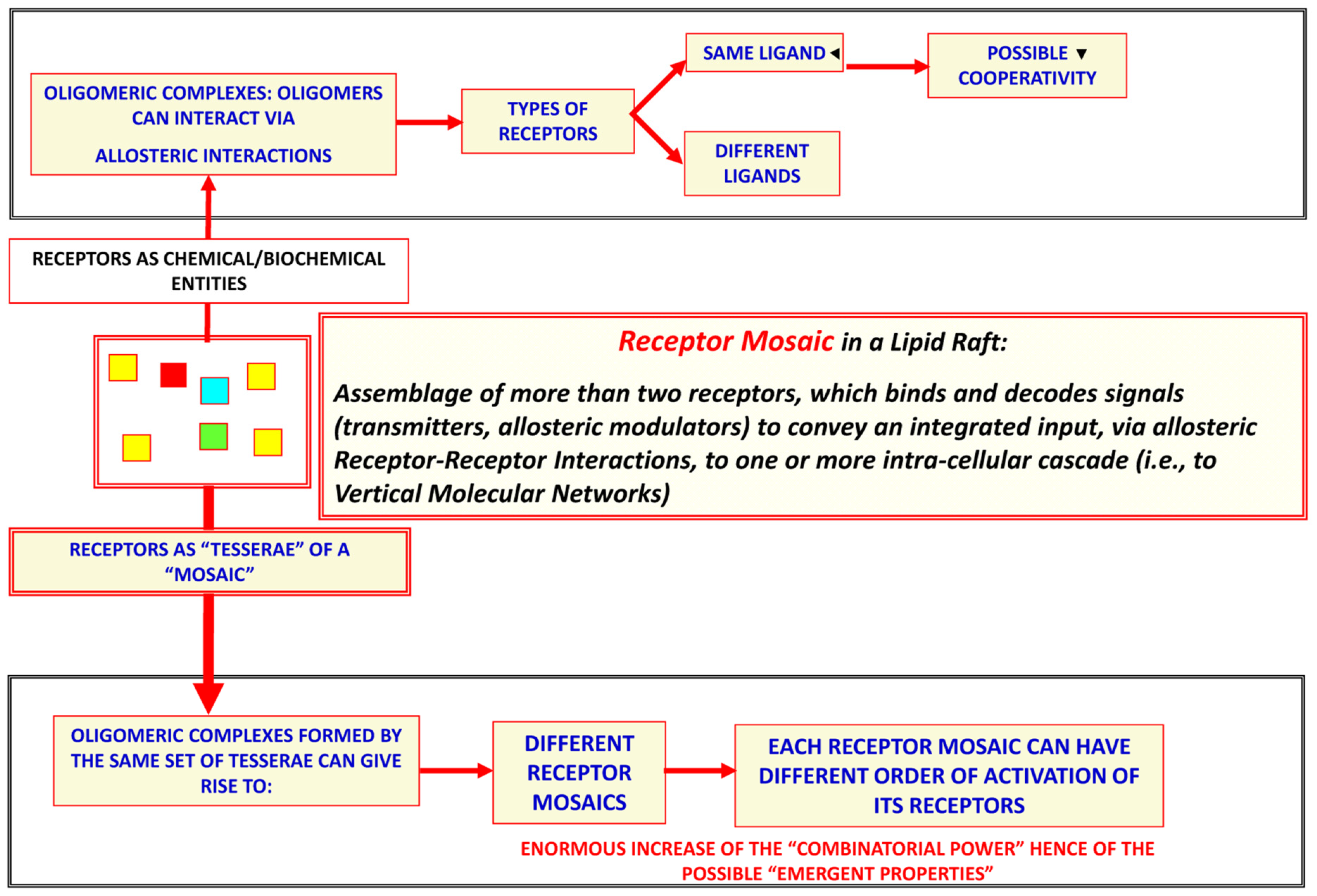
3.2. Evidence of the Existence of Horizontal Molecular Networks at Cell Membrane Levels and of the Integrative Role of GPCR Aggregates
4. Future Investigations on Integrative Functions of the Brain
5. Final Comment: Epistemological Considerations
- −
- The Penrose and Hameroff hypothesis that brain computations are basically due to quantum computations that involve hydrophobic areas of microtubules, whose electron clouds undergo orchestrated superposition and reduction, producing proto-conscious elements that become orchestrated into conscious experiences. The main aspects of this interesting hypothesis have recently been discussed by Schiffer [173];
- −
- Friston’s free-energy principle, according to which a basic feature of any organism—from the single cell to the human brain—is that sensory inputs and memory stores are used to build a reliable model of the organism’s environment. Thus, Friston’s free-energy principle states that every living being, at every scale of organization, is driven by an imperative: to sample the world and to ensure that its predictions become a self-fulfilling prophecy. Friston argues that this imperative can be reduced to a mathematical function [174].
- The Brain—is wider than the Sky—
- For—put them side by side—
- The one the other will contain
- With ease—and You—beside—.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| BHN | Brain Hyper-Network |
| CNS | Central Nervous System |
| GPCR | G Protein-Coupled Receptor |
| RM | Receptor Mosaic |
| RRI | Receptor–receptor interaction |
| VT | Volume Transmission |
| WT | Wiring Transmission |
References
- Kuhn, T.S. The Structure of Scientific Revolutions, 1st ed.; University of Chicago Press: Chicago, IL, USA, 1962. [Google Scholar]
- Kuhn, T.S. The Structure of Scientific Revolutions, 3rd ed.; University of Chicago Press: Chicago, IL, USA, 1996. [Google Scholar]
- Björklund, A.; Hökfelt, T. Handbook of Chemical Neuroanatomy, Vol. 1: Methods in Chemical Neuroanatomy; Elsevier Science Publishers: Amsterdam, The Netherlands, 1983; ISBN 0444902813. [Google Scholar]
- Kuhar, M.J.; Unnerstall, J.R.; De Souza, E.B. Receptor mapping in neuropharmacology by autoradiography: Some technical problems. NIDA Res. Monogr. 1985, 62, 1–12. [Google Scholar] [PubMed]
- Agnati, L.F.; Fuxe, K. Quantitative Neuroanatomy in Transmitter Research; Macmillan Press Ltd.: London, UK, 1985. [Google Scholar]
- Sporns, O. Discovering the Human Connectome; MIT Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, Z.; Wang, P.; Sun, Z. Current application and future directions of photobiomodulation in central nervous diseases. Neural Regen. Res. 2021, 16, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- May, L.T.; Leach, K.; Sexton, P.M.; Christopoulos, A. Allosteric modulation of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- Nussinov, R.; Tsai, C.J. Allostery in disease and in drug discovery. Cell 2013, 153, 293–305. [Google Scholar] [CrossRef]
- Nickols, H.H.; Conn, P.J. Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol. Dis. 2014, 61, 55–71. [Google Scholar] [CrossRef]
- Agnati, L.F.; Baluška, F.; Barlow, P.W.; Guidolin, D. Mosaic, self-similarity logic and biological attraction principles: Three explanatory instruments in biology. Commun. Integr. Biol. 2009, 2, 552–563. [Google Scholar] [CrossRef]
- Milligan, G. G protein-coupled receptor hetero-dimerization: Contribution to pharmacology and function. Br. J. Pharmacol. 2009, 158, 5–14. [Google Scholar] [CrossRef]
- Agnati, L.F.; Guidolin, D.; Leo, G.; Carone, C.; Genedani, S.; Fuxe, K. Receptor-receptor interactions: A novel concept in brain integration. Prog. Neurobiol. 2010, 90, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.R.; Donthamsetti, P.; Shonberg, J.; Draper-Joyce, C.J.; Dentry, S.; Michino, M.; Shi, L.; López, L.; Scammells, P.J.; Capuano, B.; et al. A new mechanism of allostery in a G protein-coupled receptor dimer. Nat. Chem. Biol. 2014, 10, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Farran, B. An update on the physiological and therapeutic relevance of GPCR oligomers. Pharmacol. Res. 2017, 117, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Marcoli, M.; Tortorella, C.; Maura, G.; Agnati, L.F. G protein-coupled receptor-receptor interactions give integrative dynamics to intercellular communication. Rev. Neurosci. 2018, 29, 703–726. [Google Scholar] [CrossRef]
- Guidolin, D.; Marcoli, M.; Tortorella, C.; Maura, G.; Agnati, L.F. Receptor-Receptor Interactions as a Widespread Phenomenon: Novel Targets for Drug Development? Front. Endocrinol. 2019, 10, 53. [Google Scholar] [CrossRef]
- Slosky, L.M.; Caron, M.G.; Barak, L.S. Biased Allosteric Modulators: New Frontiers in GPCR Drug Discovery. Trends Pharmacol. Sci. 2021, 42, 283–299. [Google Scholar] [CrossRef]
- Agnati, L.F.; Genedani, S.; Leo, G.; Rivera, A.; Guidolin, D.; Fuxe, K. One century of progress in neuroscience founded on Golgi and Cajal’s outstanding experimental and theoretical contributions. Brain Res. Rev. 2007, 55, 167–189. [Google Scholar] [CrossRef]
- Mazzarello, P. Golgi C: A Biography of the Founder of Modern Neuroscience; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Golgi, C. The Neuron Doctrine—Theory and Facts; Nobel Lecture. 11 December 1906. Available online: https://www.nobelprize.org/prizes/medicine/1906/golgi/lecture/ (accessed on 9 November 2022).
- Cajal, S.R. The Structure and Connexions of Neurons; Nobel Lecture. 12 December 1906. Available online: https://www.nobelprize.org/prizes/medicine/1906/cajal/lecture/ (accessed on 9 November 2022).
- Sherrington, C.S. The Integrative Action of the Nervous System; Yale University Press: New Haven, CT, USA, 1906. [Google Scholar]
- Zeng, H.; Sanes, J.R. Neuronal cell-type classification: Challenges, opportunities and the path forward. Nat. Rev. Neurosci. 2017, 18, 530–546. [Google Scholar] [CrossRef]
- Dale, H.H. Chemical Transmission of the Effects of Nerve Impulses. BMJ 1934, 1, 835–841. [Google Scholar] [CrossRef]
- Dale, H. Pharmacology and Nerve-Endings. Proc. R. Soc. Med. 1935, 28, 319–332. [Google Scholar]
- Eccles, J.C. The Physiology of Nerve Cells; John Hopkins Press: Baltimore, MD, USA, 1957. [Google Scholar]
- Strata, P.; Harvey, R. Dale’s principle. Brain Res. Bull. 1999, 50, 349–350. [Google Scholar] [CrossRef]
- Palay, S.L.; Wissig, S.L. Secretory granules and Nissl substance in fresh supraoptic neurones of the rabbit. Anat. Rec. 1953, 116, 301–313. [Google Scholar] [CrossRef]
- Palade, G.E.; Palay, S.L. Electron microscope observations of interneuronal and neuromuscular synapses. Anat. Rec. 1954, 118, 335–336. [Google Scholar]
- Palay, S.L. Synapses in the central nervous system. J. Biophys. Biochem. Cytol. 1956, 2, 193–202. [Google Scholar] [CrossRef]
- Falck, B.; Hillarp, N.-Å.; Thieme, G.; Torp, A. Fluorescence of catechol amines and related compounds condensed with formaldehyde. J. Histochem. Cytochem. 1962, 10, 348–354. [Google Scholar] [CrossRef]
- Dahlstrom, A.; Fuxe, K. Localization of monoamines in the lower brain stem. Experientia 1964, 20, 398–399. [Google Scholar] [CrossRef]
- Anden, N.E.; Carlsson, A.; Dahlstroem, A.; Fuxe, K.; Hillrap, N.A.; Larsson, K. Demonstration and mapping out of nigro-neostriatal dopamine neurons. Life Sci. 1964, 3, 523–530. [Google Scholar] [CrossRef]
- Fuxe, K. Evidence for the existence of monoamine neurons in the central nervous system: IV. Distribution of monoamine nerve terminals in the central nervous system. Acta Physiol. Scand. 1965, 247, 37–45. [Google Scholar]
- Fuxe, K. Evidence for the existence of monoamine neurons in the central nervous system: 3. The monoamine nerve terminal. Z. Zellforsch. Mikrosk. Anat. 1965, 65, 573–596. [Google Scholar] [CrossRef]
- Dahlström, A.; Fuxe, K. Evidence for the existence of monoamine neurons in the central nervous system: II. Experimentally induced changes in the intraneuronal amine levels of bulbospinal neuron systems. Acta Physiol. Scand. Suppl. 1965, 247, 1–36. [Google Scholar]
- Benfenati, F.; Cimino, M.; Agnati, L.F.; Fuxe, K. Quantitative autoradiography of central neurotransmitter receptors: Methodological and statistical aspects with special reference to computer-assisted image analysis. Acta Physiol. Scand. 1986, 128, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Fuxe, K.; Benfenati, F.; Zini, I.; Zoli, M.; Fabbri, L.; Härfstrand, A. Computer assisted morphometry and microdensitometry of transmitter identified neurons with special reference to the mesostriatal dopamine pathway—I. Methodological aspects. Acta Physiol. Scand. Suppl. 1984, 52, 5–36. [Google Scholar]
- Zoli, M.; Zini, I.; Agnati, L.F.; Guidolin, D.; Ferraguti, F.; Fuxe, K. Aspects of neural plasticity in the central nervous system—I. Computer-assisted image analysis methods. Neurochem. Int. 1990, 16, 383–418. [Google Scholar] [CrossRef] [PubMed]
- Pert, C.B.; Kuhar, M.J.; Snyder, S.H. Autoradiograhic localization of the opiate receptor in rat brain. Life Sci 1975, 16, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Hökfelt, T.; Johansson, O.; Goldstein, M. Chemical anatomy of the brain. Science 1984, 225, 1326–1334. [Google Scholar] [CrossRef]
- Yelnik, J.; Bardinet, E.; Dormont, D.; Malandain, G.; Ourselin, S.; Tandé, D.; Karachi, C.; Ayache, N.; Cornu, P.; Agid, Y. A three-dimensional, histological and deformable atlas of human basal ganglia. I. Atlas construction based on immunohistochemical and MRI data. NeuroImage 2007, 34, 618–638. [Google Scholar] [CrossRef]
- Hökfelt, T.; Elfvin, L.G.; Elde, R.; Schultzberg, M.; Goldstein, M.; Luft, R. Occurrence of somatostatin-like immunoreactivity in some peripheral sympathetic noradrenergic neurons. Proc. Natl. Acad. Sci. USA 1977, 74, 3587–3591. [Google Scholar] [CrossRef]
- Hökfelt, T.; Holets, V.R.; Staines, W.; Meister, B.; Melander, T.; Schalling, M.; Schultzberg, M.; Freedman, J.; Björklund, H.; Olson, L.; et al. Coexistence of neuronal messengers—An overview. Prog. Brain Res. 1986, 68, 33–70. [Google Scholar] [CrossRef]
- Furness, J.B.; Morris, J.L.; Gibbins, I.L.; Costa, M. Chemical coding of neurons and plurichemical transmission. Annu. Rev. Pharmacol. Toxicol. 1989, 29, 289–306. [Google Scholar] [CrossRef]
- Burnstock, G. Cotransmission. Curr. Opin. Pharmacol. 2004, 4, 47–52. [Google Scholar] [CrossRef]
- Svensson, E.; Apergis-Schoute, J.; Burnstock, G.; Nusbaum, M.P.; Parker, D.; Schiöth, H.B. General Principles of Neuronal Co-transmission: Insights from Multiple Model Systems. Front. Neural Circuits 2019, 12, 117. [Google Scholar] [CrossRef]
- Hökfelt, T. Neuropeptides in perspective: The last ten years. Neuron 1991, 7, 867–879. [Google Scholar] [CrossRef]
- Lundberg, J.M. Pharmacology of cotransmission in the autonomic nervous system: Integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol. Rev. 1996, 48, 113–178. [Google Scholar]
- Hökfelt, T.; Broberger, C.; Xu, Z.D.; Sergeyev, V.; Ubink, R.; Diez, M. Neuropeptides—An overview. Neuropharmacology 2000, 39, 1337–1356. [Google Scholar] [CrossRef]
- Cifuentes, F.; Morales, M.A. Functional Implications of Neurotransmitter Segregation. Front. Neural Circuits 2021, 15, 738516. [Google Scholar] [CrossRef]
- Kandel, E.R. Cellular Basis of Behaviour; W.H. Freeman: San Francisco, CA, USA, 1977. [Google Scholar]
- Kandel, E.; Koester, J.D.; Mack, S.H.; Siegelbaum, S.A. Principles of Neuroscience, 6th ed.; McGraw Hill: New York, NY, USA, 2021. [Google Scholar]
- Agnati, L.F.; Fuxe, K.; Zoli, M.; Pich, E.M.; Benfenati, F.; Zini, I.; Goldstein, M. Aspects on the information handling by the central nervous system: Focus on cotransmission in the aged rat brain. Prog. Brain Res. 1986, 68, 291–301. [Google Scholar] [CrossRef]
- Kim, S.; Sabatini, B.L. Analytical approaches to examine gamma-aminobutyric acid and glutamate vesicular co-packaging. Front. Synaptic Neurosci. 2023, 14, 1076616. [Google Scholar] [CrossRef]
- Hnasko, T.S.; Edwards, R.H. Neurotransmitter corelease: Mechanism and physiological role. Annu. Rev. Physiol. 2012, 74, 225–243. [Google Scholar] [CrossRef]
- Tritsch, N.X.; Granger, A.J.; Sabatini, B.L. Mechanisms and fuction of GABA co-release. Nat. Rev. Neurosci. 2016, 17, 139–145. [Google Scholar] [CrossRef]
- Saunders, A.; Oldenburg, I.A.; Berezovskii, V.K.; Johnson, C.A.; Kingery, N.D.; Elliott, H.L.; Xie, T.; Gerfen, C.R.; Sabatini, B.L. A direct GABAergic output from the basal ganglia to frontal cortex. Nature 2015, 21, 85–89. [Google Scholar] [CrossRef]
- Changeaux, J.P.; Christopoulos, A. Allosteric modulation as a unifying mechanism for receptor function and regulation. Diabetes Obes. Metab. 2017, 19, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Virchow, R. Ueber das granulirte Ansehen der Wandungen der Gehirnventrikel. In Gesammelte Abhandlungen zur Wissenschaftlichen Medicin; Virchow, R., Ed.; Meidinger Sohn & Comp.: Frankfurt, Germany, 1856; pp. 885–891. [Google Scholar]
- Virchow, R. Die Cellularpathologie in Ihrer Begründung auf Physiologische und Pathologische Gewebelehre; Hirschwald: Berlin, Germany, 1858. [Google Scholar]
- Lugaro, E. Sulle funzioni della nevroglia. Riv. Pat. Nerv. Ment. 1907, 12, 225–233. [Google Scholar]
- Verkhratsky, A.; Nedergaard, M. Physiology of astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Parpura, V.; Heneka, M.T.; Montana, V.; Oliet, S.H.R.; Schousboe, A.; Haydon, P.G.; Stout, R.F., Jr.; Spray, D.C.; Reichenbach, A.; Pannicke, T.; et al. Glial cells in (patho)physiology. J. Neurochem. 2012, 121, 4–27. [Google Scholar] [CrossRef]
- Guidolin, D.; Tortorella, C.; Marcoli, M.; Cervetto, C.; Maura, G.; Agnati, L.F. Receptor-Receptor Interactions and Glial Cell Functions with a Special Focus on G Protein-Coupled Receptors. Int. J. Mol. Sci. 2021, 22, 8656. [Google Scholar] [CrossRef]
- Chvátal, A.; Syková, E. Glial influence on neuronal signaling. Prog. Brain Res. 2000, 125, 199–216. [Google Scholar] [CrossRef]
- Färber, K.; Kettenmann, H. Physiology of microglial cells. Brain Res. Rev. 2005, 48, 133–143. [Google Scholar] [CrossRef]
- Agnati, L.F.; Marcoli, M.; Maura, G.; Woods, A.; Guidolin, D. The brain as a “hyper-network”: The key role of neural networks as main producers of the integrated brain actions especially via the “broadcasted” neuroconnectomics. J. Neural Transm. 2018, 125, 883–897. [Google Scholar] [CrossRef]
- Agnati, L.F.; Guidolin, D.; Maura, G.; Marcoli, M.; Leo, G.; Carone, C.; De Caro, R.; Genedani, S.; Borroto-Escuela, D.O.; Fuxe, K. Information handling by the brain: Proposal of a new “paradigm” involving the roamer type of volume transmission and the tunneling nanotube type of wiring transmission. J. Neural Transm. 2014, 121, 1431–1449. [Google Scholar] [CrossRef]
- Fields, R.D.; Stevens-Graham, B. New insights into neuron-glia communication. Science 2002, 298, 556–562. [Google Scholar] [CrossRef]
- Kofuji, P.; Araque, A. G-Protein-Coupled Receptors in Astrocyte-Neuron Communication. Neuroscience 2021, 456, 71–84. [Google Scholar] [CrossRef]
- Sherwood, C.C.; Stimpson, C.D.; Raghanti, M.A.; Wildman, D.E.; Uddin, M.; Grossman, L.I.; Goodman, M.; Redmond, J.C.; Bonar, C.J.; Erwin, J.M.; et al. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc. Natl. Acad. Sci. USA 2006, 103, 13606–13611. [Google Scholar] [CrossRef]
- Von Bartheld, C.S.; Bahney, J.; Herculano-Houzel, S. The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J. Comp. Neurol. 2016, 524, 3865–3895. [Google Scholar] [CrossRef]
- Pelassa, S.; Guidolin, D.; Venturini, A.; Averna, M.; Frumento, G.; Campanini, L.; Bernardi, R.; Cortelli, P.; Buonaura, G.C.; Maura, G.; et al. A2A-D2 Heteromers on Striatal Astrocytes: Biochemical and Biophysical Evidence. Int. J. Mol. Sci. 2019, 20, 2457. [Google Scholar] [CrossRef]
- Guidolin, D.; Marcoli, M.; Tortorella, C.; Maura, G.; Agnati, L.F. Adenosine A2A-Dopamine D2 Receptor-Receptor Interaction in Neurons and Astrocytes: Evidence and Perspectives. Prog. Med. Biol. Transl. Sci. 2020, 169, 247–277. [Google Scholar]
- Amato, S.; Averna, M.; Guidolin, D.; Pedrazzi, M.; Pelassa, S.; Capraro, M.; Passalacqua, M.; Bozzo, M.; Gatta, E.; Anderlini, D.; et al. Heterodimer of A2A and oxytocin receptors regulating glutamate release in adult striatal astrocytes. Int. J. Mol. Sci. 2022, 23, 2326. [Google Scholar] [CrossRef]
- Sporns, O. Cerebral cartography and connectomics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140173. [Google Scholar] [CrossRef]
- Lord, L.-D.; Stevner, A.B.; Deco, G.; Kringelbach, M.L. Understanding principles of integration and segregation using whole-brain computational connectomics: Implications for neuropsychiatric disorders. Phil. Trans. R. Soc. A 2017, 375, 20160283. [Google Scholar] [CrossRef]
- Harvey, R.J. Can computers think? Differences and similarities between computers and brains. Prog. Neurobiol. 1995, 45, 99–127. [Google Scholar] [CrossRef]
- Shapshak, P. Artificial Intelligence and brain. Bioinformation 2018, 14, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Singer, W. Differences Between Natural and Artificial Cognitive Systems. In Robotics, AI and Humanity; von Braun, J., Archer, M.S., Reichberg, G.M., Sánchez Sorondo, M., Eds.; Springer: Cham, Switzerland, 2021; pp. 17–27. [Google Scholar] [CrossRef]
- El Zaatari, S.; Marei, M.; Li, W.; Usman, Z. Cobot programming for collaborative industrial tasks: An overview. Robot. Auton. Syst. 2019, 116, 162–180. [Google Scholar] [CrossRef]
- Guidolin, D.; Albertin, G.; Guescini, M.; Fuxe, K.; Agnati, L.F. Central nervous system and computation. Q. Rev. Biol. 2011, 86, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, D. Birth of Intelligence: From RNA to Artificial Intelligence; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Agnati, L.F.; Anderlini, D.; Guidolin DMarcoli, M.; Maura, G. Man is a “Rope” Stretched Between Virosphere and Humanoid Robots: On the Urgent Need of an Ethical Code for Ecosystem Survival. Found. Sci. 2022, 27, 311–325. [Google Scholar] [CrossRef]
- Guidolin, D.; Marcoli, M.; Maura, G.; Agnati, L.F. New dimensions of connectomics and network plasticity in the central nervous system. Rev. Neurosci. 2017, 28, 113–132. [Google Scholar] [CrossRef]
- Agnati, L.F.; Fuxe, K.; Zoli, M.; Rondanini, C.; Ogren, S.O. New vistas on synaptic plasticity: The receptor mosaic hypothesis of the engram. Med. Biol. 1982, 60, 183–190. [Google Scholar]
- Agnati, L.F.; Guidolin, D.; Leo, G.; Fuxe, K. A boolean network modelling of receptor mosaics relevance of topology and cooperativity. J. Neural. Transm. 2007, 114, 77–92. [Google Scholar] [CrossRef]
- Dityatev, A.; Rusakov, D.A. Molecular signals of plasticity at the tetrapartite synapse. Curr. Opin. Neurobiol. 2011, 21, 353–359. [Google Scholar] [CrossRef]
- Aggarwal, S.; Mortensen, O.V. Overview of Monoamine Transporters. Curr. Protoc. Pharmacol. 2017, 79, 12.16.1–12.16.17. [Google Scholar] [CrossRef]
- Fuxe, K.; Dahlström, A.B.; Jonsson, G.; Marcellino, D.; Guescini, M.; Dam, M.; Manger, P.; Agnati, L. The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog. Neurobiol. 2010, 90, 82–100. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural. Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef]
- Bernardinelli, Y.; Muller, D.; Nikonenko, I. Astrocyte-synapse structural plasticity. Neural Plast. 2014, 2014, 232105. [Google Scholar] [CrossRef]
- Agnati, L.F.; Fuxe, K.; Zoli, M.; Ozini, I.; Toffano, G.; Ferraguti, F. A correlation analysis of the regional distribution of central enkephalin and beta endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: The volume transmission and the wiring transmission. Acta Physiol. Scand. 1986, 128, 201–207. [Google Scholar] [CrossRef]
- Fuxe, K.; Agnati, L.F. Volume Transmission in the Brain, Novel Mechanisms for Neural Transmission; Raven Press: New York, NY, USA, 1991; Volume 1. [Google Scholar]
- Agnati, L.F.; Fuxe, K. Volume transmission as a key feature of information handling in the central nervous system: Possible new interpretative value of the Turing’s B-type machine. Progr. Brain Res. 2000, 125, 3–19. [Google Scholar] [CrossRef]
- Marcoli, M.; Agnati, L.F.; Benedetti, F.; Genedani, S.; Guidolin, D.; Ferraro, L.; Maura, G.; Fuxe, K. On the role of the extracellular space on the holistic behavior of the brain. Rev. Neurosci. 2015, 26, 489–506. [Google Scholar] [CrossRef]
- Kiss, J.P.; Vizi, E.S. Nitric oxide: A novel link between synaptic and nonsynaptic transmission. Trends Neurosci. 2001, 24, 211–215. [Google Scholar] [CrossRef]
- Agnati, L.F.; Guidolin, D.; Guescini, M.; Genedani, S.; Fuxe, K. Understanding wiring and volume transmission. Brain Res. Rev. 2010, 64, 137–159. [Google Scholar] [CrossRef]
- Taber, K.H.; Hurley, R.A. Volume transmission in the brain: Beyond the synapse. J. Neuropsychiatry Clin. Neurosci. 2014, 26, 4. [Google Scholar] [CrossRef]
- Greitz, D. Cerebrospinal fluid circulation and associated intracranial dynamics. A radiologic investigation using MR imaging and radionuclide cisternography. Acta Radiol. Suppl. 1993, 386, 1–23. [Google Scholar]
- Yablonskiy, D.A.; Ackerman, J.J.; Raichle, M.E. Coupling between changes in human brain temperature and oxidative metabolism during prolonged visual stimulation. Proc. Natl. Acad. Sci. USA 2000, 97, 7603–7608. [Google Scholar] [CrossRef]
- Bullock, T.H. Signals and signs in the nervous system: The dynamic anatomy of electrical activity is probably information-rich. Proc. Natl. Acad. Sci. USA 1997, 94, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Poo, M.M. Mobility and localization of proteins in excitable membranes. Annu. Rev. Neurosci. 1985, 8, 369–406. [Google Scholar] [CrossRef] [PubMed]
- Isakovic, J.; Dobbs-Dixon, I.; Chaudhuri, D.; Mitrecic, D. Modeling of inhomogeneous electromagnetic fields in the nervous system: A novel paradigm in understanding cell interactions, disease ethiology and therapy. Sci. Rep. 2018, 8, 12909. [Google Scholar] [CrossRef] [PubMed]
- Guescini, M.; Guidolin, D.; Vallorani, L.; Casadei, L.; Gioacchini, A.M.; Tibollo, P.; Battistelli, M.; Falcieri, E.; Battistin, L.; Agnati, L.F.; et al. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp. Cell Res. 2010, 316, 1977–1984. [Google Scholar] [CrossRef]
- Venturini, A.; Passalacqua, M.; Pelassa, S.; Pastorino, F.; Tedesco, M.; Cortese, K.; Gagliani, M.C.; Leo, G.; Maura, G.; Guidolin, D.; et al. Exosomes from Astrocyte Processes: Signaling to Neurons. Front. Pharmacol. 2019, 10, 1452. [Google Scholar] [CrossRef]
- Guescini, M.; Leo, G.; Genedani, S.; Carone, C.; Pederzoli, F.; Ciruela, F.; Guidolin, D.; Stocchi, V.; Mantuano, M.; Borroto-Escuela, D.O.; et al. Microvesicle and tunneling nanotube mediated intercellular transfer of g-protein coupled receptors in cell cultures. Exp. Cell Res. 2012, 318, 603–613. [Google Scholar] [CrossRef]
- Venero, J.L.; Vizuete, M.L.; Machado, A.; Cano, J. Aquaporins in the central nervous system. Prog. Neurobiol. 2001, 63, 321–366. [Google Scholar] [CrossRef]
- Niermann, H.; Amiry-Moghaddam, M.; Holthoff, K.; Witte, O.W.; Ottersen, O.P. A novel role of vasopressin in the brain: Modulation of activity-dependent water flux in the neocortex. J. Neurosci. 2001, 21, 3045–3051. [Google Scholar] [CrossRef]
- Sperelakis, N. Cell Physiology; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Jacob, F. Evolution and tinkering. Science 1977, 196, 1161–1166. [Google Scholar] [CrossRef]
- Kandel, E.R.; Schwartz, J.H.; Jessel, T.M. Principles of Neural Science; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- McCulloch, W.S.; Pitts, W. A logical calculus of the ideas immanent in nervous activity. Bull. Math. Biophys. 1943, 5, 115–133. [Google Scholar] [CrossRef]
- Woo, J.; Choi, K.; Kim, S.H.; Han, K.; Choi, M. Characterization of multiscale logic operations in the neural circuits. Front. Biosci. 2021, 26, 723–739. [Google Scholar] [CrossRef]
- Agnati, L.F.; Fuxe, K.; Zini, I.; Lenzi, P.; Hökfelt, T. Aspects on receptor regulation and isoreceptor identification. Med. Biol. 1980, 58, 182–187. [Google Scholar]
- Fuxe, K.; Agnati, L.F.; Benfenati, F.; Cimmino, M.; Algeri, S.; Hokfelt, T.; Mutt, V. Modulation by cholecystokinins of 3H-spiroperidol binding in rat striatum: Evidence for increased affinity and reduction in the number of binding sites. Acta Physiol. Scand. 1981, 113, 567–569. [Google Scholar] [CrossRef]
- Fuxe, K.; Agnati, L.F.; Benfenati, F.; Celani, M.; Zini, I.; Zoli, M.; Mutt, V. Evidence for the Existence of Receptor—Receptor Interactions in the Central Nervous System. Studies on the Regulation of Monoamine Receptors by Neuropeptides. J. Neural Transm. 1983, 18, 165–179. [Google Scholar] [CrossRef]
- Agnati, L.F.; Guidolin, D.; Vilardaga, J.P.; Ciruela, F.; Fuxe, K. On the expanding terminology in the GPCR field: The meaning of receptor mosaics and receptor heteromers. J. Recept. Signal Transduct. Res. 2010, 30, 287–303. [Google Scholar] [CrossRef]
- Agnati, L.F.; Fuxe, K.; Ferré, S. How receptor mosaics decode transmitter signals. Possible relevance of cooperativity. Trends Biochem. Sci. 2005, 30, 188–193. [Google Scholar] [CrossRef]
- Agnati, L.F.; Celani, M.F.; Fuxe, K. Cholecystokinin peptides in vitro modulate the characteristics of the striatal 3H-Npropylnorapomorphine sites. Acta Physiol. Scand. 1983, 118, 79–81. [Google Scholar] [CrossRef]
- Agnati, L.F.; Fuxe, K.; Benfenati, F.; Zini, I.; Hökfelt, T. On the functional role of coexistence of 5-HT and substance P in bulbospinal 5-HT neurons. Substance P reduces affinity and increases density of 3H-5-HT binding sites. Acta Physiol. Scand. 1983, 117, 299–301. [Google Scholar] [CrossRef]
- Fuxe, K.; von Euler, G.; van der Ploeg, I.; Fredholm, B.B.; Agnati, L.F. Pertussis toxin treatment counteracts the cardiovascular effects of neuropeptide Y and clonidine in the awake unrestrained rat. Neurosci. Lett. 1989, 101, 337–341. [Google Scholar] [CrossRef]
- Fuxe, K.; Agnati, L.F.; Harfstrand, A.; Zoli, M.; von Euler, G.; Grimaldi, R.; Merlo Pich, E.; Bjelke, B.; Eneroth, P.; Benfenati, F. On the role of neuropeptide Y in information handling in the central nervous system in normal and physio- pathological states. Focus on volume transmission and neuropeptide Y/alpha 2 receptor interactions. Ann. N. Y. Acad. Sci. 1990, 579, 28–67. [Google Scholar] [CrossRef]
- Monod, J. Chance and Necessity: An Essay on the Natural Philosophy of Modern Biology; William Collins Sons & Co. Ltd.: Glasgow, Scotland, 1979. [Google Scholar]
- Fenton, A.W. Allostery: An illustrated definition for the ‘second secret of life’. Trends Biochem. Sci. 2008, 33, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Tarakanov, A.O.; Guidolin, D. A simple mathematical model of cooperativity in receptor mosaics based on the “symmetry rule”. BioSystems 2005, 80, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Dehner, C. Non-allosteric proteins. Why do proteins have quaternary structure? In Molecular Life Sciences. An Encyclopedic Reference; Wells, R.D., Bond, J.S., Klinman, J., Siler Masters, B.S., Eds.; Springer: New York, NY, USA, 2018; pp. 812–818. [Google Scholar] [CrossRef]
- Kenakin, T. Allosteric Theory: Taking Therapeutic Advantage of the Malleable Nature of GPCRs. Curr. Neuropharmacol. 2007, 5, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Guidolin, D.; Leo, G.; Guescini, M.; Pizzi, M.; Stocchi, V.; Spano, P.F.; Ghidoni, R.; Ciruela, F.; Genedani, S.; et al. Possible new targets for GPCR modulation: Allosteric interactions, plasma membrane domains, intercellular transfer and epigenetic mechanisms. J. Recept. Signal Transduct. Res. 2011, 31, 315–331. [Google Scholar] [CrossRef]
- Nussinov, R. The spatial structure of cell signaling systems. Phys. Biol. 2013, 10, 045004. [Google Scholar] [CrossRef]
- Jordan, B.A.; Devi, L.A. G-protein-coupled receptor heterodimerization modulates receptor function. Nature 1999, 399, 697–700. [Google Scholar] [CrossRef]
- George, S.R.; Fan, T.; Xie, Z.; Tse, R.; Tam, V.; Varghese, G.; O’Dowd, B.F. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J. Biol. Chem. 2000, 275, 26128–26135. [Google Scholar] [CrossRef]
- Rashid, A.J.; So, C.H.; Kong, M.M.; Furtak, T.; El-Ghundi, M.; Cheng, R.; O’Dowd, B.F.; George, S.R. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. USA 2007, 104, 654–659. [Google Scholar] [CrossRef]
- Agnati, L.F.; Guidolin, D.; Cervetto, C.; Borroto-Escuela, D.O.; Fuxe, K. Role of iso-receptors in receptor-receptor interactions with a focus on dopamine iso-receptor complexes. Rev. Neurosci. 2016, 27, 1–25. [Google Scholar] [CrossRef]
- Agnati, L.F.; Guidolin, D.; Fuxe, K. The brain as a system of nested but partially overlapping networks. Heuristic relevance of the model for brain physiology and pathology. J. Neural. Transm. 2007, 114, 3–19. [Google Scholar] [CrossRef]
- Genedani, S.; Carone, C.; Guidolin, D.; Filaferro, M.; Marcellino, D.; Fuxe, K.; Agnati, L.F. Differential sensitivity of A2A and especially D2 receptor trafficking to cocaine compared with lipid rafts in cotransfected CHO cell lines. Novel actions of cocaine independent of the DA transporter. J. Mol. Neurosci. 2010, 41, 347–357. [Google Scholar] [CrossRef]
- Hillion, J.; Canals, M.; Torvinen, M.; Casado, V.; Scott, R.; Terasmaa, A.; Hansson, A.; Watson, S.; Olah, M.E.; Mallol, J.; et al. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J. Biol. Chem. 2002, 277, 18091–18097. [Google Scholar] [CrossRef]
- Genedani, S.; Guidolin, D.; Leo, G.; Filaferro, M.; Torvinen, M.; Woods, A.S.; Fuxe, K.; Ferré, S.; Agnati, L.F. Computer-assisted image analysis of caveolin-1 involvement in the internalization process of adenosine A2A-dopamine D2 receptor heterodimers. J. Mol. Neurosci. 2005, 26, 177–184. [Google Scholar] [CrossRef]
- Mores, K.L.; Cassell, R.J.; van Rijn, R.M. Arrestin recruitment and signaling by G protein-coupled receptor heteromers. Neuropharmacology 2019, 152, 15–21. [Google Scholar] [CrossRef]
- Agnati, L.F.; Guidolin, D.; Carone, C.; Dam, M.; Genedani, S.; Fuxe, K. Understanding neuronal molecular networks builds on neuronal cellular network architecture. Brain Res. Rev. 2008, 58, 379–399. [Google Scholar] [CrossRef]
- Agnati, L.F.; Fuxe, K.; Woods, A.; Genedani, S.; Guidolin, D. Theoretical considerations on the topological organization of receptor mosaics. Curr. Protein Pept. Sci. 2009, 10, 559–569. [Google Scholar] [CrossRef]
- Agnati, L.F.; Guidolin, D.; Albertin, G.; Trivello, E.; Ciruela, F.; Genedani, S.; Tarakanov, A.; Fuxe, K. An integrated view on the role of receptor mosaics at perisynaptic level: Focus on adenosine A2A, dopamine D2, cannabinoid CB1, and metabotropic glutamate mGlu5 receptors. J. Recept. Signal Transduct. Res. 2010, 30, 355–369. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Brito, I.; Romero-Fernandez, W.; Di Palma, M.; Oflijan, J.; Skieterska, K.; Duchou, J.; Van Craenenbroeck, K.; Suárez-Boomgaard, D.; Rivera, A.; et al. The G protein-coupled receptor heterodimer network (GPCR-HetNet) and its hub components. Int. J. Mol. Sci. 2014, 15, 8570. [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P.A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef]
- Insel, P.A.; Sriram, K.; Gorr, M.W.; Wiley, S.Z.; Michkov, A.; Salmerón, C.; Chinn, A.M. GPCRomics: An Approach to Discover GPCR Drug Targets. Trends Pharmacol. Sci. 2019, 40, 378–387. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, T.; Yun, Y.; Xie, X. Recent progress in assays for GPCR drug discovery. Am. J. Physiol.-Cell Physiol. 2022, 323, C583–C594. [Google Scholar] [CrossRef] [PubMed]
- Maggio, R.; Fasciani, I.; Carli, M.; Petragnano, F.; Marampon, F.; Rossi, M.; Scarselli, M. Integration and Spatial Organization of Signaling by G Protein-Coupled Receptor Homo- and Heterodimers. Biomolecules 2021, 11, 1828. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef] [PubMed]
- Scheefhals, N.; MacGillavry, H.D. Functional organization of postsynaptic glutamate receptors. Mol. Cell. Neurosci. 2018, 91, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Carlsson, J.; Ambrogini, P.; Narváez, M.; Wydra, K.; Tarakanov, A.O.; Li, X.; Millón, C.; Ferraro, L.; Cuppini, R.; et al. Understanding the Role of GPCR Heteroreceptor Complexes in Modulating the Brain Networks in Health and Disease. Front. Cell. Neurosci. 2017, 11, 37. [Google Scholar] [CrossRef]
- Valle-León, M.; Callado, L.F.; Aso, E.; Cajiao-Manrique, M.M.; Sahlholm, K.; López-Cano, M.; Soler, C.; Altafaj, X.; Watanabe, M.; Ferré, S.; et al. Decreased striatal adenosine A2A-dopamine D2 receptor heteromerization in schizophrenia. Neuropsychopharmacology 2021, 46, 665–672. [Google Scholar] [CrossRef]
- Valle-León, M.; Casajuana-Martin, N.; Del Torrent, C.L.; Argerich, J.; Gómez-Acero, L.; Sahlholm, K.; Ferré, S.; Pardo, L.; Ciruela, F. Unique effect of clozapine on adenosine A2A-dopamine D2 receptor heteromerization. Biomed. Pharmacother. 2023, 160, 114327. [Google Scholar] [CrossRef]
- Fernández-Dueñas, V.; Gómez-Soler, M.; Valle-León, M.; Watanabe, M.; Ferrer, I.; Ciruela, F. Revealing Adenosine A2A-Dopamine D2 Receptor Heteromers in Parkinson’s Disease Post-Mortem Brain through a New AlphaScreen-Based Assay. Int. J. Mol. Sci. 2019, 20, 3600. [Google Scholar] [CrossRef]
- Marcoli, M.; Agnati, L.F.; Franco, R.; Cortelli, P.; Anderlini, D.; Guidolin, D.; Cervetto, C.; Maura, G. Modulating brain integrative actions as a new perspective on pharmacological approaches to neuropsychiatric diseases. Front. Endocrinol. 2023, 13, 1038874. [Google Scholar] [CrossRef]
- Li, K.; Li, J.; Zheng, J.; Qin, S. Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis. 2019, 10, 664–675. [Google Scholar] [CrossRef]
- Cragnolini, A.B.; Lampitella, G.; Virtuoso, A.; Viscovo, I.; Panetsos, F.; Papa, M.; Cirillo, G. Regional brain susceptibility to neurodegeneration: What is the role of glial cells? Neural Regen. Res. 2020, 15, 838–842. [Google Scholar] [CrossRef]
- Diderot, D. Le Reve de d’Alembert; Paris, France, 1830. [Google Scholar]
- Guidolin, D.; Anderlini, D.; Maura, G.; Marcoli, M.; Cortelli, P.; Calandra-Buonaura, G.; Woods, A.S.; Agnati, L.F. A New Integrative Theory of Brain-Body-Ecosystem Medicine: From the Hippocratic Holistic View of Medicine to Our Modern Society. Int. J. Environ. Res. Public Health 2019, 16, 3136. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life. Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, J.; Li, Z.; Huang, Y.; Yuan, Y.; Wang, J.; Zhang, M.; Hu, S.; Liang, Y. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: A cross-sectional study. Schizophr. Res. 2018, 197, 470–477. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Lo Monaco, M.R.; Landi, F.; Bernabei, R.; Marzetti, E. Of Microbes and Minds: A Narrative Review on the Second Brain Aging. Front. Med. 2018, 5, 53. [Google Scholar] [CrossRef]
- Fülling, C.; Dinan, T.G.; Cryan, J.F. Gut Microbe to Brain Signaling: What Happens in Vagus…. Neuron 2019, 101, 998–1002. [Google Scholar] [CrossRef]
- Fil, J.; Dalchau, N.; Chu, D. Programming Molecular Systems to Emulate a Learning Spiking Neuron. ACS Synth. Biol. 2022, 11, 2055–2069. [Google Scholar] [CrossRef]
- Agnati, L.F.; Ferré, S.; Genedani, S.; Leo, G.; Guidolin, D.; Filaferro, M.; Carriba, P.; Casado, V.; Lluis, C.; Franco, R.; et al. Allosteric Modulation of Dopamine D2 Receptors by Homocysteine. J. Proteome Res. 2006, 5, 3077–3083. [Google Scholar] [CrossRef]
- Nussinov, R.; Tsai, C.-J.; Liu, J. Principles of Allosteric Interactions in Cell Signaling. J. Am. Chem. Soc. 2014, 136, 17692–17701. [Google Scholar] [CrossRef]
- Schiffer, F. The physical nature of subjective experience and its interaction with the Brain. Med. Hypotheses 2019, 125, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J. Waves of prediction. PLoS Biol. 2019, 17, e3000426. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R.P. The Meaning of It All Thoughts of a Citizen Scientist; Addison-Wesley: Boston, MA, USA, 1998. [Google Scholar]
- Dickinson, E. J632 (1862). In The Complete Poems of Emily Dickinson; Johnson, T.H., Ed.; Little Brown: Boston, MA, USA, 1890; p. 312. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnati, L.F.; Guidolin, D.; Cervetto, C.; Maura, G.; Marcoli, M. Brain Structure and Function: Insights from Chemical Neuroanatomy. Life 2023, 13, 940. https://doi.org/10.3390/life13040940
Agnati LF, Guidolin D, Cervetto C, Maura G, Marcoli M. Brain Structure and Function: Insights from Chemical Neuroanatomy. Life. 2023; 13(4):940. https://doi.org/10.3390/life13040940
Chicago/Turabian StyleAgnati, Luigi F., Diego Guidolin, Chiara Cervetto, Guido Maura, and Manuela Marcoli. 2023. "Brain Structure and Function: Insights from Chemical Neuroanatomy" Life 13, no. 4: 940. https://doi.org/10.3390/life13040940
APA StyleAgnati, L. F., Guidolin, D., Cervetto, C., Maura, G., & Marcoli, M. (2023). Brain Structure and Function: Insights from Chemical Neuroanatomy. Life, 13(4), 940. https://doi.org/10.3390/life13040940








