Phytochemicals as Invaluable Sources of Potent Antimicrobial Agents to Combat Antibiotic Resistance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Phytochemicals as a Source of Antimicrobial Compounds
2.1. Antibacterial Agents Derived from Plants
2.1.1. Apigenin
2.1.2. 18-β-Glycyrrhetinic Acid
2.1.3. Honokiol
2.1.4. Kaempferol
2.1.5. Naringin and Naringenin
2.1.6. Nimbolide
2.1.7. Resveratrol
2.1.8. Sanguinarine
2.1.9. Withaferin A
| Sl. No. | Phytocompound | Sources | Microorganisms Affected by the Title Compound and Dose | Mechanism of Action | Ref. |
|---|---|---|---|---|---|
| 1 | Allicin | Allium sativum, Allium spp. | Streptococcus pneumoniae (MIC: 64 µg/mL), Streptococcus pyogenes (MIC: 32 µg/mL) | ND | [58] |
| 2 | Conessine | Holarrhena floribunda, Holarrhena antidysenterica, Funtumia elastica | P. aeruginosa (MIC: 20 mg/L) | Inhibition of MexAB-OprM efflux pump | [59] |
| 3 | Thymol | Thymus vulgaris, Thymus capitatus | K. pneumoniae (MIC: 128 µg/mL) | Inhibition of biofilm formation | [60,61] |
| S. aureus (MIC: 72 µg/mL) | Reversal of efflux pump action | ||||
| 4 | Carvacrol | Origanum vulgare | S. aureus (MIC: 256 µg/mL) | Reversal of efflux pump action | [61] |
| 5 | Eugenol | Syzygium aromaticum, Eugenia caryophyllus | A. baumannii, Salmonella enteritidis, Campylobacter Jejuni, P. aeruginosa, E. coli | ND | [62] |
| 6 | Berberine | Berberis vulgaris, Berberis fremontii, Hydrastis Canadensis | H. pylori | Increased the sensitivity of amoxicillin and tetracycline, and reduced the expression of hefA mRNA upon treatment with amoxicillin, tetracycline, and berberine | [63] |
| 7 | Curcumin I | Curcuma longa | P. aeruginosa | Damage to the bacterial membrane | [64] |
| H. pylori | Inhibition of biofilm formation | ||||
| 8 | Quercetin | Capparis spinosa, Polymnia fruticose, Ginkgo biloba | Salmonella enterica serotype Typhimurium (MIC: 0.0072 µm/mL), S. aureus (MIC: 0.0068 µm/mL), P. aeruginosa (MIC: 0.0085 µm/mL) | Disruption of cell membrane integrity, thereby causing cell lysis | [65] |
| 9 | Epigallocatechin | Camellia sinensis | S. aureus (MIC: 62.5 µg/mL), P. aeruginosa (MIC: 125 µg/mL) | Increased the sensitivity of gentamycin against S. aureus and P. aeruginosa | [66] |
| 10 | Catechin | Fructus Crataegi | MRSA (MIC: 0.1 g/L) | Inhibition of biofilm formation via suppression of fibronectin-binding protein A and B (fnbA and fnbB) | [67] |
| 11 | Genistein | Glycine max | Aeromonas hydrophila | Disruption of QS, biofilm formation, and aerolysin production | [68] |
| 12 | Protocatechuic acid | Scrophularia frutescens | Yersinia enterocolitica (MIC: 2.5 mg/mL) | Cell membrane depolarization, reduction of intracellular pH and adenosine triphosphate (ATP), leakage of potassium ions | [69] |
| 13 | Gallic acid | Vitis rotundifolia | P. aeruginosa (MIC: 500 µg/mL), S. aureus (MIC: 1750 μg/mL), Listeria monocytogenes (MIC: 2000 μg/mL) | Membrane permeabilization, the release of intracellular potassium ions, disruption of the physicochemical surface properties of the cell | [70,71] |
| Shigella flexneri (MIC: 2 mg/mL) | Inhibition of biofilm formation via regulation of mdoH gene expression and the OpgH protein | ||||
| 14 | Hydroquinone | Vaccinium myrtillus | P. aeruginosa (MIC: 7.81 µg/mL), S. aureus (MIC: 15.625 µg/mL) | Depolarization of the cell membrane potential, increase in cell permeability, and leakage of intracellular potassium ions | [72] |
| 15 | Osthole | Cnidium monnieri, Angelica archangelica, Angelica pubescens | S. typhimurium (MIC: 1.67 ± 0.58 µg/mL), K. pneumoniae (MIC: 3.33 ± 1.15 µg/mL), A. baumannii (MIC: 1.68 ± 0.58 µg/mL) | ND | [73] |
| 16 | Taxifolin | Silybum marianum, Allium cepa, Pseudotsuga taxifolia, Pinus pinaster | E. faecalis (MIC: 128 µg/mL), VREF (MIC: 512 µg/mL) | Based on docking data, taxifolin showed a good binding affinity for β-ketoacyl acyl carrier protein synthase III, which is an important enzyme for bacterial fatty acid biosynthesis | [74] |
| 17 | Piperine | Piper nigrum | MRSA (MIC: 100 µg/mL) | Liposomal formulation of piperine and gentamicin acts as an efflux pump inhibitor | [75] |
| S. aureus (MIC: >16 µg/mL) | Piperine, in combination with ciprofloxacin, causes inhibition of efflux pump | ||||
| 18 | Sophoraflavanone B | Desmodium caudatum | MRSA (MIC: 15.6–31.25 µg/mL) | Disturbance of the cell membrane and leakage of cell contents | [76] |
| 19 | Farnesol | Vachellia farnesiana | S. aureus (MIC: 184 µg/mL), L. monocytogenes (MIC: 133 µg/mL) | ND | [77] |
| 20 | Rosthornin | Rabdosia rosthornii | Propionibacterium acnes (MIC: 3.17–25 µg/mL) | ND | [78] |
| 21 | Ellagic acid | Rosa rugosa | H. pylori (MIC: 5–30 mg/L) | ND | [79] |
| 22 | Chebulagic acid | Terminalia chebula | A. baumannii | ND | [80] |
| 23. | Hexahydroxy diphenoyl ester vescalagin | Lythrum salicaria | S. aureus (MIC: 62 µg/mL), P. mirabilis (MIC: 62 µg/mL) | ND | [81] |
| 24 | Stigmasterol | Neocarya macrophylla | MRSA (MIC: 6.25–25 µg/mL), Streptococcus faecalis (MIC: 6.25–25 µg/mL), S. aureus (MIC: 6.25–25 µg/mL) | Broad spectrum antibacterial activity | [82] |
| 25 | Chlorogenic acid | Fruits, vegetables, and graminaceous plants | Streptococcus pneumoniae (MIC: 20 µg/mL), Salmonella typhimurium (MIC: 20 µg/mL), Shigella dysenteriae (MIC: 10 µg/mL) | An increase in cell membrane permeability binds to bacterial DNA and thereby inhibits cellular functions | [83] |
| 26 | Thymoquinone | Nigella sativa | S. flexneri (MIC: 0.4 mg/mL) | Disruption of the cell membrane integrity | [84] |
| 27 | Guggulsterone | Commiphora wightii (Arn.) Bhandari | E. coli (MIC: 0.5 mg/mL), K. pneumoniae (MIC: 2 mg/mL), P. aeruginosa (MIC: >2 mg/mL), Salmonella typhi (MIC: >2 mg/mL), E. faecalis (MIC: 0.5 mg/mL), S. aureus (MIC: 2 mg/mL) | ND | [85] |
| 28 | Isoliquiritigenin | Glycyrrhiza uralensis | Staphylococcus xylosus (MIC: 80 µg/mL) | Downregulation of the IGPD gene | [86] |
| 29 | Celastrol | Tripterygium Wilfordii | S. aureus (MIC:1.25 µg/mL), E. faecalis (MIC: 1.25 µg/mL) | Disruption of DNA and protein synthesis | [87] |
| 30 | Cryptotanshinone | Salvia miltiorrhiza Bunge | S. aureus (MIC:12.5 µg/mL) | Dissipation of membrane Potential. Respiratory chain inhibition probably by targeting type II NADH:quinone dehydrogenase | [88] |
| 31 | Oridonin | Rabdosia rubescens, Isodon rubescens | MRSA (MIC: 64 µg/mL) | Permeability of cell membrane, disruption in protein and DNA metabolism | [89] |
| 32 | Magnolol | Magnolia officinalis | S. aureus (MIC: 16 ppm) | Based on simulation studies magnolol exhibited a high binding affinity for cell division Protein FtsZ | [90,91] |
| MRSA (MIC: 10 µg/mL) | Repression of mecA, mecI, and upregulation of mecR1 | ||||
| 33 | Hesperidin | citrus fruits, Poncirus trifoliata | S. aureus (MIC: 1 mg/mL), Bacillus cereus (MIC: 2 mg/mL), E. coli (MIC: >2 mg/mL) P. aeruginosa (MIC: 2 mg/mL) | ND | [92] |
| 35 | Evocarpine | Evodiae fructus | Mycobacterium smegmatis (MIC: 2–4 mg/mL), Mycobacterium tuberculosis (MIC: 5 mg/mL) | ND | [93] |
| 36 | Ursolic acid | Malus domestica | K. pneumoniae (MIC: 400 µg/mL), CRKP-1 (MIC: 800 µg/mL), CRKP-2 (MIC: 800 µg/mL), CRKP-8 (MIC: 800 µg/mL), CRKP-10 (MIC: 800 µg/mL) | Increase in membrane integrity, reduction in membrane potential, and intracellular ATP | [94] |
| 37 | Ferulic aid | All plants | E. coli (MIC: 100 µg/mL), P. aeruginosa (MIC: 100 µg/mL), S. aureus (MIC: 1100 µg/mL), L. monocytogenes (MIC: 1200 µg/mL) | Disruption of membrane integrity, cell surface hydrophobicity, and potassium ion leakage | [71] |
| 38 | Morusin | Morus alba | S. aureus (MIC:14.9 μmol/L) | Disruption of membrane integrity, Modulation of expression of phosphatidic acid biosynthesis-associated genes | [95] |
| 39 | Lonicerin | Lonicera japonica | P. aeruginosa | Inhibition of alginate secretion protein (AlgE) and inhibition of biofilm formation | [96] |
| 40 | Galangin | Allium sativum | VISA (MIC: 32 μg/mL) | Inhibition of murein hydrolase activity and growth of VISA strain-Mu50 | [97] |
| 41 | Artemisinin | Artemisia annua | S. aureus (MIC: 0.09 mg/mL) | ND | [98] |
| 42 | Punicalagin | Punica granatum | S. aureus (MIC: 0.25 mg/mL) | Disruption of the cell membrane, leakage of potassium ions, Inhibition of biofilm formation | [99] |
| 43 | Aloe-emodin | Cassia occidentalis, Aloe vera, Polygonum multiflorum Thunb. | S. aureus (MIC: 32 μg/mL), MRSA (MIC: 16 μg/mL), Staphylococcus epidermidis (MIC: 4 μg/mL), P. aeruginosa (MIC: 256 μg/mL) | Transcriptional profile studies have revealed alterations of genes involved in sulfur metabolism, L-lysine, peptidoglycan biosynthesis, and biofilm formation | [100] |
| 44 | Skullcapflavone II | Scutellaria baicalensis | M. smegmatis (MIC99: 128 mg/L), Mycobacterium aurum (MIC99: 7.8 mg/L), Mycobacterium bovis (MIC99: 31.25 mg/L) | Efflux pump inhibition in M. aurum and M. smegmatis | [101] |
| 45 | Wogonin | Agrimonia pilosa | P. aeruginosa | Reduction of the quorum sensing-related genes. decreased production of virulence factors, inhibition of biofilm formation | [102] |
| 46 | Sulforaphane | Brassica oleracea and other cruciferous plants | H. pylori (MBC: 2.8–5.6 µg/mL) | Inhibition of bacterial urease | [103] |
| 47 | Arjunolic acid | Syzygium guineense, Syzygium cordatum | Shigella sonnei (MIC: 30 µg/spot) | ND | [104,105] |
| 48 | Terminolic acid | Syzygium guineense | S. sonnei (MIC: 50 µg/spot) | ND | [106] |
| 49 | Asiatic acid | Centella asiatica | Clostridium difficile (MIC: 10–20 μg/mL) | Disruption of membrane permeability, inhibition of cell motility | [107] |
| 50 | Cinnamic acid | Cinnamomum cassia | M. tuberculosis (MIC: 270 µM) Neisseria gonorrhoeae (MIC: 6.75 mM) | ND | [108,109] |
| 51 | Caffeic acid | Abundantly present in fruits and vegetables, such as olives, cinnamon, nutmeg, blueberries, apple, star anise | S. aureus (MIC: 256 µg/mL) | ND | [110] |
| 52 | Andrographolide | Andrographis paniculata | Burkholderia pseudomallei (MIC: 0.5 µg/mL) | Andrographolide-stabilized silver nanoparticle binding and charge neutralization at the membrane surface, and the production of Ag+ and ROS | [111,112] |
| P. aeruginosa | Suppression of QS regulators LasR and RhlR, which control the expression of many genes in P. aeruginosa | ||||
| 53 | Diosgenin | Rhizoma polgonati, Smilax china, Trigonella foenumgraecum | Porphyromonas gingivalis, Prevotella intermedia | Inhibition of biofilm formation | [113] |
| 54 | Rhein | Rheum palmatum, Reynoutria japonica (Houtt.), Fallopia multiflora | Cutibacterium acnes (MIC: 6.25 µg/mL) | Inhibition of C. acnes NADH dehydrogenase-2 activity | [114,115] |
| MRSA (MIC: 62.5–250 μg/mL) | Rhein in combination with oxacillin causes a reduction of mecA/mecI/mecR1 and blaZ/blaI/blaR1 gene expressions | ||||
| 55 | Riccardin C derivatives | Reboulia hemisphaerica | MRSA (MIC: 1 µg/mL), E. faecalis (MIC: 4 µg/mL), P. aeruginosa (MIC: >128 µg /mL), Vibrio parahaemolyticus (MIC: >128 µg/mL) | Disruption of membrane permeability and cell morphology, Alterations in intracellular Na+ and K+ concentrations, Mutation in FabI (an enoyl-acyl carrier protein reductase) in the S. aureus mutants | [116] |
| 56 | Artesunate | Artemisia annua | M. tuberculosis (MIC: 75 µg/mL) | ND | [117] |
| 57 | Betulinic acid | Mikania cordata | P. aeruginosa (MIC: 256 µg/mL), S. aureus (MIC: 256 µg /mL) | Increased production of a superoxide anion radical and malondialdehyde, elevated NAD+/NADH ratio, reduced glutathione, and DNA fragmentation | [118] |
| 58 | Sakuranetin | Polymnia fruticosa | H. pylori (MIC: 87.3 µM /mL) | Inhibition of β-hydroxy acyl-acyl carrier protein dehydratase | [119] |
| 59 | Protoanemonin | Ranunculus bulbosus | S. aureus (MIC: 31.25 µg/mL), P. aeruginosa (MIC: 62.5 µg/mL), Serratia marcescens (MIC: 15.625 µg/mL), K. pneumoniae (MIC: 31.25 µg /mL), Providencia stuartii (MIC: 15.625 µg/mL), P. acnes (MIC: 31.25 µg/mL), Clostridium perfringens (MIC: 62.5 µg/mL) | Broad spectrum antibacterial activity | [120] |
| 60 | Capsaicin | Piper nigrum, Capsicum annuum | Streptococcus pyogenes (MIC: 64–128 μg/mL) | Cell membrane damage, reduction of cell invasion and hemolytic activity, inhibition of biofilm formation | [121,122] |
| 61 | Thymoquinone | Nigella sativa | P. aeruginosa (MIC: 1.56 µg/mL), S. aureus (MIC: 3.125 µg/mL) | Depolarization of the membrane, production of ROS, and inhibition of biofilm formation | [84,123,124] |
| V. paraheamolyticus (MIC: 32µg/mL), Vibrio alginolyticus (MIC: 256µg/mL), Salmonella enterica serovar Typhimurium (MIC: >512 µg/mL), Staphylococcus epidermidis (MIC: 8 µg /mL), S. aureus (MIC: 8 µg/mL) | Inhibition of biofilm formation | ||||
| S. flexneri (MIC: 0.4 mg/mL) | Disruption of cell membrane integrity, inhibition of biofilm formation | ||||
| 62 | Piceatannol | Grapes, white tea, passion fruit, Japanese knotweed | Streptococcus mutans, Streptococcus sanguinis, Streptococcus gordonii | Inhibition of Streptococcus glucosyl transferase-GtfC | [125] |
| 63 | Curcumin | Curcuma longa | MRSA (MIC: 125–250 μg/mL), E. faecalis, P. aeruginosa | Membrane damage, inhibition of FtsZ proteins, inhibition of mecA gene transcription, reduced expression of PBP2α proteins | [64,126] |
| 64 | Reserpine | Rauvolfia serpentina | S. aureus (MIC:1200 µg/mL) | Inhibition of biofilm formation and virulence-regulatory proteins | [127,128] |
| M. tuberculosis | ND | ||||
| 65 | Tomatidine | Solanum lycopersicum | S. aureus, L. monocytogenes, Bacillus species. | Inhibition of ATP synthase subunit C | [129] |
| 66 | Isoliquirtigenin | Dalbergia odorifera, Glycyrrhiza uralensis | M. bovis (MIC: 50 µg/mL), MRSA (MIC: 50–100 µg/mL) | Inhibition of FAS I and FAS II | [130] |
| 67 | 2,2′,4-Trihydroxy chalcone | Dalbergia odorifera | M. bovis (MIC: 55 µg/mL) | Inhibition of FAS I and FAS II | [131] |
| 68 | Fisetin | Rhus cotinus | M. bovis (MIC: 63 µg/mL) | Inhibition of FAS II | [131] |
| 69 | Butein | Rhus verniciflua | M. bovis (MIC: 43 µg/mL) | Inhibition of FAS II | [131] |
| 70 | Coumarin | All plants | S. typhimurium (MIC: 2.5 mg/mL), Enterobacter aerogenes (MIC: 0.625 mg/mL) | ND | [132] |
| 71 | Plumbagin | Plumbago rosea, Plumbago zeylanica | S. aureus (MIC: 5 μg/mL), MRSA (MIC: 4–8 μg/mL) | Inhibition of DNA gyrase | [133] |
| 72 | Hibiscetin | Hibiscus sabdariffa | K. pneumoniae (MIC: 1024 μg/mL), E. aerogenes (MIC: 1024 μg/mL) | ND | [134] |
| 73 | Terchebulin | Terminalia chebula | A. baumannii (MIC: 500 μg/mL) | ND | [135] |
| 74 | Norwogonin | Scutellaria baicalensis | A. baumannii (MIC: 128 µg/mL) | ND | [135] |
2.2. Antifungal Agents Derived from Plants
2.2.1. Carvacrol
| Sl. No. | Phytocompound | Sources | Microorganisms Affected by the Title Compound and Dose | Mechanism of Action | Ref. |
|---|---|---|---|---|---|
| 1 | Carvone | Carum carvi, Anethum graveolens, Mentha spicata | C. albicans | Inhibition of the transition from yeast form to filamentous form | [146] |
| 2 | Thymol | Present in the plants belong to genera such as Thymus, Ocimum, Origanum, Satureja, Thymbra, Monarda | Candida species (MIC: 100 µg/mL) | Inhibition of H+ ATPase | [147] |
| C. neoformans | Interferes in intracellular Ca2+ homeostasis, reduction in ergosterol content through HOG-dependent pathway, reduction in protein glycosylation | ||||
| 3 | Menthol | Mentha piperita, Mentha longifolia, etc. | Aspergillus niger (MIC: 150 µg/mL), Aspergillus fumigatus (MIC: 150 µg/mL), Aspergillus flavus (MIC: 100 µg/mL), Aspergillus ochraceus (MIC: 100 µg/mL), Alternaria alternate (MIC: 450 µg/mL), Botrytis cinerea (MIC: 400 µg/mL), Cladosporium spp. (MIC: 125 µg/mL), Penicillium citrinum (MIC: 100 µg/mL), Penicillium chrysogenum (MIC: 300 µg/mL), Fusarium oxysporum (MIC: 200 µg/mL), and Rhizopus oryzae (MIC: 250 µg/mL) | Decreased the fungal growth dose-dependently | [148] |
| 4 | Cinnamaldehyde | Cinnamomum Cassia, Cinnamomum burmannii | Geotrichum citri-aurantii | Disruption of cell wall permeability and integrity | [149] |
| C. neoformans var. grubii (MIC90: 0.683 mg/mL) | Damage to the cell wall, induction of cell gigantism | ||||
| 5 | Citronellal | Cymbopogon citrates | C. albicans (MIC: 1 mg/mL) | Disruption of membrane homeostasis, inhibition of yeast to hyphal transition and biofilm formation | [150] |
| 6 | Wogonin | Scutellaria baicalensis Georgi | T. rubrum (MIC50: 0.06 mM), A. fumigatus (MIC50: 0.23 mM) | Perturbance in cell wall synthesis, | [151] |
| Trichophyton mentagrophytes (MIC50: 0.03 mM) | Perturbance in cell wall synthesis and generation of reactive oxygen species | ||||
| 7 | Gallic acid | Punica granatum | T. rubrum (MIC: 43.75 μg/mL) | Inhibition of ergosterol biosynthesis, reduction in sterol 14α-demethylase P450 (CYP51) and squalene epoxidase activity | [152] |
| T.mentagrophytes, Trichophyton violaceum, Trichophyton verrucosum, Trichophyton schoenleinii (Mean MIC: 54.17–83.33 μg/mL), C. albicans (Mean MIC: 12.5 μg/mL) | ND | ||||
| 8 | α-pinene | Eucalyptus plants | C. parapsilosis (MFC: 128 μg/mL) | Inhibition of pseudo-hyphae and promoting a marked reduction in blastoconidia | [153] |
| 9 | β-Asarone | Acorus calamus | A. niger | Reduces ergosterol content in the plasma membrane | [154] |
| 10 | Quercetin | Morus alba, Camellia sinensis, Allium fistulosum, Calamus scipionum, Centella asiatica, Lactuca sativa | C. albicans | Programmed cell death through mitochondrial dysfunction | [155,156] |
| 11 | Osthole | Cnidii Fructus, Cnidium monnieri | Microsporum canis (MIC: 1.95 µg/mL) | Decrease in 1,3-β-D-glucan and chitin contents | [157] |
| 12 | Plagiochin E | Marchantia polymorpha | C. albicans | Induction of the metacaspase-dependent apoptotic pathway, inhibition of chitin biosynthesis | [158,159] |
| 13 | Riccardin D | Dumortiera hirsute | C. albicans | Down-regulation of hypha-specific genes, such as ALS1, ALS3, ECE1, EFG1, HWP1 and CDC35, leading to retardation of hypha formation | [160,161] |
| Azole-resistant C. albicans strains (MIC80: 16 µg/mL) | Interferes in sterol biosynthesis | ||||
| 14 | Silibinin | Silybum marianum | C. albicans | Inhibition of biofilm development, disruption of cell membrane | [162] |
| 15 | Chlorogenic acid | Present in a wide variety of fruits, vegetables, olive oil, wine, and coffee | C. albicans | Induction of apoptosis by mitochondrial depolarization, production of reactive oxygen species, DNA fragmentation, externalization of phosphatidyl serine. | [163,164] |
2.2.2. Emodin
2.2.3. Eucalyptol
2.2.4. Eugenol
2.2.5. Geraniol
2.2.6. Hibiscuslide C
2.2.7. Magnoflorine
2.2.8. Tea Saponin
2.3. Antiviral Agents Derived from Plants
2.3.1. Betulinic Acid
2.3.2. Guggulsterone
2.3.3. Salvianolic Acids
2.3.4. Silvestrol
| Sl. No. | Phytocompound | Sources | Microorganisms Affected by the Title Compound and Dose | Mechanism of Action | Ref. |
|---|---|---|---|---|---|
| 1 | Berberine | Berberis vulgaris, Berberis fremontii, Hydrastis Canadensis | Chikungunya virus (EC50: 37.6–50.9 µM) | Reduction in viral RNA and protein synthesis | [218] |
| 2 | Baicalein | Polygonatum sibiricum, Scutellaria baicalensis | Japanese encephalitis virus (IC50: 14.28 µg/mL, CC50: 115.2 ± 0.2 µg/mL) | ND | [219,220] |
| 3 | Rosmarinic acid | Salvia miltiorrhiza | EV-A71 (CC50: 327.68 ± 14.43 µM, EC50: 31.57 ± 4.14–114 ± 4.10 µM, SI: 2.87–10.36) | Interferes with virus-host receptor interaction | [221] |
| 4 | Raoulic acid | Raoulia australis | HRV 2 (CC50: 201.78 µg/mL, IC50: <0.1 µg/mL, TI: 2017.8), HRV 3 (CC50: 201.78 µg/mL, IC50: 0.197 ± 0.11 µg/mL, TI: 1090.7), CV B3 (CC50: 65.86 µg/mL IC50: 0.337 ± 0.02, TI; 199.58), CV B4 (CC50: 65.86 IC50: 0.40 ± 0.05, TI: 164.65), EV 71 (CC50: 65.86 µg/mL, IC50: <0.1, TI: >658.6) | Broad spectrum antiviral activity | [222,223] |
| 5 | Tetra-O-galloyl-β-d-glucose (TGG) | Galla chinensis | SARS-CoV (CC50: 1.08 mM, EC50: 4.5 μM, SI: 240) | Interferes with viral entry into host cells | [224] |
| 6 | Saikosaponin B2 | Bupleurum spp., Heteromorpha spp., Scrophularia scorodonia | HCoV-229E (IC50: 1.7 ± 0.1 mmol/L, CC50: 383.3 ± 0.2 μmol/L, SI: 221.9) | Interferes in virus absorption and penetration into host cells | [225] |
| 7 | Patentiflorin A | Justica gendarussa | HIV (IC50: 24–37 nM, CC50: 75 μM) | Inhibition of reverse transcriptase | [226] |
| 8 | Oligonol | Litchi chinensis | Influenza virus (H3N2) | Inhibition of the proliferation of the influenza virus by blocking ROS-dependent ERK phosphorylation | [227] |
| 9 | Punicalagin | Punica granatum | Influenza virus (H3N2) | Inhibition of agglutination of RBCs | [228] |
| 10 | 3-hydroxy caruilignan C | Swietenia macrophylla | HCV (EC50: 10.5 ± 1.2 μM) | Inhibition of viral RNA and protein synthesis | [229] |
| 11 | Lycorine | Lycoris radiate, Narcissus pseudonarcissus | Zika virus (CC50: 4.29–21 μM, EC50: 0.22–0.39 μM, SI: 19.5–54) | Inhibition of viral RNA synthesis and protein synthesis, inhibition of viral RDRP activity | [230] |
| 12 | Quercetin | Houttuynia cordata | HSV-1 (CC50: 485.69 μg/mL, EC50: 52.9 μg/mL, SI: 9.18) | Inhibition of viral entry and NF-κB activation | [231] |
| HSV-2 (CC50: 485.69 μg/mL, EC50: 70.01 μg/mL, SI: 6.94) | ND | ||||
| 13 | Shikonin | Radix Lithospermi | ADV-3 | Inhibition of hexon protein expression | [232] |
| 14 | Naringenin | Citrus paradisi, Citrus aurantium, Prunus cerasus, Solanum lycopersicum, Origanum vulgare | HCV | Reduction in HCV secretion in infected cells | [233] |
| 15 | Ursolic acid | Ocimum basilicum | CV B1 (CC50: 100.5 mg/L, EC50: 0.4 ± 0.1 mg/L, SI: 251.3), EV 71 (CC50: 100.5 mg/L, EC50: 0.5 ± 0.2 mg/L, SI: 201) | Interferes in the viral replication phase | [234] |
| HSV-1 (CC50: 100.5 mg/L, EC50: 6.6 ± 1.8 mg/L, SI: 15.2), ADV-8 (CC50: 100.5 mg/L, EC50: 4.2 ± 0.3 mg/L, SI: 23.8) | ND | ||||
| 16 | Myricetin | Abundant in fruit, vegetables, tea, berries | SARS-CoV-2 | Inhibition of SARS-CoV-2 Mpro activity | [235] |
| 17 | Emetine | Cephaelisipecacuanha | SARS-CoV-2 (CC50: 1603.8 nM, EC50: 0.147 nM, SI: 10,910.4) | Inhibition of SARS-CoV-2 mRNA/eIF4E interaction | [236] |
| 18 | Ladanein | Marrubium Peregrinum | HCV (EC50: 2.54 μmol/L, toxic dose 50 %: 98.04 μmol/L) | Interferes with virus entry into host cells | [237] |
| 19 | Samarangenin B | Limonium sinense | HSV-1 (IC50: 11.4 ± 0.9 μM) | Inhibition of HSV-1 α gene expression, inhibition of HSV-1 DNA synthesis, and structural protein expression | [238] |
| 20 | Pterocarnin A | Pterocarya stenoptera | HSV-2 (IC50: 5.4 ± 0.3 μM, CC50: 31.7 ± 1.6 μM, SI: 5.9) | Inhibition of virus attachment and penetration into host cells and inhibition of virus replication | [239] |
3. Synergistic Antimicrobial Effects of Plant Metabolites with Standard Antibiotics
4. Plant-Derived Drugs That Are in Clinical Practice for the Treatment of Human Ailments
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Youssef, F.S.; Ashour, M.L.; Singab, A.N.B.; Wink, M. A Comprehensive Review of Bioactive Peptides from Marine Fungi and Their Biological Significance. Mar. Drugs 2019, 17, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Razek, A.S.; El-Naggar, M.E.; Allam, A.; Morsy, O.M.; Othman, S.I. Microbial Natural Products in Drug Discovery. Processes 2020, 8, 470. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [Green Version]
- García, J.; García-Galán, M.J.; Day, J.W.; Boopathy, R.; White, J.R.; Wallace, S.; Hunter, R.G. A review of emerging organic contaminants (EOCs), antibiotic resistant bacteria (ARB), and antibiotic resistance genes (ARGs) in the environment: Increasing removal with wetlands and reducing environmental impacts. Bioresour. Technol. 2020, 307, 123228. [Google Scholar] [CrossRef]
- Rather, I.A.; Kim, B.-C.; Bajpai, V.K.; Park, Y.-H. Self-medication and antibiotic resistance: Crisis, current challenges, and prevention. Saudi J. Biol. Sci. 2017, 24, 808–812. [Google Scholar] [CrossRef]
- Subramaniam, G.; Girish, M. Antibiotic resistance—A cause for reemergence of infections. Indian J. Pediatr. 2020, 87, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Jadimurthy, R.; Mayegowda, S.B.; Nayak, S.C.; Mohan, C.D.; Rangappa, K.S. Escaping mechanisms of ESKAPE pathogens from antibiotics and their targeting by natural compounds. Biotechnol. Rep. 2022, 34, e00728. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.D.; Rangappa, S.; Preetham, H.D.; Chandra Nayaka, S.; Gupta, V.K.; Basappa, S.; Sethi, G.; Rangappa, K.S. Targeting STAT3 signaling pathway in cancer by agents derived from Mother Nature. Semin. Cancer Biol. 2022, 80, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.D.; Hari, S.; Preetham, H.D.; Rangappa, S.; Barash, U.; Ilan, N.; Nayak, S.C.; Gupta, V.K.; Basappa; Vlodavsky, I.; et al. Targeting Heparanase in Cancer: Inhibition by Synthetic, Chemically Modified, and Natural Compounds. iScience 2019, 15, 360–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, C.D.; Rangappa, S.; Nayak, S.C.; Sethi, G.; Rangappa, K.S. Paradoxical functions of long noncoding RNAs in modulating STAT3 signaling pathway in hepatocellular carcinoma. Biochim. Biophys. Acta BBA—Rev. Cancer 2021, 1876, 188574. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; Naliyadhara, N.; Kumar, A.; Alqahtani, M.S.; Abbas, M.; Mohan, C.D.; Warrier, S.; Hui, K.M.; Rangappa, K.S. Natural compounds targeting nuclear receptors for effective cancer therapy. Cancer Metastasis Rev. 2022, 1–58. [Google Scholar] [CrossRef]
- Mohan, C.D.; Yang, M.H.; Rangappa, S.; Chinnathambi, A.; Alharbi, S.A.; Alahmadi, T.A.; Deivasigamani, A.; Hui, K.M.; Sethi, G.; Rangappa, K.S.; et al. 3-Formylchromone Counteracts STAT3 Signaling Pathway by Elevating SHP-2 Expression in Hepatocellular Carcinoma. Biology 2022, 11, 29. [Google Scholar] [CrossRef]
- Mohan, C.D.; Kim, C.; Siveen, K.S.; Manu, K.A.; Rangappa, S.; Chinnathambi, A.; Alharbi, S.A.; Rangappa, K.S.; Kumar, A.P.; Ahn, K.S. Crocetin imparts antiproliferative activity via inhibiting STAT3 signaling in hepatocellular carcinoma. IUBMB Life 2021, 73, 1348–1362. [Google Scholar] [CrossRef]
- Mohan, C.D.; Liew, Y.Y.; Jung, Y.Y.; Rangappa, S.; Preetham, H.D.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Lin, Z.-X.; Rangappa, K.S.; et al. Brucein D modulates MAPK signaling cascade to exert multi-faceted anti-neoplastic actions against breast cancer cells. Biochimie 2021, 182, 140–151. [Google Scholar] [CrossRef]
- Kim, N.Y.; Mohan, C.D.; Chinnathambi, A.; Alharbi, S.A.; Sethi, G.; Rangappa, K.S.; Ahn, K.S. Euphorbiasteroid Abrogates EGFR and Wnt/beta-Catenin Signaling in Non-Small-Cell Lung Cancer Cells to Impart Anticancer Activity. Molecules 2022, 27, 3824. [Google Scholar] [CrossRef]
- Sin, Z.W.; Mohan, C.D.; Chinnathambi, A.; Govindasamy, C.; Rangappa, S.; Rangappa, K.S.; Jung, Y.Y.; Ahn, K.S. Leelamine Exerts Antineoplastic Effects in Association with Modulating Mitogen-Activated Protein Kinase Signaling Cascade. Nutr. Cancer 2022, 74, 3375–3387. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Garg, V.K.; Buttar, H.S.; Setzer, W.N.; Sethi, G. Apigenin: A natural bioactive flavone-type molecule with promising therapeutic function. J. Funct. Foods 2018, 48, 457–471. [Google Scholar] [CrossRef]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. D-Alanine:D-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.R.; Ettefagh, K.A.; Todd, D.; Cole, P.S.; Egan, J.M.; Foil, D.H.; Graf, T.N.; Schindler, B.D.; Kaatz, G.W.; Cech, N.B. A mass spectrometry-based assay for improved quantitative measurements of efflux pump inhibition. PLoS ONE 2015, 10, e0124814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuli, H.S.; Garg, V.K.; Mehta, J.K.; Kaur, G.; Mohapatra, R.K.; Dhama, K.; Sak, K.; Kumar, A.; Varol, M.; Aggarwal, D.; et al. Licorice (Glycyrrhiza glabra L.)-Derived Phytochemicals Target Multiple Signaling Pathways to Confer Oncopreventive and Oncotherapeutic Effects. OncoTargets Ther. 2022, 15, 1419–1448. [Google Scholar] [CrossRef] [PubMed]
- Long, D.R.; Mead, J.; Hendricks, J.M.; Hardy, M.E.; Voyich, J.M. 18β-Glycyrrhetinic acid inhibits methicillin-resistant Staphylococcus aureus survival and attenuates virulence gene expression. Antimicrob. Agents Chemother. 2013, 57, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Banik, K.; Ranaware, A.M.; Deshpande, V.; Nalawade, S.P.; Padmavathi, G.; Bordoloi, D.; Sailo, B.L.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; et al. Honokiol for cancer therapeutics: A traditional medicine that can modulate multiple oncogenic targets. Pharmacol. Res. 2019, 144, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yin, F.; Zhao, X.; Guo, Y.; Wang, W.; Wang, P.; Zhu, H.; Yin, Y.; Wang, X. Colistin Resistance Gene mcr-1 Mediates Cell Permeability and Resistance to Hydrophobic Antibiotics. Front. Microbiol. 2020, 10, 3015. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Lv, X.; Wang, Y.; Zhou, Y.; Lu, N.; Deng, X.; Wang, J. Honokiol restores polymyxin susceptibility to MCR-1-positive pathogens both in vitro and in vivo. Appl. Environ. Microbiol. 2020, 86, e02346-19. [Google Scholar] [CrossRef]
- Guo, Y.; Hou, E.; Wen, T.; Yan, X.; Han, M.; Bai, L.-P.; Fu, X.; Liu, J.; Qin, S. Development of membrane-active honokiol/magnolol amphiphiles as potent antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). J. Med. Chem. 2021, 64, 12903–12916. [Google Scholar] [CrossRef]
- Li, W.-L.; Zhao, X.-C.; Zhao, Z.-W.; Huang, Y.-J.; Zhu, X.-Z.; Meng, R.-Z.; Shi, C.; Yu, L.; Guo, N. In vitro antimicrobial activity of honokiol against Staphylococcus aureus in biofilm mode. J. Asian Nat. Prod. Res. 2016, 18, 1178–1185. [Google Scholar] [CrossRef]
- Guo, N.; Liu, Z.; Yan, Z.; Liu, Z.; Hao, K.; Liu, C.; Wang, J. Subinhibitory concentrations of Honokiol reduce α-Hemolysin (Hla) secretion by Staphylococcus aureus and the Hla-induced inflammatory response by inactivating the NLRP3 inflammasome. Emerg. Microbes Infect. 2019, 8, 707–716. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-H.; Huang, C.-C.; Chen, C.-C.; Yang, K., Jr.; Huang, C.-Y. Inhibition of Staphylococcus aureus PriA helicase by flavonol kaempferol. Protein J. 2015, 34, 169–172. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, M.; Guo, W.; Yao, Z.; Du, X.; Chen, L.; Sun, Y.; Shi, S.; Cao, J.; Zhou, T. The Antibacterial Activity of Kaempferol Combined with Colistin against Colistin-Resistant Gram-Negative Bacteria. Microbiol. Spectr. 2022, 10, e02265-22. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Q.; Hou, R.; Sun, H.; Tang, Q.; Wang, H.; Hao, Z.; Kang, S.; Xu, T.; Wu, S. Kaempferol-3-O-glucorhamnoside inhibits inflammatory responses via MAPK and NF-κB pathways in vitro and in vivo. Toxicol. Appl. Pharmacol. 2019, 364, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X. Kaempferol inhibits the primary attachment phase of biofilm formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef] [Green Version]
- Jeong, K.-W.; Lee, J.-Y.; Kim, Y.-M. Homology Modeling and Docking Study of β-Ketoacyl Acyl Carrier Protein Synthase Ⅲ from Enterococcus faecalis. Bull. Korean Chem. Soc. 2007, 28, 1335–1340. [Google Scholar]
- Tomar, A.; Broor, S.; Kaushik, S.; Bharara, T.; Arya, D. Synergistic effect of naringenin with conventional antibiotics against methicillin resistant Staphylococcus aureus. Eur. J. Mol. Clin. Med. 2021, 7, 2020. [Google Scholar]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: Design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015, 22, 552–561. [Google Scholar] [CrossRef]
- Dey, P.; Parai, D.; Banerjee, M.; Hossain, S.T.; Mukherjee, S.K. Naringin sensitizes the antibiofilm effect of ciprofloxacin and tetracycline against Pseudomonas aeruginosa biofilm. Int. J. Med. Microbiol. 2020, 310, 151410. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.M.; Perveen, K.; Qais, F.A.; Ahmad, I.; Alfarhan, A.H.; El-Sheikh, M.A. Naringin inhibits the biofilms of metallo-β-lactamases (MβLs) producing Pseudomonas species isolated from camel meat. Saudi J. Biol. Sci. 2021, 28, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, S. Bioactivity of naringin and related mechanisms. Die Pharm.-Int. J. Pharm. Sci. 2021, 76, 359–363. [Google Scholar]
- Zhang, J.; Jung, Y.Y.; Mohan, C.D.; Deivasigamani, A.; Chinnathambi, A.; Alharbi, S.A.; Rangappa, K.S.; Hui, K.M.; Sethi, G.; Ahn, K.S. Nimbolide enhances the antitumor effect of docetaxel via abrogation of the NF-κB signaling pathway in prostate cancer preclinical models. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2022, 1869, 119344. [Google Scholar] [CrossRef]
- Blum, F.C.; Singh, J.; Merrell, D.S. In vitro activity of neem (Azadirachta indica) oil extract against Helicobacter pylori. J. Ethnopharmacol. 2019, 232, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Wylie, M.R.; Windham, I.H.; Blum, F.C.; Wu, H.; Merrell, D.S. In vitro antibacterial activity of nimbolide against Helicobacter pylori. J. Ethnopharmacol. 2022, 285, 114828. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Acharyya, S.; Banerjee, A.; Patra, A.; Thankamani, K.; Koley, H.; Bag, P.K. Intracellular, biofilm-inhibitory and membrane-damaging activities of nimbolide isolated from Azadirachta indica A. Juss (Meliaceae) against meticillin-resistant Staphylococcus aureus. J. Med. Microbiol. 2016, 65, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Ko, J.-H.; Lee, H.; Jung, J.; Kong, M.; Lee, J.-W.; Lee, J.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; et al. Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: Role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomedicine 2016, 23, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.S.; Gadi, V.; Kaur, G.; Chintamaneni, M.; Tuli, H.S.; Ramniwas, S.; Sethi, G. Resveratrol and Its Role in the Management of B-Cell Malignancies—A Recent Update. Biomedicines 2023, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.S.; Tan, L.T.-H.; Chan, K.-G.; Yap, W.H.; Pusparajah, P.; Chuah, L.-H.; Ming, L.C.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Resveratrol—Potential antibacterial agent against foodborne pathogens. Front. Pharmacol. 2018, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yu, J.; Shen, X.; Cao, X.; Zhan, Q.; Guo, Y.; Yu, F. Resveratrol enhances the antimicrobial effect of polymyxin B on Klebsiella pneumoniae and Escherichia coli isolates with polymyxin B resistance. BMC Microbiol. 2020, 20, 306. [Google Scholar] [CrossRef]
- Subramanian, M.; Soundar, S.; Mangoli, S. DNA damage is a late event in resveratrol-mediated inhibition of Escherichia coli. Free Radic. Res. 2016, 50, 708–719. [Google Scholar] [CrossRef]
- Hwang, D.; Lim, Y.-H. Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FtsZ expression. Sci. Rep. 2015, 5, 10029. [Google Scholar] [CrossRef] [Green Version]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Raneri, M.; Pinatel, E.; Peano, C.; Rampioni, G.; Leoni, L.; Bianconi, I.; Jousson, O.; Dalmasio, C.; Ferrante, P.; Briani, F. Pseudomonas aeruginosa mutants defective in glucose uptake have pleiotropic phenotype and altered virulence in non-mammal infection models. Sci. Rep. 2018, 8, 16912. [Google Scholar] [CrossRef] [PubMed]
- Falchi, F.A.; Borlotti, G.; Ferretti, F.; Pellegrino, G.; Raneri, M.; Schiavoni, M.; Caselli, A.; Briani, F. Sanguinarine Inhibits the 2-Ketogluconate Pathway of Glucose Utilization in Pseudomonas aeruginosa. Front. Microbiol. 2021, 12, 2552. [Google Scholar] [CrossRef] [PubMed]
- Obiang-Obounou, B.W.; Kang, O.-H.; Choi, J.-G.; Keum, J.-H.; Kim, S.-B.; Mun, S.-H.; Shin, D.-W.; Kim, K.W.; Park, C.-B.; Kim, Y.-G. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J. Toxicol. Sci. 2011, 36, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Obiang-Obounou, B.W.; Kang, O.-H.; Choi, J.-G.; Keum, J.-H.; Kim, S.-B.; Mun, S.-H.; Shin, D.-W.; Park, C.-B.; Kim, Y.-G.; Han, S.-H. In vitro potentiation of ampicillin, oxacillin, norfloxacin, ciprofloxacin, and vancomycin by sanguinarine against methicillin-resistant Staphylococcus aureus. Foodborne Pathog. Dis. 2011, 8, 869–874. [Google Scholar] [CrossRef]
- Kumar, S.; Mathew, S.O.; Aharwal, R.P.; Tulli, H.S.; Mohan, C.D.; Sethi, G.; Ahn, K.S.; Webber, K.; Sandhu, S.S.; Bishayee, A. Withaferin A: A Pleiotropic Anticancer Agent from the Indian Medicinal Plant Withania somnifera (L.) Dunal. Pharmaceuticals 2023, 16, 160. [Google Scholar] [CrossRef]
- Murugan, R.; Rajesh, R.; Seenivasan, B.; Haridevamuthu, B.; Sudhakaran, G.; Guru, A.; Rajagopal, R.; Kuppusamy, P.; Juliet, A.; Gopinath, P. Withaferin A targets the membrane of Pseudomonas aeruginosa and mitigates the inflammation in zebrafish larvae; an in vitro and in vivo approach. Microb. Pathog. 2022, 172, 105778. [Google Scholar] [CrossRef]
- Reiter, J.; Levina, N.; Van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a volatile antimicrobial from garlic (Allium sativum), kills human lung pathogenic bacteria, including MDR strains, as a vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siriyong, T.; Srimanote, P.; Chusri, S.; Yingyongnarongkul, B.-E.; Suaisom, C.; Tipmanee, V.; Voravuthikunchai, S.P. Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2017, 17, 405. [Google Scholar] [CrossRef] [Green Version]
- Bisso Ndezo, B.; Tokam Kuaté, C.R.; Dzoyem, J.P. Synergistic antibiofilm efficacy of thymol and piperine in combination with three aminoglycoside antibiotics against Klebsiella pneumoniae biofilms. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 7029944. [Google Scholar] [CrossRef]
- Sousa Silveira, Z.D.; Macêdo, N.S.; Sampaio dos Santos, J.F.; Sampaio de Freitas, T.; Rodrigues dos Santos Barbosa, C.; Júnior, D.L.D.S.; Muniz, D.F.; Castro de Oliveira, L.C.; Júnior, J.P.S.; Cunha, F.A.B.D. Evaluation of the antibacterial activity and efflux pump reversal of thymol and carvacrol against Staphylococcus aureus and their toxicity in Drosophila melanogaster. Molecules 2020, 25, 2103. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.-K.; Kamal, M.; Ayuba, S.; Sakirolla, R.; Kang, Y.-B.; Mohandas, K.; Balijepalli, M.; Ahmad, S.; Pichika, M. A comprehensive review on eugenol’s antimicrobial properties and industry applications: A transformation from ethnomedicine to industry. Pharmacogn. Rev. 2019, 13, 1–9. [Google Scholar]
- Huang, Y.-Q.; Huang, G.-R.; Wu, M.-H.; Tang, H.-Y.; Huang, Z.-S.; Zhou, X.-H.; Yu, W.-Q.; Su, J.-W.; Mo, X.-Q.; Chen, B.-P. Inhibitory effects of emodin, baicalin, schizandrin and berberine on hefA gene: Treatment of Helicobacter pylori-induced multidrug resistance. World J. Gastroenterol. WJG 2015, 21, 4225. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Bazzaz, B.S.F.; Sarabandi, S.; Khameneh, B.; Hosseinzadeh, H. Effect of catechins, green tea extract and methylxanthines in combination with gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa-combination therapy against resistant bacteria. J. Pharmacopunct. 2016, 19, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Qu, Y.; Tang, J.; Chen, J.; Liu, J. Tea Catechin Inhibits Biofilm Formation of Methicillin-Resistant S. aureus. J. Food Qual. 2021, 2021, 8873091. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, D.; Li, J.; Liu, Y.; Zhou, S.; Yang, Y.; Xu, N.; Yang, Q.; Ai, X. Genistein Inhibits the Pathogenesis of Aeromonas hydrophila by Disrupting Quorum Sensing Mediated Biofilm Formation and Aerolysin Production. Front. Pharmacol. 2021, 12, 753581. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tian, L.; Fu, J.; Liao, S.; Li, H.; Gai, Z.; Gong, G. Antibacterial mechanism of Protocatechuic acid against Yersinia enterocolitica and its application in pork. Food Control 2022, 133, 108573. [Google Scholar] [CrossRef]
- Kang, J.; Liu, L.; Liu, M.; Wu, X.; Li, J. Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression. Food Control 2018, 94, 147–154. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Jeyanthi, V.; Velusamy, P.; Kumar, G.V.; Kiruba, K. Effect of naturally isolated hydroquinone in disturbing the cell membrane integrity of Pseudomonas aeruginosa MTCC 741 and Staphylococcus aureus MTCC 740. Heliyon 2021, 7, e07021. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Guo, Y.; Liu, X.; Liu, S.; Niu, X.; Wang, Y.; Deng, X. Discovery of a potential MCR-1 inhibitor that reverses polymyxin activity against clinical mcr-1-positive Enterobacteriaceae. J. Infect. 2019, 78, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.-W.; Lee, J.-Y.; Kang, D.-I.; Lee, J.-U.; Shin, S.Y.; Kim, Y. Screening of flavonoids as candidate antibiotics against Enterococcus faecalis. J. Nat. Prod. 2009, 72, 719–724. [Google Scholar] [CrossRef]
- Alves, F.S.; Cruz, J.N.; de Farias Ramos, I.N.; do Nascimento Brandão, D.L.; Queiroz, R.N.; da Silva, G.V.; da Silva, G.V.; Dolabela, M.F.; da Costa, M.L.; Khayat, A.S. Evaluation of Antimicrobial Activity and Cytotoxicity Effects of Extracts of Piper nigrum L. and Piperine. Separations 2023, 10, 21. [Google Scholar] [CrossRef]
- Mun, S.-H.; Joung, D.-K.; Kim, S.-B.; Park, S.-J.; Seo, Y.-S.; Gong, R.; Choi, J.-G.; Shin, D.-W.; Rho, J.-R.; Kang, O.-H. The mechanism of antimicrobial activity of sophoraflavanone B against methicillin-resistant Staphylococcus aureus. Foodborne Pathog. Dis. 2014, 11, 234–239. [Google Scholar] [CrossRef]
- Togashi, N.; Inoue, Y.; Hamashima, H.; Takano, A. Effects of two terpene alcohols on the antibacterial activity and the mode of action of farnesol against Staphylococcus aureus. Molecules 2008, 13, 3069–3076. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, V.; Roy, R.; Tiwari, M. Antimicrobial active herbal compounds against Acinetobacter baumannii and other pathogens. Front. Microbiol. 2015, 6, 618. [Google Scholar] [CrossRef] [Green Version]
- De, R.; Sarkar, A.; Ghosh, P.; Ganguly, M.; Karmakar, B.C.; Saha, D.R.; Halder, A.; Chowdhury, A.; Mukhopadhyay, A.K. Antimicrobial activity of ellagic acid against Helicobacter pylori isolates from India and during infections in mice. J. Antimicrob. Chemother. 2018, 73, 1595–1603. [Google Scholar] [CrossRef] [Green Version]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2021, 11, 46. [Google Scholar] [CrossRef]
- Becker, H.; Scher, J.M.; Speakman, J.-B.; Zapp, J. Bioactivity guided isolation of antimicrobial compounds from Lythrum salicaria. Fitoterapia 2005, 76, 580–584. [Google Scholar] [CrossRef]
- Mailafiya, M.M.; Yusuf, A.J.; Abdullahi, M.I.; Aleku, G.A.; Ibrahim, I.A.; Yahaya, M.; Abubakar, H.; Sanusi, A.; Adamu, H.W.; Alebiosu, C.O. Antimicrobial activity of stigmasterol from the stem bark of Neocarya macrophylla. J. Med. Plants Econ. Dev. 2018, 2, 1–5. [Google Scholar]
- Tan, S.; Yan, F.; Li, Q.; Liang, Y.; Yu, J.; Li, Z.; He, F.; Li, R.; Li, M. Chlorogenic acid promotes autophagy and alleviates Salmonella Typhimurium infection through the lncRNAGAS5/miR-23a/PTEN axis and the p38 MAPK pathway. Front. Cell Dev. Biol. 2020, 8, 552020. [Google Scholar] [CrossRef]
- Fan, Q.; Yuan, Y.; Jia, H.; Zeng, X.; Wang, Z.; Hu, Z.; Gao, Z.; Yue, T. Antimicrobial and anti-biofilm activity of thymoquinone against Shigella flexneri. Appl. Microbiol. Biotechnol. 2021, 105, 4709–4718. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Nadgauda, R.S. Commiphora wightii (Arnott) Bhandari—A natural source of guggulsterone: Facing a high risk of extinction in its natural habitat. Am. J. Plant Sci. 2013, 4, 33323. [Google Scholar] [CrossRef] [Green Version]
- Qu, Q.; Wang, J.; Cui, W.; Zhou, Y.; Xing, X.; Che, R.; Liu, X.; Chen, X.; Bello-Onaghise, G.S.; Dong, C. In vitro activity and In vivo efficacy of Isoliquiritigenin against Staphylococcus xylosus ATCC 700404 by IGPD target. PLoS ONE 2019, 14, e0226260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilla-Montaño, N.; de León Guerra, L.; Moujir, L. Antimicrobial Activity and Mode of Action of Celastrol, a Nortriterpen Quinone Isolated from Natural Sources. Foods 2021, 10, 591. [Google Scholar] [CrossRef]
- Chen, B.-C.; Ding, Z.-S.; Dai, J.-S.; Chen, N.-P.; Gong, X.-W.; Ma, L.-F.; Qian, C.-D. New insights into the antibacterial mechanism of Cryptotanshinone, a representative diterpenoid quinone from Salvia miltiorrhiza bunge. Front. Microbiol. 2021, 12, 647289. [Google Scholar] [CrossRef]
- Yuan, Z.; Ouyang, P.; Gu, K.; Rehman, T.; Zhang, T.; Yin, Z.; Fu, H.; Lin, J.; He, C.; Shu, G. The antibacterial mechanism of oridonin against methicillin-resistant Staphylococcus aureus (MRSA). Pharm. Biol. 2019, 57, 710–716. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Pan, Y.; Lai, R. New mechanism of magnolol and honokiol from Magnolia officinalis against Staphylococcus aureus. Nat. Prod. Commun. 2014, 9, 1934578X1400900922. [Google Scholar] [CrossRef] [Green Version]
- Chiu, K.-C.; Shih, Y.-H.; Wang, T.-H.; Lan, W.-C.; Li, P.-J.; Jhuang, H.-S.; Hsia, S.-M.; Shen, Y.-W.; Chen, M.Y.-C.; Shieh, T.-M. In vitro antimicrobial and antipro-inflammation potential of honokiol and magnolol against oral pathogens and macrophages. J. Formos. Med. Assoc. 2021, 120, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Lee, S.-H.; Lee, K.-A. A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef]
- Hochfellner, C.; Evangelopoulos, D.; Zloh, M.; Wube, A.; Guzman, J.; McHugh, T.; Kunert, O.; Bhakta, S.; Bucar, F. Antagonistic effects of indoloquinazoline alkaloids on antimycobacterial activity of evocarpine. J. Appl. Microbiol. 2015, 118, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Wang, W.; Zhang, J.; Wang, T.; Liu, M.; Yang, M.; Sun, Z.; Li, X.; Li, Y. Antimicrobial and antibiofilm activities of ursolic acid against carbapenem-resistant Klebsiella pneumoniae. J. Antibiot. 2020, 73, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.; Liao, S.; Wang, W.; Mu, L.; Li, E.; Shen, W.; Liu, F.; Zou, Y. Destruction of the cell membrane and inhibition of cell phosphatidic acid biosynthesis in Staphylococcus aureus: An explanation for the antibacterial mechanism of morusin. Food Funct. 2019, 10, 6438–6446. [Google Scholar] [CrossRef]
- Xu, Z.; Li, K.; Pan, T.; Liu, J.; Li, B.; Li, C.; Wang, S.; Diao, Y.; Liu, X. Lonicerin, an anti-algE flavonoid against Pseudomonas aeruginosa virulence screened from Shuanghuanglian formula by molecule docking based strategy. J. Ethnopharmacol. 2019, 239, 111909. [Google Scholar] [CrossRef]
- Ouyang, J.; Sun, F.; Feng, W.; Xie, Y.; Ren, L.; Chen, Y. Antimicrobial activity of galangin and its effects on murein hydrolases of vancomycin-intermediate Staphylococcus aureus (VISA) strain Mu50. Chemotherapy 2018, 63, 20–28. [Google Scholar] [CrossRef]
- Appalasamy, S.; Lo, K.Y.; Ch’ng, S.J.; Nornadia, K.; Othman, A.S.; Chan, L.-K. Antimicrobial activity of artemisinin and precursor derived from in vitro plantlets of Artemisia annua L. BioMed Res. Int. 2014, 2014, 215872. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Shi, C.; Wu, Q.; Zheng, Z.; Liu, P.; Li, G.; Peng, X.; Xia, X. Antimicrobial activity of punicalagin against Staphylococcus aureus and its effect on biofilm formation. Foodborne Pathog. Dis. 2017, 14, 282–287. [Google Scholar] [CrossRef]
- Li, T.; Lu, Y.; Zhang, H.; Wang, L.; Beier, R.C.; Jin, Y.; Wang, W.; Li, H.; Hou, X. Antibacterial Activity and Membrane-Targeting Mechanism of Aloe-Emodin against Staphylococcus epidermidis. Front. Microbiol. 2021, 12, 621866. [Google Scholar] [CrossRef]
- Solnier, J.; Martin, L.; Bhakta, S.; Bucar, F. Flavonoids as novel efflux pump inhibitors and antimicrobials against both environmental and pathogenic intracellular mycobacterial species. Molecules 2020, 25, 734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Feng, Y.; Han, X.; Cai, X.; Yang, L.; Liu, C.; Shen, L. Inhibition of virulence factors and biofilm formation by Wogonin attenuates pathogenicity of Pseudomonas aeruginosa PAO1 via targeting pqs quorum-sensing system. Int. J. Mol. Sci. 2021, 22, 12699. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Stephenson, K.K.; Wade, K.L.; Talalay, P. Urease from Helicobacter pylori is inactivated by sulforaphane and other isothiocyanates. Biochem. Biophys. Res. Commun. 2013, 435, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, J.; Sil, P.C. Arjunolic acid: A new multifunctional therapeutic promise of alternative medicine. Biochimie 2013, 95, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Djoukeng, J.; Abou-Mansour, E.; Tabacchi, R.; Tapondjou, A.; Bouda, H.; Lontsi, D. Antibacterial triterpenes from Syzygium guineense (Myrtaceae). J. Ethnopharmacol. 2005, 101, 283–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, G.F.; Amponsah, I.K.; Harley, B.K.; Jibira, Y.; Baah, M.K.; Adjei, S.; Armah, F.A.; Mensah, A.Y. Triterpenoids mediate the antimicrobial, antioxidant, and anti-inflammatory activities of the stem bark of Reissantia indica. J. Appl. Pharm. Sci. 2021, 11, 039–048. [Google Scholar]
- Harnvoravongchai, P.; Chankhamhaengdecha, S.; Ounjai, P.; Singhakaew, S.; Boonthaworn, K.; Janvilisri, T. Antimicrobial effect of asiatic acid against Clostridium difficile is associated with disruption of membrane permeability. Front. Microbiol. 2018, 9, 2125. [Google Scholar] [CrossRef]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.; Froufe, H.J.; Abreu, R.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial potential of caffeic acid against Staphylococcus aureus clinical strains. BioMed Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Bao, M.; Liu, B.; Zhao, H.; Zhang, Y.; Ji, X.; Zhao, N.; Zhang, C.; He, X.; Yi, J. Effect of andrographolide and its analogs on bacterial infection: A review. Pharmacology 2020, 105, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Thammawithan, S.; Talodthaisong, C.; Srichaiyapol, O.; Patramanon, R.; Hutchison, J.A.; Kulchat, S. Andrographolide stabilized-silver nanoparticles overcome ceftazidime-resistant Burkholderia pseudomallei: Study of antimicrobial activity and mode of action. Sci. Rep. 2022, 12, 10701. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Tong, Q.; Peng, Q.; Shen, T.; Zhu, X.; Xu, Y.; Qi, S. In vitro anti-bacterial activity of diosgenin on Porphyromonas gingivalis and Prevotella intermedia. Mol. Med. Rep. 2020, 22, 5392–5398. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Kim, K.-Y. Rhein inhibits the growth of Propionibacterium acnes by blocking NADH dehydrogenase-2 activity. J. Med. Microbiol. 2020, 69, 689–696. [Google Scholar] [CrossRef]
- Gong, R.; Lee, D.Y.; Lee, J.W.; Choi, D.J.; Kim, G.-S.; Lee, S.H.; Lee, Y.-S. Potentiating activity of rhein in targeting of resistance genes in methicillin-resistant Staphylococcus aureus. Asian Pac. J. Trop. Med. 2019, 12, 14. [Google Scholar]
- Morita, D.; Sawada, H.; Ogawa, W.; Miyachi, H.; Kuroda, T. Riccardin C derivatives cause cell leakage in Staphylococcus aureus. Biochim. Biophys. Acta BBA-Biomembr. 2015, 1848, 2057–2064. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Xia, L.; Wei, L.; Li, D.; Jiang, W. Artesunate inhibits Staphylococcus aureus biofilm formation by reducing alpha-toxin synthesis. Arch. Microbiol. 2021, 203, 707–717. [Google Scholar] [CrossRef]
- Oloyede, H.; Ajiboye, H.; Salawu, M.; Ajiboye, T. Influence of oxidative stress on the antibacterial activity of betulin, betulinic acid and ursolic acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three flavonoids targeting the β-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: Crystal structure characterization with enzymatic inhibition assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef] [Green Version]
- Didry, N.; Dubreuil, L.; Pinkas, M. Microbiological properties of protoanemonin isolated from Ranunculus bulbosus. Phytother. Res. 1993, 7, 21–24. [Google Scholar] [CrossRef]
- Marini, E.; Magi, G.; Mingoia, M.; Pugnaloni, A.; Facinelli, B. Antimicrobial and anti-virulence activity of capsaicin against erythromycin-resistant, cell-invasive group a streptococci. Front. Microbiol. 2015, 6, 1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, P.; Das, C.; Dias, O.; Shanbhag, T. Antimicrobial property of capsaicin. Int. Res. J. Biol. Sci. 2017, 6, 7–11. [Google Scholar]
- Goel, S.; Mishra, P. Thymoquinone inhibits biofilm formation and has selective antibacterial activity due to ROS generation. Appl. Microbiol. Biotechnol. 2018, 102, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, K.; Kouidhi, B.; Jrah, H.; Mahdouani, K.; Bakhrouf, A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement. Altern. Med. 2011, 11, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nijampatnam, B.; Zhang, H.; Cai, X.; Michalek, S.M.; Wu, H.; Velu, S.E. Inhibition of Streptococcus mutans biofilms by the natural stilbene piceatannol through the inhibition of glucosyltransferases. ACS Omega 2018, 3, 8378–8385. [Google Scholar] [CrossRef] [Green Version]
- Teow, S.-Y.; Liew, K.; Ali, S.A.; Khoo, A.S.-B.; Peh, S.-C. Antibacterial action of curcumin against Staphylococcus aureus: A brief review. J. Trop. Med. 2016, 2016, 2853045. [Google Scholar] [CrossRef] [Green Version]
- Parai, D.; Banerjee, M.; Dey, P.; Mukherjee, S.K. Reserpine attenuates biofilm formation and virulence of Staphylococcus aureus. Microb. Pathog. 2020, 138, 103790. [Google Scholar] [CrossRef]
- Begum, S.; Naqvi, S.Q.Z.; Ahmed, A.; Tauseef, S.; Siddiqui, B.S. Antimycobacterial and antioxidant activities of reserpine and its derivatives. Nat. Prod. Res. 2012, 26, 2084–2088. [Google Scholar] [CrossRef]
- Lamontagne Boulet, M.; Isabelle, C.; Guay, I.; Brouillette, E.; Langlois, J.-P.; Jacques, P.-É.; Rodrigue, S.; Brzezinski, R.; Beauregard, P.B.; Bouarab, K. Tomatidine is a lead antibiotic molecule that targets Staphylococcus aureus ATP synthase subunit C. Antimicrob. Agents Chemother. 2018, 62, e02197-17. [Google Scholar] [CrossRef] [Green Version]
- Gaur, R.; Gupta, V.K.; Singh, P.; Pal, A.; Darokar, M.P.; Bhakuni, R.S. Drug Resistance Reversal Potential of Isoliquiritigenin and Liquiritigenin Isolated from Glycyrrhiza glabra Against Methicillin-Resistant Staphylococcus aureus (MRSA). Phytother. Res. 2016, 30, 1708–1715. [Google Scholar] [CrossRef]
- Brown, A.K.; Papaemmanouil, A.; Bhowruth, V.; Bhatt, A.; Dover, L.G.; Besra, G.S. Flavonoid inhibitors as novel antimycobacterial agents targeting Rv0636, a putative dehydratase enzyme involved in Mycobacterium tuberculosis fatty acid synthase II. Microbiology 2007, 153, 3314–3322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitiema, L.W.; Savadogo, A.; Simpore, J.; Dianou, D.; Traore, A.S. In vitro antimicrobial activity of some phenolic compounds (coumarin and quercetin) against gastroenteritis bacterial strains. Int. J. Microbiol. Res. 2012, 3, 183–187. [Google Scholar]
- Periasamy, H.; Iswarya, S.; Pavithra, N.; Senthilnathan, S.; Gnanamani, A. In vitro antibacterial activity of plumbagin isolated from Plumbago zeylanica L. against methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2019, 69, 41–49. [Google Scholar] [PubMed]
- Djeussi, D.E.; Noumedem, J.A.; Seukep, J.A.; Fankam, A.G.; Voukeng, I.K.; Tankeo, S.B.; Nkuete, A.H.; Kuete, V. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement. Altern. Med. 2013, 13, 164. [Google Scholar] [CrossRef] [Green Version]
- Miyasaki, Y.; Rabenstein, J.D.; Rhea, J.; Crouch, M.-L.; Mocek, U.M.; Kittell, P.E.; Morgan, M.A.; Nichols, W.S.; Van Benschoten, M.; Hardy, W.D. Isolation and characterization of antimicrobial compounds in plant extracts against multidrug-resistant Acinetobacter baumannii. PLoS ONE 2013, 8, e61594. [Google Scholar] [CrossRef] [Green Version]
- Firacative, C. Invasive fungal disease in humans: Are we aware of the real impact? Memórias Inst. Oswaldo Cruz 2020, 115, e200430. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Kainz, K.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. Fungal infections in humans: The silent crisis. Microb. Cell 2020, 7, 143. [Google Scholar] [CrossRef]
- Alastruey-Izquierdo, A.; World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. First Meeting of the WHO Antifungal Expert Group on Identifying Priority Fungal Pathogens: Meeting Report; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Normile, T.G.; Bryan, A.M.; Del Poeta, M. Animal models of Cryptococcus neoformans in identifying immune parameters associated with primary infection and reactivation of latent infection. Front. Immunol. 2020, 11, 581750. [Google Scholar] [CrossRef]
- Okoye, C.A.; Nweze, E.; Ibe, C. Invasive candidiasis in Africa, what is the current picture? Pathog. Dis. 2022, 80, ftac012. [Google Scholar] [CrossRef]
- Zotti, M.; Colaianna, M.; Morgese, M.G.; Tucci, P.; Schiavone, S.; Avato, P.; Trabace, L. Carvacrol: From ancient flavoring to neuromodulatory agent. Molecules 2013, 18, 6161–6172. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Wang, C.; Yang, Y.; Chen, R.; Zhang, J.; Chen, H.; Zhuge, Y.; Li, J.; Cheng, J.; Xu, K. Carvacrol induces Candida albicans apoptosis associated with Ca2+/calcineurin pathway. Front. Cell. Infect. Microbiol. 2020, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Chaillot, J.; Tebbji, F.; Remmal, A.; Boone, C.; Brown, G.W.; Bellaoui, M.; Sellam, A. The monoterpene carvacrol generates endoplasmic reticulum stress in the pathogenic fungus Candida albicans. Antimicrob. Agents Chemother. 2015, 59, 4584–4592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Terzi, V. Carvone (Mentha spicata L.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 309–316. [Google Scholar]
- Jung, K.-W.; Chung, M.-S.; Bai, H.-W.; Chung, B.-Y.; Lee, S. Investigation of Antifungal Mechanisms of Thymol in the Human Fungal Pathogen, Cryptococcus Neoformans. Molecules 2021, 26, 3476. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.; Abbaszadeh, A. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J. Mycol. Med. 2014, 24, e51–e56. [Google Scholar] [CrossRef]
- OuYang, Q.; Duan, X.; Li, L.; Tao, N. Cinnamaldehyde exerts its antifungal activity by disrupting the cell wall integrity of Geotrichum citri-aurantii. Front. Microbiol. 2019, 10, 55. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Fatima, Z.; Hameed, S. Citronellal-induced disruption of membrane homeostasis in Candida albicans and attenuation of its virulence attributes. Rev. Soc. Bras. Med. Trop. 2016, 49, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Da, X.; Nishiyama, Y.; Tie, D.; Hein, K.Z.; Yamamoto, O.; Morita, E. Antifungal activity and mechanism of action of Ou-gon (Scutellaria root extract) components against pathogenic fungi. Sci. Rep. 2019, 9, 1683. [Google Scholar] [CrossRef]
- Li, Z.J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.G.; Aibai, S. Antifungal activity of gallic acid in vitro and in vivo. Phytother. Res. 2017, 31, 1039–1045. [Google Scholar] [CrossRef]
- Nóbrega, J.R.; Silva, D.D.F.; Andrade Júnior, F.P.D.; Sousa, P.M.S.; Figueiredo, P.T.R.D.; Cordeiro, L.V.; Lima, E.D.O. Antifungal action of α-pinene against Candida spp. isolated from patients with otomycosis and effects of its association with boric acid. Nat. Prod. Res. 2021, 35, 6190–6193. [Google Scholar] [CrossRef]
- Venkatesan, R.; Karuppiah, P.S.; Arumugam, G.; Balamuthu, K. β-Asarone exhibits antifungal activity by inhibiting ergosterol biosynthesis in Aspergillus niger ATCC 16888. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 173–184. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar]
- Kwun, M.S.; Lee, D.G. Quercetin-induced yeast apoptosis through mitochondrial dysfunction under the accumulation of magnesium in Candida albicans. Fungal Biol. 2020, 124, 83–90. [Google Scholar] [CrossRef]
- Liu, C.; Yang, R.; Jiang, P.; Sun, T.; Zhang, T.; Han, C. Antifungal activity of Osthole on Microsporum canis through interfering with biosynthesis of fungal cell wall. Indian J. Pharm. Sci. 2018, 80, 852–857. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.-Z.; Chang, W.-Q.; Cheng, A.-X.; Sun, L.-M.; Lou, H.-X. Plagiochin E, an antifungal active macrocyclic bis (bibenzyl), induced apoptosis in Candida albicans through a metacaspase-dependent apoptotic pathway. Biochim. Biophys. Acta BBA-Gen. Subj. 2010, 1800, 439–447. [Google Scholar] [CrossRef]
- Wu, X.-Z.; Cheng, A.-X.; Sun, L.-M.; Lou, H.-X. Effect of plagiochin E, an antifungal macrocyclic bis (bibenzyl), on cell wall chitin synthesis in Candida albicans. Acta Pharmacol. Sin. 2008, 29, 1478–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Ma, Y.; Zhang, L.; Guo, F.; Ren, L.; Yang, R.; Li, Y.; Lou, H. In vivo inhibitory effect on the biofilm formation of Candida albicans by liverwort derived riccardin D. PLoS ONE 2012, 7, e35543. [Google Scholar] [CrossRef]
- Hong-Zhuo, S.; Chang, W.-Q.; Zhang, M.; Hong-Xiang, L. Two natural molecules preferentially inhibit azole-resistant Candida albicans with MDR1 hyperactivation. Chin. J. Nat. Med. 2019, 17, 209–217. [Google Scholar]
- Yun, D.G.; Lee, D.G. Assessment of silibinin as a potential antifungal agent and investigation of its mechanism of action. IUBMB Life 2017, 69, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Rocha da Silva, C.; Sá, L.G.D.A.V.; Dos Santos, E.V.; Ferreira, T.L.; Coutinho, T.D.N.P.; Moreira, L.E.A.; de Sousa Campos, R.; de Andrade, C.R.; Barbosa da Silva, W.M.; de Sá Carneiro, I. Evaluation of the antifungal effect of chlorogenic acid against strains of Candida spp. resistant to fluconazole: Apoptosis induction and in silico analysis of the possible mechanisms of action. J. Med. Microbiol. 2022, 71, 001526. [Google Scholar] [CrossRef]
- Veljkovic, E.; Xia, W.; Phillips, B.; Wong, E.T.; Ho, J.; Oviedo, A.; Hoeng, J.; Peitsch, M. Nicotine and Other Tobacco Compounds in Neurodegenerative and Psychiatric Diseases: Overview of Epidemiological Data on Smoking and Preclinical and Clinical Data on Nicotine; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Subramaniam, A.; Shanmugam, M.K.; Ong, T.H.; Li, F.; Perumal, E.; Chen, L.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; et al. Emodin inhibits growth and induces apoptosis in an orthotopic hepatocellular carcinoma model by blocking activation of STAT3. Br. J. Pharmacol. 2013, 170, 807–821. [Google Scholar] [CrossRef] [Green Version]
- Manu, K.A.; Shanmugam, M.K.; Ong, T.H.; Subramaniam, A.; Siveen, K.S.; Perumal, E.; Samy, R.P.; Bist, P.; Lim, L.H.K.; Kumar, A.P.; et al. Emodin Suppresses Migration and Invasion through the Modulation of CXCR4 Expression in an Orthotopic Model of Human Hepatocellular Carcinoma. PLoS ONE 2013, 8, e57015. [Google Scholar] [CrossRef] [Green Version]
- Stompor-Gorący, M. The health benefits of Emodin, a natural anthraquinone derived from rhubarb—A summary update. Int. J. Mol. Sci. 2021, 22, 9522. [Google Scholar] [CrossRef]
- Janeczko, M. Emodin Reduces the Activity of (1, 3)-D-Glucan Synthase from and Does Not Interact with Caspofungin. Pol. J. Microbiol. 2018, 67, 463–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janeczko, M.; Masłyk, M.; Kubiński, K.; Golczyk, H. Emodin, a natural inhibitor of protein kinase CK2, suppresses growth, hyphal development, and biofilm formation of Candida albicans. Yeast 2017, 34, 253–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.; Liu, C.; Li, J.; Hao, M.; Ji, Y.; Zeng, X. The effects of aloe emodin-mediated antimicrobial photodynamic therapy on drug-sensitive and resistant Candida albicans. Photochem. Photobiol. Sci. 2020, 19, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Mączka, W.; Duda-Madej, A.; Górny, A.; Grabarczyk, M.; Wińska, K. Can eucalyptol replace antibiotics? Molecules 2021, 26, 4933. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Pruthi, V.; Poluri, K.M. Mechanistic insights into Candida biofilm eradication potential of eucalyptol. J. Appl. Microbiol. 2021, 131, 105–123. [Google Scholar] [CrossRef]
- Mishra, P.; Gupta, P.; Srivastava, A.K.; Poluri, K.M.; Prasad, R. Eucalyptol/β-cyclodextrin inclusion complex loaded gellan/PVA nanofibers as antifungal drug delivery system. Int. J. Pharm. 2021, 609, 121163. [Google Scholar] [CrossRef]
- Bendre, R.S.; Rajput, J.D.; Bagul, S.D.; Karandikar, P. Outlooks on medicinal properties of eugenol and its synthetic derivatives. Nat. Prod. Chem. Res. 2016, 4, 100021. [Google Scholar] [CrossRef]
- de Oliveira Pereira, F.; Mendes, J.M.; de Oliveira Lima, E. Investigation on mechanism of antifungal activity of eugenol against Trichophyton rubrum. Med. Mycol. 2013, 51, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didehdar, M.; Chegini, Z.; Shariati, A. Eugenol: A novel therapeutic agent for the inhibition of Candida species infection. Front. Pharmacol. 2022, 13, 872127. [Google Scholar] [CrossRef]
- Gupta, P.; Gupta, S.; Sharma, M.; Kumar, N.; Pruthi, V.; Poluri, K.M. Effectiveness of phytoactive molecules on transcriptional expression, biofilm matrix, and cell wall components of Candida glabrata and its clinical isolates. ACS Omega 2018, 3, 12201–12214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, J.C.; Ferreira, G.F.; Santos, J.R.; Silva, L.C.; Rodrigues, J.F.; Neto, W.R.; Farah, E.I.; Santos, Á.R.; Mendes, B.S.; Sousa, L.V. Eugenol induces phenotypic alterations and increases the oxidative burst in Cryptococcus. Front. Microbiol. 2017, 8, 2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saracino, I.M.; Foschi, C.; Pavoni, M.; Spigarelli, R.; Valerii, M.C.; Spisni, E. Antifungal Activity of Natural Compounds vs. Candida spp.: A Mixture of Cinnamaldehyde and Eugenol Shows Promising In Vitro Results. Antibiotics 2022, 11, 73. [Google Scholar] [CrossRef]
- Jafri, H.; Khan, M.S.A.; Ahmad, I. In vitro efficacy of eugenol in inhibiting single and mixed-biofilms of drug-resistant strains of Candida albicans and Streptococcus mutans. Phytomedicine 2019, 54, 206–213. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, A.M.; Mosbah, R.A.; Goda, R.M.; Mansour, B.; Sultana, T.; Dahms, T.E.; El-Ganiny, A.M. Back to nature: Combating Candida albicans biofilm, phospholipase and hemolysin using plant essential oils. Antibiotics 2021, 10, 81. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, A.M. Geraniol—A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Miron, D.; Battisti, F.; Silva, F.K.; Lana, A.D.; Pippi, B.; Casanova, B.; Gnoatto, S.; Fuentefria, A.; Mayorga, P.; Schapoval, E.E. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Rev. Bras. Farmacogn. 2014, 24, 660–667. [Google Scholar] [CrossRef]
- Sharma, Y.; Khan, L.; Manzoor, N. Anti-Candida activity of geraniol involves disruption of cell membrane integrity and function. J. Mycol. Med. 2016, 26, 244–254. [Google Scholar] [CrossRef]
- Pereira, F.D.O.; Mendes, J.M.; Lima, I.O.; Mota, K.S.D.L.; Oliveira, W.A.D.; Lima, E.D.O. Antifungal activity of geraniol and citronellol, two monoterpenes alcohols, against Trichophyton rubrum involves inhibition of ergosterol biosynthesis. Pharm. Biol. 2015, 53, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Leite, M.C.A.; de Brito Bezerra, A.P.; de Sousa, J.P.; de Oliveira Lima, E. Investigating the antifungal activity and mechanism (s) of geraniol against Candida albicans strains. Med. Mycol. 2015, 53, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Dalleau, S.; Cateau, E.; Bergès, T.; Berjeaud, J.-M.; Imbert, C. In vitro activity of terpenes against Candida biofilms. Int. J. Antimicrob. Agents 2008, 31, 572–576. [Google Scholar] [CrossRef]
- Hwang, J.H.; Jin, Q.; Woo, E.R.; Lee, D.G. Antifungal property of hibicuslide C and its membrane-active mechanism in Candida albicans. Biochimie 2013, 95, 1917–1922. [Google Scholar] [CrossRef]
- Hwang, J.H.; Choi, H.; Kim, A.R.; Yun, J.W.; Yu, R.; Woo, E.-R.; Lee, D.G. Hibicuslide C-induced cell death in Candida albicans involves apoptosis mechanism. J. Appl. Microbiol. 2014, 117, 1400–1411. [Google Scholar] [CrossRef]
- Xu, T.; Kuang, T.; Du, H.; Li, Q.; Feng, T.; Zhang, Y.; Fan, G. Magnoflorine: A review of its pharmacology, pharmacokinetics and toxicity. Pharmacol. Res. 2020, 152, 104632. [Google Scholar] [CrossRef]
- Kim, J.; Ha Quang Bao, T.; Shin, Y.-K.; Kim, K.-Y. Antifungal activity of magnoflorine against Candida strains. World J. Microbiol. Biotechnol. 2018, 34, 167. [Google Scholar] [CrossRef]
- Luo, N.; Jin, L.; Yang, C.; Zhu, Y.; Ye, X.; Li, X.; Zhang, B. Antifungal activity and potential mechanism of magnoflorine against Trichophyton rubrum. J. Antibiot. 2021, 74, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Deng, Z.; Wang, T.; Yang, W.; Wang, K. Synthesis of Natural Tea-Saponin-Based Succinic Acid Sulfonate as Anionic Foaming Agent. J. Surfactants Deterg. 2018, 21, 303–312. [Google Scholar] [CrossRef]
- Yan, J.; Wu, Z.; Zhao, Y.; Jiang, C. Separation of tea saponin by two-stage foam fractionation. Sep. Purif. Technol. 2011, 80, 300–305. [Google Scholar] [CrossRef]
- Li, Y.; Shan, M.; Li, S.; Wang, Y.; Yang, H.; Chen, Y.; Gu, B.; Zhu, Z. Teasaponin suppresses Candida albicans filamentation by reducing the level of intracellular cAMP. Ann. Transl. Med. 2020, 8, 175. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, X.; He, J. Study on the antifungal activity and mechanism of tea saponin from Camellia oleifera cake. Eur. Food Res. Technol. 2022, 248, 783–795. [Google Scholar] [CrossRef]
- Weiss, R.A.; Esparza, J. The prevention and eradication of smallpox: A commentary on Sloane (1755)‘An account of inoculation’. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140378. [Google Scholar] [CrossRef] [Green Version]
- Zerbini, F.M.; Kitajima, E.W. From Contagium vivum fluidum to Riboviria: A Tobacco Mosaic Virus-Centric History of Virus Taxonomy. Biomolecules 2022, 12, 1363. [Google Scholar] [CrossRef]
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. History and impact of virology. Fenner White’s Med. Virol. 2017, 3–14. [Google Scholar]
- Siegel, R.D. Classification of human viruses. Princ. Pract. Pediatr. Infect. Dis. 2018, 1044–1048.e1. [Google Scholar]
- Available online: https://covid19.who.int/?mapFilter=deaths (accessed on 28 March 2023).
- Meyers, L.; Frawley, T.; Goss, S.; Kang, C. Ebola virus outbreak 2014: Clinical review for emergency physicians. Ann. Emerg. Med. 2015, 65, 101–108. [Google Scholar] [CrossRef]
- Sudhan, S.S.; Sharma, P. Human Viruses: Emergence and Evolution. In Emerging and Reemerging Viral Pathogens; Elsevier: Amsterdam, The Netherlands, 2020; pp. 53–68. [Google Scholar]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A review on preparation of betulinic acid and its biological activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Máñez, S. New pharmacological opportunities for betulinic acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Hong, E.-H.; Song, J.H.; Kang, K.B.; Sung, S.H.; Ko, H.-J.; Yang, H. Anti-influenza activity of betulinic acid from Zizyphus jujuba on influenza A/PR/8 virus. Biomol. Ther. 2015, 23, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loe, M.W.C.; Hao, E.; Chen, M.; Li, C.; Lee, R.C.H.; Zhu, I.X.Y.; Teo, Z.Y.; Chin, W.-X.; Hou, X.; Deng, J. Betulinic acid exhibits antiviral effects against dengue virus infection. Antivir. Res. 2020, 184, 104954. [Google Scholar] [CrossRef]
- Bouslama, L.; Kouidhi, B.; Alqurashi, Y.M.; Chaieb, K.; Papetti, A. Virucidal Effect of guggulsterone isolated from Commiphora gileadensis. Planta Med. 2019, 85, 1225–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baliga, M.; Nandhini, J.; Emma, F.; Venkataranganna, M.; Venkatesh, P.; Fayad, R. Indian medicinal plants and spices in the prevention and treatment of ulcerative colitis. In Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease: Bioactive Foods in Chronic Disease States; Academic Press: Cambridge, MA, USA, 2012; p. 173. [Google Scholar]
- Chen, W.-C.; Wei, C.-K.; Hossen, M.; Hsu, Y.-C.; Lee, J.-C. (E)-Guggulsterone Inhibits Dengue Virus Replication by Upregulating Antiviral Interferon Responses through the Induction of Heme Oxygenase-1 Expression. Viruses 2021, 13, 712. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Tang, L.; Yi, Q. Salvianolic acids: Potential source of natural drugs for the treatment of fibrosis disease and cancer. Front. Pharmacol. 2019, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Wang, J.; Zhang, Y.; Bai, H.; Wang, C.; Wang, N.; He, L. Three salvianolic acids inhibit 2019-nCoV spike pseudovirus viropexis by binding to both its RBD and receptor ACE2. J. Med. Virol. 2021, 93, 3143–3151. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.-F.; Xu, W.; Liu, S.-W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Yang, C.; Pan, X.; Xu, X.; Cheng, C.; Huang, Y.; Li, L.; Jiang, S.; Xu, W.; Xiao, G.; Liu, S. Salvianolic acid C potently inhibits SARS-CoV-2 infection by blocking the formation of six-helix bundle core of spike protein. Signal Transduct. Target. Ther. 2020, 5, 220. [Google Scholar] [CrossRef] [PubMed]
- Biedenkopf, N.; Lange-Grünweller, K.; Schulte, F.W.; Weißer, A.; Müller, C.; Becker, D.; Becker, S.; Hartmann, R.K.; Grünweller, A. The natural compound silvestrol is a potent inhibitor of Ebola virus replication. Antivir. Res. 2017, 137, 76–81. [Google Scholar] [CrossRef]
- Müller, C.; Schulte, F.W.; Lange-Grünweller, K.; Obermann, W.; Madhugiri, R.; Pleschka, S.; Ziebuhr, J.; Hartmann, R.K.; Grünweller, A. Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona-and picornaviruses. Antivir. Res. 2018, 150, 123–129. [Google Scholar] [CrossRef]
- Varghese, F.S.; Thaa, B.; Amrun, S.N.; Simarmata, D.; Rausalu, K.; Nyman, T.A.; Merits, A.; McInerney, G.M.; Ng, L.F.; Ahola, T. The antiviral alkaloid berberine reduces chikungunya virus-induced mitogen-activated protein kinase signaling. J. Virol. 2016, 90, 9743–9757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johari, J.; Kianmehr, A.; Mustafa, M.R.; Abubakar, S.; Zandi, K. Antiviral activity of baicalein and quercetin against the Japanese encephalitis virus. Int. J. Mol. Sci. 2012, 13, 16785–16795. [Google Scholar] [CrossRef]
- Singla, R.K.; He, X.; Chopra, H.; Tsagkaris, C.; Shen, L.; Kamal, M.A.; Shen, B. Natural Products for the Prevention and Control of the COVID-19 Pandemic: Sustainable Bioresources. Front. Pharmacol. 2021, 12, 758159. [Google Scholar] [CrossRef]
- Hsieh, C.-F.; Jheng, J.-R.; Lin, G.-H.; Chen, Y.-L.; Ho, J.-Y.; Liu, C.-J.; Hsu, K.-Y.; Chen, Y.-S.; Chan, Y.F.; Yu, H.-M. Rosmarinic acid exhibits broad anti-enterovirus A71 activity by inhibiting the interaction between the five-fold axis of capsid VP1 and cognate sulfated receptors. Emerg. Microbes Infect. 2020, 9, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Song, J.-H.; Lim, C.-H.; Baek, S.-H.; Kwon, D.-H. Anti-human rhinovirus activity of raoulic acid from Raoulia australis. J. Med. Food 2010, 13, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-J.; Lim, C.; Song, J.; Baek, S.; Kwon, D.H. Antiviral activity of raoulic acid from Raoulia australis against Picornaviruses. Phytomedicine 2009, 16, 35–39. [Google Scholar] [CrossRef]
- Yi, L.; Li, Z.; Yuan, K.; Qu, X.; Chen, J.; Wang, G.; Zhang, H.; Luo, H.; Zhu, L.; Jiang, P. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004, 78, 11334–11339. [Google Scholar] [CrossRef] [Green Version]
- Cheng, P.W.; Ng, L.T.; Chiang, L.C.; Lin, C.C. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin. Exp. Pharmacol. Physiol. 2006, 33, 612–616. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Rumschlag-Booms, E.; Guan, Y.-F.; Wang, D.-Y.; Liu, K.-L.; Li, W.-F.; Nguyen, V.H.; Cuong, N.M.; Soejarto, D.D.; Fong, H.H. Potent inhibitor of drug-resistant HIV-1 strains identified from the medicinal plant Justicia gendarussa. J. Nat. Prod. 2017, 80, 1798–1807. [Google Scholar] [CrossRef] [Green Version]
- Gangehei, L.; Ali, M.; Zhang, W.; Chen, Z.; Wakame, K.; Haidari, M. Oligonol a low molecular weight polyphenol of lychee fruit extract inhibits proliferation of influenza virus by blocking reactive oxygen species-dependent ERK phosphorylation. Phytomedicine 2010, 17, 1047–1056. [Google Scholar] [CrossRef]
- Haidari, M.; Ali, M.; Casscells, S.W., III; Madjid, M. Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine 2009, 16, 1127–1136. [Google Scholar] [CrossRef]
- Wu, S.F.; Lin, C.K.; Chuang, Y.S.; Chang, F.R.; Tseng, C.K.; Wu, Y.C.; Lee, J.C. Anti-hepatitis C virus activity of 3-hydroxy caruilignan C from Swietenia macrophylla stems. J. Viral Hepat. 2012, 19, 364–370. [Google Scholar] [CrossRef]
- Chen, H.; Lao, Z.; Xu, J.; Li, Z.; Long, H.; Li, D.; Lin, L.; Liu, X.; Yu, L.; Liu, W. Antiviral activity of lycorine against Zika virus in vivo and in vitro. Virology 2020, 546, 88–97. [Google Scholar] [CrossRef]
- Hung, P.-Y.; Ho, B.-C.; Lee, S.-Y.; Chang, S.-Y.; Kao, C.-L.; Lee, S.-S.; Lee, C.-N. Houttuynia cordata targets the beginning stage of herpes simplex virus infection. PLoS ONE 2015, 10, e0115475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andújar, I.; Ríos, J.L.; Giner, R.M.; Recio, M.C. Pharmacological properties of shikonin–a review of literature since 2002. Planta Med. 2013, 79, 1685–1697. [Google Scholar] [CrossRef] [Green Version]
- Nahmias, Y.; Goldwasser, J.; Casali, M.; Van Poll, D.; Wakita, T.; Chung, R.T.; Yarmush, M.L. Apolipoprotein B–dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology 2008, 47, 1437–1445. [Google Scholar] [CrossRef] [Green Version]
- Chiang, L.C.; Ng, L.T.; Cheng, P.W.; Chiang, W.; Lin, C.C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef]
- Xiao, T.; Cui, M.; Zheng, C.; Wang, M.; Sun, R.; Gao, D.; Bao, J.; Ren, S.; Yang, B.; Lin, J. Myricetin inhibits SARS-CoV-2 viral replication by targeting Mpro and ameliorates pulmonary inflammation. Front. Pharmacol. 2021, 12, 1012. [Google Scholar] [CrossRef] [PubMed]
- Bleasel, M.D.; Peterson, G.M. Emetine, ipecac, ipecac alkaloids and analogues as potential antiviral agents for coronaviruses. Pharmaceuticals 2020, 13, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haid, S.; Novodomská, A.; Gentzsch, J.; Grethe, C.; Geuenich, S.; Bankwitz, D.; Chhatwal, P.; Jannack, B.; Hennebelle, T.; Bailleul, F. A plant-derived flavonoid inhibits entry of all HCV genotypes into human hepatocytes. Gastroenterology 2012, 143, 213–222. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Lin, L.-C.; Tsai, W.-J.; Chou, C.-J.; Kung, S.-H.; Ho, Y.-H. Samarangenin B from Limonium sinense suppresses herpes simplex virus type 1 replication in Vero cells by regulation of viral macromolecular synthesis. Antimicrob. Agents Chemother. 2002, 46, 2854–2864. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.-Y.; Lin, T.-C.; Yang, C.-M.; Wang, K.-C.; Lin, C.-C. Mechanism of action of the suppression of herpes simplex virus type 2 replication by pterocarnin A. Microbes Infect. 2004, 6, 738–744. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Dilbato, T.; Begna, F.; Joshi, R.K. Reviews on challenges, opportunities and future prospects of antimicrobial activities of medicinal plants: Alternative solutions to combat antimicrobial resistance. Int. J. Herb. Med. 2019, 7, 10–18. [Google Scholar]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Khameneh, B.; Iranshahy, M.; Ghandadi, M.; Ghoochi Atashbeyk, D.; Fazly Bazzaz, B.S.; Iranshahi, M. Investigation of the antibacterial activity and efflux pump inhibitory effect of co-loaded piperine and gentamicin nanoliposomes in methicillin-resistant Staphylococcus aureus. Drug Dev. Ind. Pharm. 2015, 41, 989–994. [Google Scholar] [CrossRef]
- Dwivedi, G.R.; Maurya, A.; Yadav, D.K.; Singh, V.; Khan, F.; Gupta, M.K.; Singh, M.; Darokar, M.P.; Srivastava, S.K. Synergy of clavine alkaloid ‘chanoclavine’with tetracycline against multi-drug-resistant E. coli. J. Biomol. Struct. Dyn. 2019, 37, 1307–1325. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Lafrance, M.; Boulanger, S.; Séguin, D.L.; Guay, I.; Gattuso, M.; Marsault, E.; Bouarab, K.; Malouin, F. Tomatidine acts in synergy with aminoglycoside antibiotics against multiresistant Staphylococcus aureus and prevents virulence gene expression. J. Antimicrob. Chemother. 2012, 67, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Sharifzadeh, A.; Khosravi, A.R.; Shokri, H.; Shirzadi, H. Potential effect of 2-isopropyl-5-methylphenol (thymol) alone and in combination with fluconazole against clinical isolates of Candida albicans, C. glabrata and C. krusei. J. Mycol. Med. 2018, 28, 294–299. [Google Scholar] [CrossRef]
- Behbehani, J.; Irshad, M.; Shreaz, S.; Karched, M. Synergistic effects of tea polyphenol epigallocatechin 3-O-gallate and azole drugs against oral Candida isolates. J. Mycol. Med. 2019, 29, 158–167. [Google Scholar] [CrossRef]
- Quave, C.L. Antibiotics from nature: Traditional medicine as a source of new solutions for combating antimicrobial resistance. AMR Control 2016, 98–102. [Google Scholar]
- Day, J. Botany meets archaeology: People and plants in the past. J. Exp. Bot. 2013, 64, 5805–5816. [Google Scholar] [CrossRef] [Green Version]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Samuel, S.M.; Kubatka, P.; Büsselberg, D. Treating Cancers Using Nature’s Medicine: Significance and Challenges. Biomolecules 2021, 11, 1698. [Google Scholar] [CrossRef]
- Li, D.-D.; Zhang, Y.-H.; Zhang, W.; Zhao, P. Meta-analysis of randomized controlled trials on the efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease. Front. Neurosci. 2019, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.P.; Or, T.C.T.; Ip, N.Y. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochem. Int. 2015, 89, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Das, A.M. Clinical utility of nitisinone for the treatment of hereditary tyrosinemia type-1 (HT-1). Appl. Clin. Genet. 2017, 10, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Thangapazham, R.L.; Sharad, S.; Maheshwari, R.K. Skin regenerative potentials of curcumin. Biofactors 2013, 39, 141–149. [Google Scholar] [CrossRef]
- Duarte, N.B.A.; Takahashi, J.A. Plant spices as a source of antimicrobial synergic molecules to treat bacterial and viral co-infections. Molecules 2022, 27, 8210. [Google Scholar] [CrossRef] [PubMed]
- Uzma, F.; Mohan, C.D.; Hashem, A.; Konappa, N.M.; Rangappa, S.; Kamath, P.V.; Singh, B.P.; Mudili, V.; Gupta, V.K.; Siddaiah, C.N.; et al. Endophytic Fungi—Alternative Sources of Cytotoxic Compounds: A Review. Front. Pharmacol. 2018, 9, 309. [Google Scholar] [CrossRef]
- Mohan, C.D.; Rangappa, S.; Nayak, S.C.; Jadimurthy, R.; Wang, L.; Sethi, G.; Garg, M.; Rangappa, K.S. Bacteria as a treasure house of secondary metabolites with anticancer potential. Semin. Cancer Biol. 2022, 86, 998–1013. [Google Scholar] [CrossRef] [PubMed]
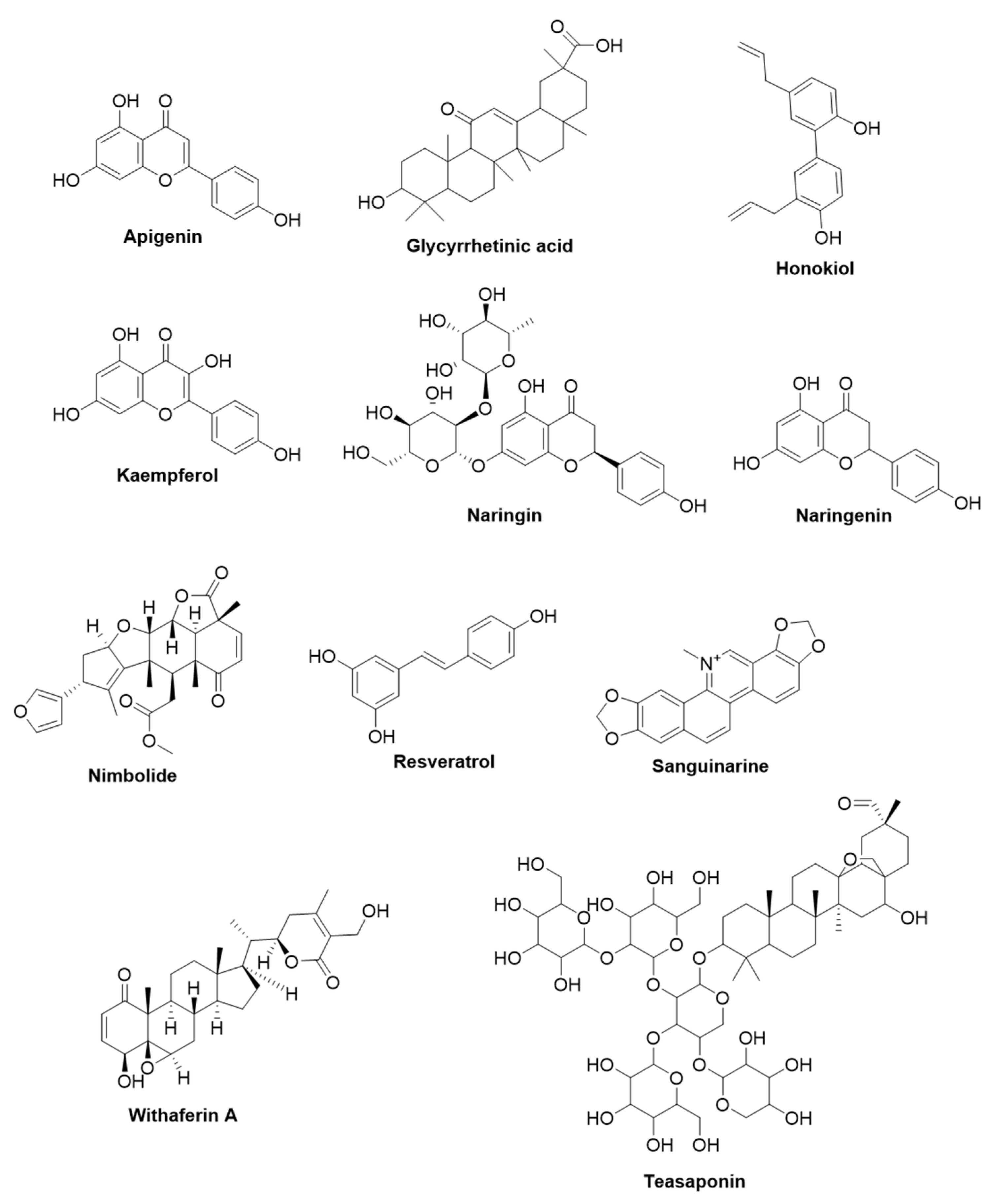
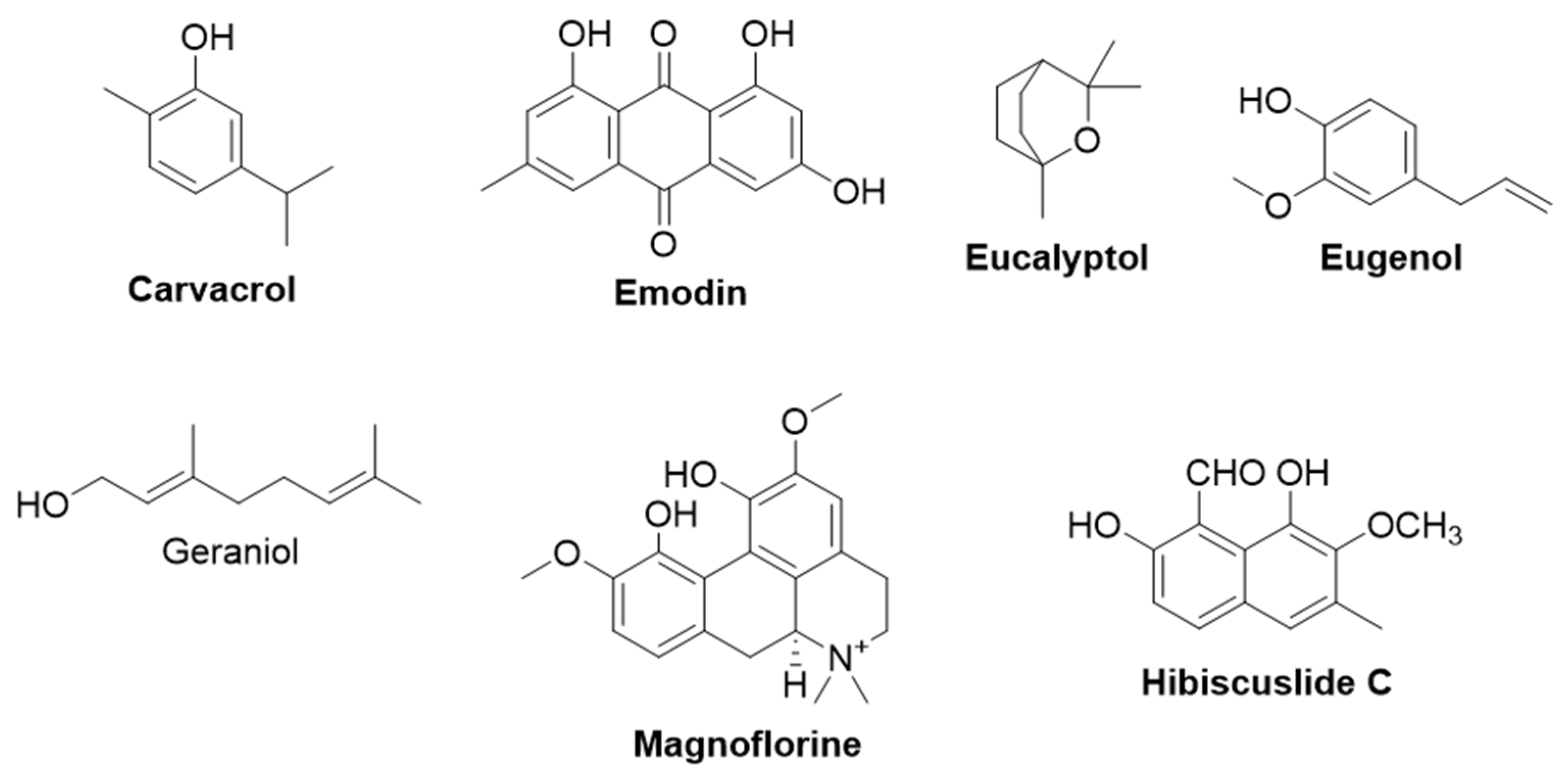

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadimurthy, R.; Jagadish, S.; Nayak, S.C.; Kumar, S.; Mohan, C.D.; Rangappa, K.S. Phytochemicals as Invaluable Sources of Potent Antimicrobial Agents to Combat Antibiotic Resistance. Life 2023, 13, 948. https://doi.org/10.3390/life13040948
Jadimurthy R, Jagadish S, Nayak SC, Kumar S, Mohan CD, Rangappa KS. Phytochemicals as Invaluable Sources of Potent Antimicrobial Agents to Combat Antibiotic Resistance. Life. 2023; 13(4):948. https://doi.org/10.3390/life13040948
Chicago/Turabian StyleJadimurthy, Ragi, Swamy Jagadish, Siddaiah Chandra Nayak, Sumana Kumar, Chakrabhavi Dhananjaya Mohan, and Kanchugarakoppal S. Rangappa. 2023. "Phytochemicals as Invaluable Sources of Potent Antimicrobial Agents to Combat Antibiotic Resistance" Life 13, no. 4: 948. https://doi.org/10.3390/life13040948
APA StyleJadimurthy, R., Jagadish, S., Nayak, S. C., Kumar, S., Mohan, C. D., & Rangappa, K. S. (2023). Phytochemicals as Invaluable Sources of Potent Antimicrobial Agents to Combat Antibiotic Resistance. Life, 13(4), 948. https://doi.org/10.3390/life13040948






