Out of Box Thinking to Tangible Science: A Benchmark History of 3D Bio-Printing in Regenerative Medicine and Tissues Engineering
Abstract
:1. Introduction
2. Sequel of Skin Tissue Models
3. Sequel of Bones and Cartilaginous Tissue Models
4. Sequel of 3D-BP Cardiac Tissue Models
5. Sequel of 3D-BP Kidney and Liver Tissue Models
6. Sequel of Neural Tissue
7. Bioprinters and Bioinks for 3D Printing
8. 3D-BP and Micro Fluidics
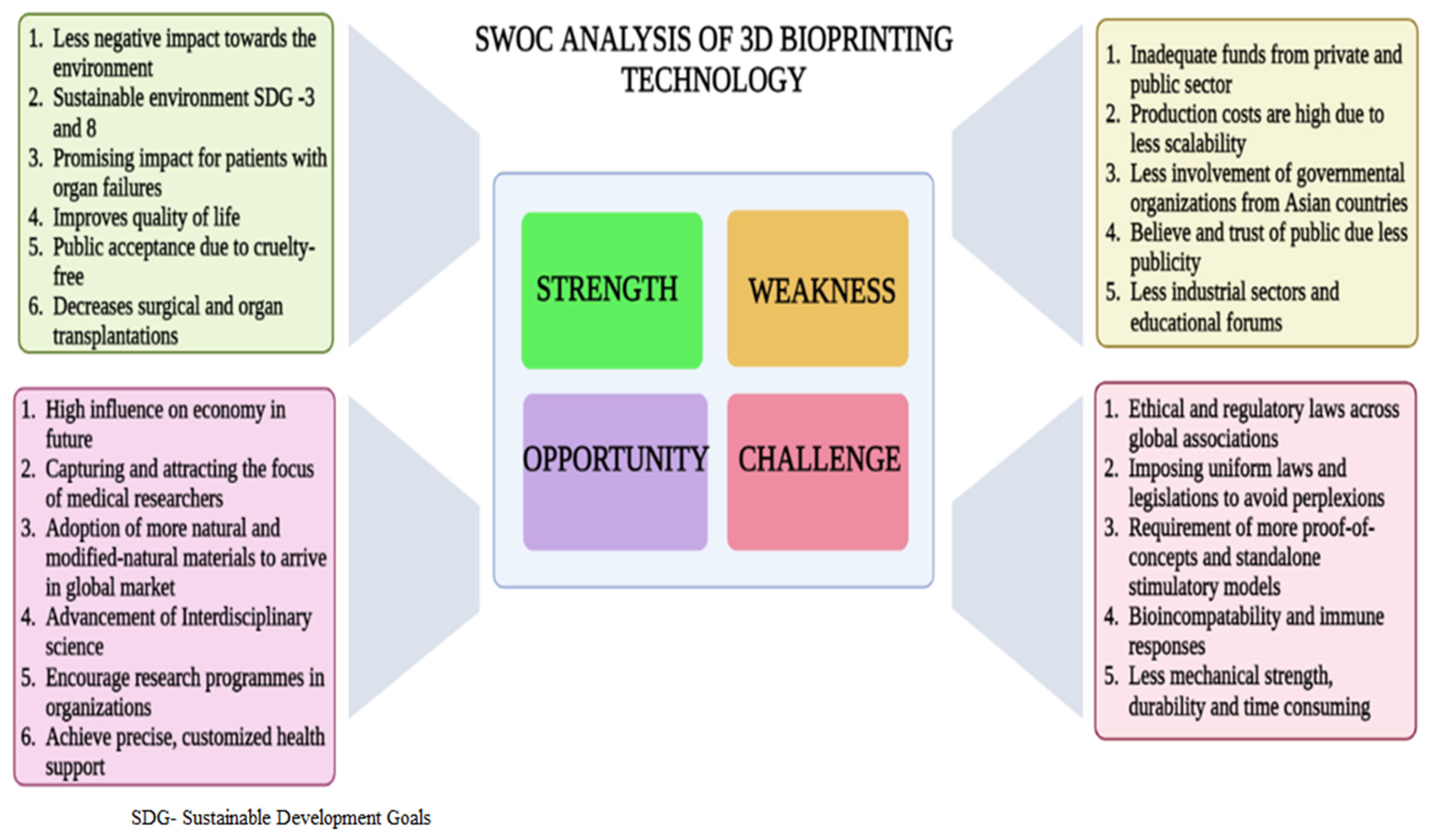
9. Significance of 3D-BP in Tissue Regeneration
10. Limitations
11. Tangible Outcomes and Future Outlook of 3D-BP
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Etheredge, H.R. Assessing Global Organ Donation Policies: Opt-In vs Opt-Out. Risk Manag. Healthc. Policy 2021, 14, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kapadia, W.; Li, C.; Lin, F.; Pereira, R.F.; Granja, P.L.; Sarmento, B.; Cui, W. Tissue-specific engineering: 3D bioprinting in regenerative medicine. J. Control. Release 2021, 329, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [Green Version]
- Parihar, A.; Pandita, V.; Kumar, A.; Parihar, D.S.; Puranik, N.; Bajpai, T.; Khan, R. 3D Printing: Advancement in Biogenerative Engineering to Combat Shortage of Organs and Bioapplicable Materials. Regen. Eng. Transl. Med. 2022, 8, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Kathuria, H.; Dubey, N. Advances in 3D bioprinting of tissues/organs for regenerative medicine and in-vitro models. Biomaterials 2022, 287, 121639. [Google Scholar] [CrossRef]
- Liaw, C.-Y.; Guvendiren, M. Current and emerging applications of 3D printing in medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef]
- Kneser, U.; Polykandriotis, E.; Ohnolz, J.; Heidner, K.; Grabinger, L.; Euler, S.; Amann, K.U.; Hess, A.; Brune, K.; Greil, P.; et al. Engineering of vascularized transplantable bone tissues: Induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng. 2006, 12, 1721–1731. [Google Scholar] [CrossRef] [Green Version]
- Zervantonakis, I.K.; Hughes-Alford, S.K.; Charest, J.L.; Condeelis, J.S.; Gertler, F.B.; Kamm, R.D. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl. Acad. Sci. USA 2012, 109, 13515–13520. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, S.; Klar, A. Bioengineered skin substitutes: Advances and future Trends. Appl. Sci. 2021, 11, 1493. [Google Scholar] [CrossRef]
- Savoji, H.; Godau, B.; Hassani, M.S.; Akbari, M. Skin Tissue Substitutes and Biomaterial Risk Assessment and Testing. Front. Bioeng. Biotechnol. 2018, 6, 86. [Google Scholar] [CrossRef]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [Green Version]
- Duan, B. State-of-the-Art review of 3D bioprinting for cardiovascular tissue engineering. Ann. Biomed. Eng. 2017, 45, 195–209. [Google Scholar] [CrossRef]
- Schütz, K.; Placht, A.-M.; Paul, B.; Brüggemeier, S.; Gelinsky, M.; Lode, A. Three-dimensional plotting of a cell-laden alginate/methylcellulose blend: Towards biofabrication of tissue engineering constructs with clinically relevant dimensions. J. Tissue Eng. Regen. Med. 2017, 11, 1574–1587. [Google Scholar] [CrossRef]
- Blaeser, A.; Campos, D.F.D.; Fischer, H. 3D bioprinting of cell-laden hydrogels for advanced tissue engineering. Curr. Opin. Biomed. Eng. 2017, 2, 58–66. [Google Scholar] [CrossRef]
- Wang, P.; Berry, D.; Moran, A.; He, F.; Tam, T.; Chen, L.; Chen, S. Controlled Growth Factor Release in 3D-Printed Hydrogels. Adv. Healthc. Mater. 2020, 9, 1900977. [Google Scholar] [CrossRef]
- Jose, R.R.; Rodriguez, M.J.; Dixon, T.A.; Omenetto, F.; Kaplan, D.L. Evolution of bioinks and additive manufacturing technologies for 3D bioprinting. ACS Biomater. Sci. Eng. 2016, 2, 1662–1678. [Google Scholar] [CrossRef]
- Abdelaal, O.A.M.; Darwish, S.M.H. Review of rapid prototyping techniques for tissue engineering scaffolds fabrication. In Characterization and Development of Biosystems and Biomaterials; Springer: Berlin/Heidelberg, Germany, 2013; pp. 33–54. [Google Scholar]
- Tavafoghi, M.; Darabi, M.A.; Mahmoodi, M.; Tutar, R.; Xu, C.; Mirjafari, A.; Billi, F.; Swieszkowski, W.; Nasrollahi, F.; Ahadian, S.; et al. Multimaterial bioprinting towards the fabrication of biomimetic tissues and organs. Biofabrication 2021, 13, 042002. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Su, Y.; Liao, Z.; Liu, Z.; Huang, X.; Zhao, L.; Duan, R.; Hu, Y.; Wei, Y.; Lian, X.; et al. Coaxial bioprinting vascular constructs: A review. Eur. Polym. J. 2022, 179, 111549. [Google Scholar] [CrossRef]
- Yang, G.H.; Kang, D.; An, S.; Ryu, J.Y.; Lee, K.; Kim, J.S.; Song, M.-Y.; Kim, Y.-S.; Kwon, S.-M.; Jung, W.-K.; et al. Advances in the development of tubular structures using extrusion-based 3D cell-printing technology for vascular tissue regenerative applications. Biomater. Res. 2022, 26, 73. [Google Scholar] [CrossRef]
- Wattanaanek, N.; Suttapreyasri, S.; Samruajbenjakun, B. 3D printing of calcium phosphate/calcium sulfate with alginate/cellulose-based scaffolds for bone regeneration: Multilayer fabrication and characterization. J. Funct. Biomater. 2022, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Zuev, D.M.; Golubchikov, D.O.; Evdokimov, P.V.; Putlyaev, V.I. Synthesis of Amorphous Calcium Phosphate Powders for Production of Bioceramics and Composites by 3D Printing. Russ. J. Inorg. Chem. 2022, 67, 940–951. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Synthetic amorphous calcium phosphates (ACPs): Preparation, structure, properties, and biomedical applications. Biomater. Sci. 2021, 9, 7748–7798. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Obaid, R.F.; Smaisim, G.F.; Esfahani, M.M.; Alsaikhan, F.; Baghaei, S.; Hadrawi, S.K.; Yusof, M.; Yadav, A. Investigation of addition of calcium phosphate ceramic to multilayer scaffold for bone applications with improved mechanical properties: Fuzzy logic analysis. Ceram. Int. 2023, 49, 8339–8349. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, H.; Sun, Y.; Jiang, Y.; Fang, S.; Kan, Z.; Lu, Y.; Liu, S.; Zhou, X.; Li, Z. Using Platelet-Rich Plasma Hydrogel to Deliver Mesenchymal Stem Cells into Three-Dimensional PLGA Scaffold for Cartilage Tissue Engineering. ACS Appl. Bio. Mater. 2021, 4, 8607–8614. [Google Scholar] [CrossRef]
- Xia, D.; Chen, J.; Zhang, Z.; Dong, M. Emerging polymeric biomaterials and manufacturing techniques in regenerative medicine. Aggregate 2022, 3, e176. [Google Scholar] [CrossRef]
- Pishavar, E.; Luo, H.; Naserifar, M.; Hashemi, M.; Toosi, S.; Atala, A.; Ramakrishna, S.; Behravan, J. Advanced hydrogels as exosome delivery systems for osteogenic differentiation of MSCS: Application in bone regeneration. Int. J. Mol. Sci. 2021, 22, 6203. [Google Scholar] [CrossRef]
- Jansen, L.E.; Kim, H.; Hall, C.L.; McCarthy, T.P.; Lee, M.J.; Peyton, S.R. A poly(ethylene glycol) three-dimensional bone marrow hydrogel. Biomaterials 2022, 280, 121–270. [Google Scholar] [CrossRef]
- Mahendiran, B.; Muthusamy, S.; Sampath, S.; Jaisankar, S.; Popat, K.C.; Selvakumar, R.; Krishnakumar, G.S. Recent trends in natural polysaccharide based bioinks for multiscale 3D printing in tissue regeneration: A review. Int. J. Biol. Macromol. 2021, 183, 564–588. [Google Scholar] [CrossRef]
- Dufaud, M.; Solé, L.; Maumus, M.; Simon, M.; Perrier-Groult, E.; Subra, G.; Jorgensen, C.; Noël, D. 3D bioprinting of articular cartilage: Recent advances and perspectives. Bioprinting 2022, 28, e00253. [Google Scholar] [CrossRef]
- Semba, J.A.; Mieloch, A.A.; Rybka, J.D. Introduction to the state-of-the-art 3D bioprinting methods, design, and applications in orthopedics. Bioprinting 2020, 18, e00070. [Google Scholar] [CrossRef]
- Blaeser, A.; Campos, D.F.D.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Sorg, H.; Peck, C.-T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Gao, G.; Cui, X. Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotechnol. Lett. 2016, 38, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Blokzijl, M.M.; Levato, R.; Peiffer, Q.C.; de Ruijter, M.; Hennink, W.E.; Vermonden, T.; Malda, J. Hydrogel-based reinforcement of 3D bioprinted constructs. Biofabrication 2016, 8, 035004. [Google Scholar] [CrossRef] [Green Version]
- Irmak, G.; Demirtaş, T.T.; Gümüşderelioǧlu, M. Highly methacrylated gelatin bioink for bone tissue engineering. ACS Biomater. Sci. Eng. 2018, 5, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Maxson, E.L.; Young, M.D.; Noble, C.; Go, J.L.; Heidari, B.; Khorramirouz, R.; Morse, D.W.; Lerman, A. In vivo remodeling of a 3D-Bioprinted tissue engineered heart valve scaffold. Bioprinting 2019, 16, e00059. [Google Scholar] [CrossRef]
- Zamani, M.; Karaca, E.; Huang, N.F. Multicellular interactions in 3D engineered myocardial tissue. Front. Cardiovasc. Med. 2018, 5, 147. [Google Scholar] [CrossRef] [Green Version]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.S.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, E.; Fukunishi, T.; Bai, Y.; Bedja, D.; Pitaktong, I.; Mattson, G.; Jeyaram, A.; Lui, C.; Ong, C.S.; Inoue, T.; et al. Cardiac regeneration using human-induced pluripotent stem cell-derived biomaterial-free 3D-bioprinted cardiac patch in vivo. J. Tissue Eng. Regen. Med. 2019, 13, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Wragg, N.M.; Burke, L.; Wilson, S.L. A critical review of current progress in 3D kidney biomanufacturing: Advances, challenges, and recommendations. Ren. Replace. Ther. 2019, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Peloso, A.; Katari, R.; Murphy, S.V.; Zambon, J.P.; DeFrancesco, A.; Farney, A.C.; Rogers, J.; Stratta, R.J.; Manzia, T.M.; Orlando, G. Prospect for kidney bioengineering: Shortcomings of the status quo. Expert Opin. Biol. Ther. 2015, 15, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Sateesh, J.; Guha, K.; Dutta, A.; Sengupta, P.; Rao, K.S. Regenerating re-absorption function of proximal convoluted tubule using microfluidics for kidney-on-chip applications. SN Appl. Sci. 2020, 2, 39. [Google Scholar] [CrossRef] [Green Version]
- Morizane, R.; Bonventre, J.V. Kidney organoids: A translational journey. Trends Mol. Med. 2017, 23, 246–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz-Abraham, A.S.; Rodriguez-Davalos, M.I.; Bertacco, A.; Wengerter, B.; Geibel, J.P.; Mulligan, D.C. 3D printing of organs for transplantation: Where are we and where are we heading? Curr. Transplant. Rep. 2016, 3, 93–99. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef] [Green Version]
- Arslan-Yildiz, A.; El Assal, R.; Chen, P.; Guven, S.; Inci, F.; Demirci, U. Towards artificial tissue models: Past, present, and future of 3D bioprinting. Biofabrication 2016, 8, 014103. [Google Scholar] [CrossRef] [Green Version]
- Faulkner-Jones, A.; Fyfe, C.; Cornelissen, D.-J.; Gardner, J.; King, J.; Courtney, A.; Shu, W. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015, 7, 044102. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, K.; Jeong, J.; Paik, S.S.; Kim, J.S.; Park, S.A.; Kim, W.D.; Park, J.; Choi, D. Three-dimensional (3D) printing of mouse primary hepatocytes to generate 3D hepatic structure. Ann. Surg. Treat. Res. 2017, 92, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cidonio, G.; Glinka, M.; Kim, Y.-H.; Kanczler, J.; Lanham, S.A.; Ahlfeld, T.; Lode, A.; Dawson, J.; Gelinsky, M.; Oreffo, R.O.C. Nanoclay-based 3D printed scaffolds promote vascular ingrowth ex vivo and generate bone mineral tissue in vitro and in vivo. Biofabrication 2020, 12, 035010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Sani, E.S.; Spencer, A.R.; Guan, Y.; Weiss, A.S.; Annabi, N. Human-recombinant-Elastin-based bioinks for 3D bioprinting of vascularized soft tissues. Adv. Mater. 2020, 32, 2003915. [Google Scholar] [CrossRef] [PubMed]
- Lorber, B.; Hsiao, W.-K.; Hutchings, I.; Martin, K. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication 2013, 6, 015001. [Google Scholar] [CrossRef] [PubMed]
- Fantini, V.; Bordoni, M.; Scocozza, F.; Conti, M.; Scarian, E.; Carelli, S.; Di Giulio, A.M.; Marconi, S.; Pansarasa, O.; Auricchio, F.; et al. Bioink composition and printing parameters for 3D modeling neural tissue. Cells 2019, 8, 830. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Tiwari, S.K.; Agrawal, K.; Tan, M.; Dang, J.; Tam, T.; Tian, J.; Wan, X.; Schimelman, J.; You, S.; et al. Rapid 3D bioprinting of glioblastoma model mimicking native biophysical heterogeneity. Small 2021, 17, e2006050. [Google Scholar] [CrossRef]
- Ning, L.; Sun, H.; Lelong, T.; Guilloteau, R.; Zhu, N.; Schreyer, D.J.; Chen, X. 3D bioprinting of scaffolds with living Schwann cells for potential nerve tissue engineering applications. Biofabrication 2018, 10, 035014. [Google Scholar] [CrossRef]
- Liu, X.; Hao, M.; Chen, Z.; Zhang, T.; Huang, J.; Dai, J.; Zhang, Z. 3D bioprinted neural tissue constructs for spinal cord injury repair. Biomaterials 2021, 272, 120771. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.; Gage, J.A.; Shen, T.; Haisler, W.L.; Neeley, S.K.; Shiao, S.; Chen, J.; Desai, P.K.; Liao, A.; Hebel, C.; et al. A spheroid toxicity assay using magnetic 3D bioprinting and real-time mobile device-based imaging. Sci. Rep. 2015, 5, srep13987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimann, M.; Bono, E.; Annaheim, H.; Bleisch, M.; Graf-Hausner, U. Standardized 3D bioprinting of soft tissue models with human primary cells. SLAS Technol. Transl. Life Sci. Innov. 2016, 21, 496–509. [Google Scholar] [CrossRef] [Green Version]
- Cubo, N.; Garcia, M.; del Cañizo, J.F.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2016, 9, 015006. [Google Scholar] [CrossRef] [Green Version]
- Ng, W.L.; Qi, J.T.Z.; Yeong, W.Y.; Naing, M.W. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 2018, 10, 025005. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.Y.; Leveque, J.-C.; Brumblay, H.; Krebsbach, P.H.; Hollister, S.J.; LaMarca, F. Macro-architectures in spinal cord scaffold implants influence regeneration. J. Neurotrauma 2008, 25, 1027–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, F.-Y.; Lin, H.-H.; Hsu, S.-H. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 2015, 71, 48–57. [Google Scholar] [CrossRef]
- Silva, N.A.; Salgado, A.J.; Sousa, R.A.; Oliveira, J.T.; Pedro, A.J.; Leite-Almeida, H.; Cerqueira, R.; Almeida, A.; Mastronardi, F.; Mano, J.F.; et al. Development and characterization of a novel hybrid Tissue Engineering–based scaffold for spinal cord injury repair. Tissue Eng. Part A 2010, 16, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.N.; Yassin, M.A.; Suliman, S.; Lie, S.A.; Gjengedal, H.; Mustafa, K. The bone regeneration capacity of 3D-printed templates in calvarial defect models: A systematic review and meta-analysis. Acta Biomater. 2019, 91, 1–23. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef]
- Gaetani, R.; Feyen, D.A.; Verhage, V.; Slaats, R.; Messina, E.; Christman, K.L.; Giacomello, A.; Doevendans, P.A.; Sluijter, J.P. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 2015, 61, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, H.-J.; Kim, S.-W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [Green Version]
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting technology: A current state-of-the-art review. J. Manuf. Sci. Eng. 2014, 136, 061016. [Google Scholar] [CrossRef]
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.B.; Lee, H.; Kim, G.H. Strategy to achieve highly porous/biocompatible macroscale cell blocks, using a collagen/genipin-bioink and an optimal 3D printing process. ACS Appl. Mater. Interfaces 2016, 8, 32230–32240. [Google Scholar] [CrossRef] [PubMed]
- Mobed-Miremadi, M.; Acks, E.; Polsaward, S.; Chen, D. High throughput miniaturization of artificial cells. Artif. Cells Blood Substit. Biotechnol. 2011, 39, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef] [Green Version]
- Bacelar, A.H.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Recent progress in gellan gum hydrogels provided by functionalization strategies. J. Mater. Chem. B 2016, 4, 6164–6174. [Google Scholar] [CrossRef] [Green Version]
- Yadav, N.; Chauhan, M.K.; Chauhan, V.S. Short to ultrashort peptide-based hydrogels as a platform for biomedical applications. Biomater. Sci. 2020, 8, 84–100. [Google Scholar] [CrossRef]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef]
- Beheshtizadeh, N.; Lotfibakhshaiesh, N.; Pazhouhnia, Z.; Hoseinpour, M.; Nafari, M. A review of 3D bio-printing for bone and skin tissue engineering: A commercial approach. J. Mater. Sci. 2020, 55, 3729–3749. [Google Scholar] [CrossRef]
- Muthusamy, S.; Kannan, S.; Lee, M.; Sanjairaj, V.; Lu, W.F.; Fuh, J.Y.H.; Sriram, G.; Cao, T. 3D bioprinting and microscale organization of vascularized tissue constructs using collagen-based bioink. Biotechnol. Bioeng. 2021, 118, 3150–3163. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y. Identification of potential small molecule allosteric modulator sites on IL-1R1 ectodomain using accelerated conformational sampling method. PLoS ONE 2015, 10, e0118671. [Google Scholar] [CrossRef]
- Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogt, P.M.; et al. Skin tissue generation by laser cell printing. Biotechnol. Bioeng. 2012, 109, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Hasan, S.K.; Song, Y.S.; Xu, F.; Keles, H.O.; Manzur, F.; Mikkilineni, S.; Hong, J.W.; Nagatomi, J.; Haeggstrom, E.; et al. Layer by Layer Three-dimensional Tissue Epitaxy by Cell-Laden Hydrogel Droplets. Tissue Eng. Part C Methods 2010, 16, 157–166. [Google Scholar] [CrossRef]
- Campos, D.F.D.; Blaeser, A.; Korsten, A.; Neuss, S.; Jäkel, J.; Vogt, M.; Fischer, H. The Stiffness and structure of three-dimensional printed hydrogels direct the differentiation of mesenchymal stromal cells toward adipogenic and osteogenic lineages. Tissue Eng. Part A 2015, 21, 740–756. [Google Scholar] [CrossRef]
- Yeo, M.; Lee, J.-S.; Chun, W.; Kim, G.H. An innovative collagen-based cell-printing method for obtaining human adipose stem cell-laden structures consisting of core–sheath structures for tissue engineering. Biomacromolecules 2016, 17, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yan, K.C.; Ouyang, L.; Sun, W. Mechanical characterization of bioprinted in vitro soft tissue models. Biofabrication 2013, 5, 045010. [Google Scholar] [CrossRef] [PubMed]
- Wüst, S.; Godla, M.E.; Müller, R.; Hofmann, S. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 2014, 10, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levato, R.; Webb, W.R.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.M.; Malda, J. The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017, 61, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Iwanaga, S.; Henmi, C.; Arai, K.; Nishiyama, Y. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication 2010, 2, 014110. [Google Scholar] [CrossRef] [PubMed]
- Gruene, M.; Pflaum, M.; Hess, C.; Diamantouros, S.; Schlie, S.; Deiwick, A.; Koch, L.; Wilhelmi, M.; Jockenhoevel, S.; Haverich, A.; et al. Laser printing of three-dimensional multicellular arrays for studies of cell–cell and cell–environment interactions. Tissue Eng. Part C Methods 2011, 17, 973–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.; Boland, T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 2009, 30, 6221–6227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, Y.P.; Bandyopadhyay, A.; Mandal, B.B. 3D bioprinting using cross-linker-free silk–gelatin bioink for cartilage tissue engineering. ACS Appl. Mater. Interfaces 2019, 11, 33684–33696. [Google Scholar] [CrossRef]
- Hauptstein, J.; Böck, T.; Bartolf-Kopp, M.; Forster, L.; Stahlhut, P.; Nadernezhad, A.; Blahetek, G.; Zernecke-Madsen, A.; Detsch, R.; Jüngst, T.; et al. Hyaluronic Acid-Based Bioink Composition Enabling 3D Bioprinting and Improving Quality of Deposited Cartilaginous Extracellular Matrix. Adv. Healthc. Mater. 2020, 9, 2000737. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Y.; Zhu, J.; Liu, F.; Xue, Y.; Huang, Y.; Zhu, B.; Wu, D.; Pan, H.; Gong, T.; et al. Hyaluronic acid methacrylate/pancreatic extracellular matrix as a potential 3D printing bioink for constructing islet organoids. Acta Biomater. 2022. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Luo, C.; Zhai, C.; Li, Z.; Zhang, Y.; Yuan, T.; Dong, S.; Zhang, J.; Fan, W. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater. Sci. Eng. C 2021, 118, 111388. [Google Scholar] [CrossRef]
- Mao, Q.; Wang, Y.; Li, Y.; Juengpanich, S.; Li, W.; Chen, M.; Yin, J.; Fu, J.; Cai, X. Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater. Sci. Eng. C 2020, 109, 110625. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, Y.; Hou, C.; Li, Z.; Yang, S.; Liang, X.; Zhou, L.; Guo, J.; Zhang, J.; Huang, X. Ovary-derived Decellularized Extracellular Matrix-based Bioink for Fabricating 3D Primary Ovarian Cells-laden Structures for Mouse Ovarian Failure Correction. Int. J. Bioprinting 2022, 8, 597. [Google Scholar] [CrossRef]
- Kim, H.; Kang, B.; Cui, X.; Lee, S.-H.; Lee, K.; Cho, D.-W.; Hwang, W.; Woodfield, T.B.F.; Lim, K.S.; Jang, J. Light-activated decellularized extracellular matrix-based bioinks for volumetric tissue analogs at the centimeter scale. Adv. Funct. Mater. 2021, 31, 2011252. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Gremmels, H.; van Deventer, K.; Fledderus, J.O.; Öner, F.; Verhaar, M.C.; Dhert, W.J.; Alblas, J. Prolonged presence of VEGF promotes vascularization in 3D bioprinted scaffolds with defined architecture. J. Control. Release 2014, 184, 58–66. [Google Scholar] [CrossRef]
- Huang, C.-T.; Shrestha, L.K.; Ariga, K.; Hsu, S.-H. A graphene–polyurethane composite hydrogel as a potential bioink for 3D bioprinting and differentiation of neural stem cells. J. Mater. Chem. B 2017, 5, 8854–8864. [Google Scholar] [CrossRef]
- Cai, Y.; Chang, S.Y.; Gan, S.W.; Ma, S.; Lu, W.F.; Yen, C.-C. Nanocomposite bioinks for 3D bioprinting. Acta Biomater. 2022, 151, 45–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, H.; Hu, Q.; Shen, Z.; Rana, D.; Ramalingam, M. Designing vascular supportive albumen-rich composite bioink for organ 3D printing. J. Mech. Behav. Biomed. Mater. 2020, 104, 103642. [Google Scholar] [CrossRef]
- Dubey, N.; Ferreira, J.A.; Malda, J.; Bhaduri, S.B.; Bottino, M.C. Extracellular matrix/amorphous magnesium phosphate bioink for 3D bioprinting of craniomaxillofacial bone tissue. ACS Appl. Mater. Interfaces 2020, 12, 23752–23763. [Google Scholar] [CrossRef]
- Meng, Y.; Cao, J.; Chen, Y.; Yu, Y.; Ye, L. 3D printing of a poly(vinyl alcohol)-based nano-composite hydrogel as an artificial cartilage replacement and the improvement mechanism of printing accuracy. J. Mater. Chem. B 2020, 8, 677–690. [Google Scholar] [CrossRef]
- Skardal, A.; Aleman, J.; Forsythe, S.; Rajan, S.; Murphy, S.; Devarasetty, M.; Zarandi, N.P.; Nzou, G.; Wicks, R.; Sadri-Ardekani, H.; et al. Drug compound screening in single and integrated multi-organoid body-on-a-chip systems. Biofabrication 2020, 12, 025017. [Google Scholar] [CrossRef]
- Hassan, R.U.; Khalil, S.M.; Khan, S.A.; Ali, S.; Moon, J.; Cho, D.-H.; Byun, D. High-Resolution, Transparent, and Flexible Printing of Polydimethylsiloxane via Electrohydrodynamic Jet Printing for Conductive Electronic Device Applications. Polymers 2022, 14, 4373. [Google Scholar] [CrossRef]
- Yildirim, Ö.; Arslan-Yildiz, A. Development of a hydrocolloid bio-ink for 3D bioprinting. Biomater. Sci. 2022, 10, 6707–6717. [Google Scholar] [CrossRef] [PubMed]
- El Assal, R.; Guven, S.; Gurkan, U.A.; Gözen, I.; Shafiee, H.; Dalbeyler, S.; Abdalla, N.; Thomas, G.; Fuld, W.; Illigens, B.M.W.; et al. Bio-Inspired Cryo-Ink Preserves Red Blood Cell Phenotype and Function During Nanoliter Vitrification. Adv. Mater. 2014, 26, 5815–5822. [Google Scholar] [CrossRef] [PubMed]
- Deo, K.A.; Jaiswal, M.K.; Abasi, S.; Lokhande, G.; Bhunia, S.; Nguyen, T.-U.; Namkoong, M.; Darvesh, K.; Guiseppi-Elie, A.; Tian, L.; et al. Nanoengineered Ink for Designing 3D Printable Flexible Bioelectronics. ACS Nano 2022, 16, 8798–8811. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Liu, J. Bioprinting of 3D tissues/organs combined with microfluidics. RSC Adv. 2018, 8, 21712–21727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantini, M.; Testa, S.; Mozetic, P.; Barbetta, A.; Fuoco, C.; Fornetti, E.; Tamiro, F.; Bernardini, S.; Jaroszewicz, J.; Święszkowski, W.; et al. Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials 2017, 131, 98–110. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, C.; Jiang, L.; Qin, J. A 3D human lung-on-a-chip model for nanotoxicity testing. Toxicol. Res. 2018, 7, 1048–1060. [Google Scholar] [CrossRef] [Green Version]
- West-Livingston, L.N.; Park, J.; Lee, S.J.; Atala, A.; Yoo, J.J. The role of the microenvironment in controlling the fate of bioprinted stem Cells. Chem. Rev. 2020, 120, 11056–11092. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Lee, A.Y.W. Traumatic brain injuries: Pathophysiology and potential therapeutic targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hao, Z.; Xian, C.; Fang, Y.; Cheng, B.; Wu, J.; Xia, J. Neuro-bone tissue engineering: Multiple potential translational strategies between nerve and bone. Acta Biomater. 2022, 153, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Amirifar, L.; Shamloo, A.; Nasiri, R.; de Barros, N.R.; Wang, Z.Z.; Unluturk, B.D.; Libanori, A.; Ievglevskyi, O.; Diltemiz, S.E.; Sances, S.; et al. Brain-on-a-chip: Recent advances in design and techniques for microfluidic models of the brain in health and disease. Biomaterials 2022, 285, 121531. [Google Scholar] [CrossRef] [PubMed]
- Shettigar, N.; El Nihum, L.; Thyagarajan, A.; Banerjee, D.; Krencik, R. Design, Microfabrication and Testing of Brain-on-a-Chip (BOC) Platform Using Neural Organoids (Spheroids). In Fluids Engineering Division Summer Meeting; American Society of Mechanical Engineers: New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Ferrari, E.; Rasponi, M. Liver–Heart on chip models for drug safety. APL Bioeng. 2021, 5, 031505. [Google Scholar] [CrossRef]
- Gao, B.; Yang, Q.; Zhao, X.; Jin, G.; Ma, Y.; Xu, F. 4D Bioprinting for Biomedical Applications. Trends Biotechnol. 2016, 34, 746–756. [Google Scholar] [CrossRef]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic bioprinting of heterogeneous 3d tissue constructs using low-viscosity bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.; Unutmaz, D.; Ozbolat, I.T. Bioprinting towards physiologically relevant tissue models for pharmaceutics. Trends Biotechnol. 2016, 34, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, K.; Balasubramanian, B.; Arumugam, V.A.; Easwaran, M.; Park, S.; Issara, U.; Pushparaj, K.; Al-Dhabi, N.A.; Arasu, M.V.; Liu, W.-C.; et al. Biocompatibility of Veratric Acid–Encapsulated Chitosan/Methylcellulose Hydrogel: Biological Characterization, Osteogenic Efficiency with In Silico Molecular Modeling. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Eglen, R.M.; Randle, D.H. Drug discovery goes three-dimensional: Goodbye to flat high-throughput screening? Assay Drug Dev. Technol. 2015, 13, 262–265. [Google Scholar] [CrossRef]
- Hoque, M.E.; Chuan, Y.L.; Pashby, I. Extrusion based rapid prototyping technique: An advanced platform for tissue engineering scaffold fabrication. Biopolymers 2012, 97, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Park, J. The Design of a heterocellular 3D architecture and its application to monitoring the behavior of cancer cells in response to the spatial distribution of endothelial cells. Adv. Mater. 2012, 24, 5339–5344. [Google Scholar] [CrossRef]
- Bigham, A.; Aghajanian, A.H.; Saudi, A.; Rafienia, M. Hierarchical Porous Mg2SiO4-CoFe2O4 Nanomagnetic Scaffold for Bone Cancer Therapy and Regeneration: Surface Modification and In Vitro Studies. Mater. Sci. Eng. C 2019, 109, 110579. [Google Scholar] [CrossRef]
- King, S.M.; Presnell, S.C.; Nguyen, D.G. Abstract 2034: Development of 3D bioprinted human breast cancer for in vitro drug screening. Cancer Res 2014, 74 (Suppl. S19), 2034. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; Li, E.; Weiner, L.M. 3D culture systems for exploring cancer immunology. Cancers 2020, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Villa, F.; Fernadez, J.L.C.; Costa, D.; Zocchi, M.R.; Benelli, R. Three-dimensional culture models to study innate anti-tumor immune response: Advantages and disadvantages. Cancers 2021, 13, 3417. [Google Scholar] [CrossRef]
- Krysko, D.V.; Demuynck, R.; Efimova, I.; Naessens, F.; Krysko, O.; Catanzaro, E. In Vitro Veritas: From 2D Cultures to Organ-on-a-Chip Models to Study Immunogenic Cell Death in the Tumor Microenvironment. Cells 2022, 11, 3705. [Google Scholar] [CrossRef]
- Kačarević, .P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An introduction to 3D bioprinting: Possibilities, challenges and future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Gao, M.; Lobo, A.O.; Webster, T.J. 3D bioprinting in tissue engineering for medical applications: The classic and the hybrid. Polymers 2020, 12, 1717. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiong, J.; Yang, L.; Zhang, J.; Sun, S.; Liang, Y. Cell-free exosome-laden scaffolds for tissue repair. Nanoscale 2021, 13, 8740–8750. [Google Scholar] [CrossRef] [PubMed]
- Oveili, E.; Vafaei, S.; Bazavar, H.; Eslami, Y.; Mamaghanizadeh, E.; Yasamineh, S.; Gholizadeh, O. The potential use of mesenchymal stem cells-derived exosomes as microRNAs delivery systems in different diseases. Cell Commun. Signal. 2023, 21, 1–26. [Google Scholar] [CrossRef]
- Chen, C.W.; Wang, L.L.; Zaman, S.; Gordon, J.; Arisi, M.F.; Venkataraman, C.M.; Chung, J.J.; Hung, G.; Gaffey, A.C.; Spruce, L.A.; et al. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc. Res. 2018, 114, 1029–1040. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, P.; Gao, X.; Chang, L.; Chen, Z.; Mei, X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater. Sci. Eng. C. 2021, 120, 111671. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef]
- Shen, K.; Duan, A.; Cheng, J.; Yuan, T.; Zhou, J.; Song, H.; Chen, Z.; Wan, B.; Liu, J.; Zhang, X.; et al. Exosomes derived from hypoxia preconditioned mesenchymal stem cells laden in a silk hydrogel promote cartilage regeneration via the miR-205–5p/PTEN/AKT pathway. Acta Biomater. 2022, 143, 173–188. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, J.Y.; Peng, W.M.; Yuan, B.; Bi, Q.; Xu, Y.J. Exosomes from adipose-derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR-145 and miR-221. Mol. Med. Rep. 2020, 21, 1881–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, X.; Qi, Y.; Liu, X.; Shi, Y.; Li, H.; Zhao, L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomaterials Adv. 2022, 133, 112613. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Xu, L.; Xu, X.; Qin, Z.; Zhou, X.; Xiao, Y.; Liang, Y.; Xia, J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale 2021, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]


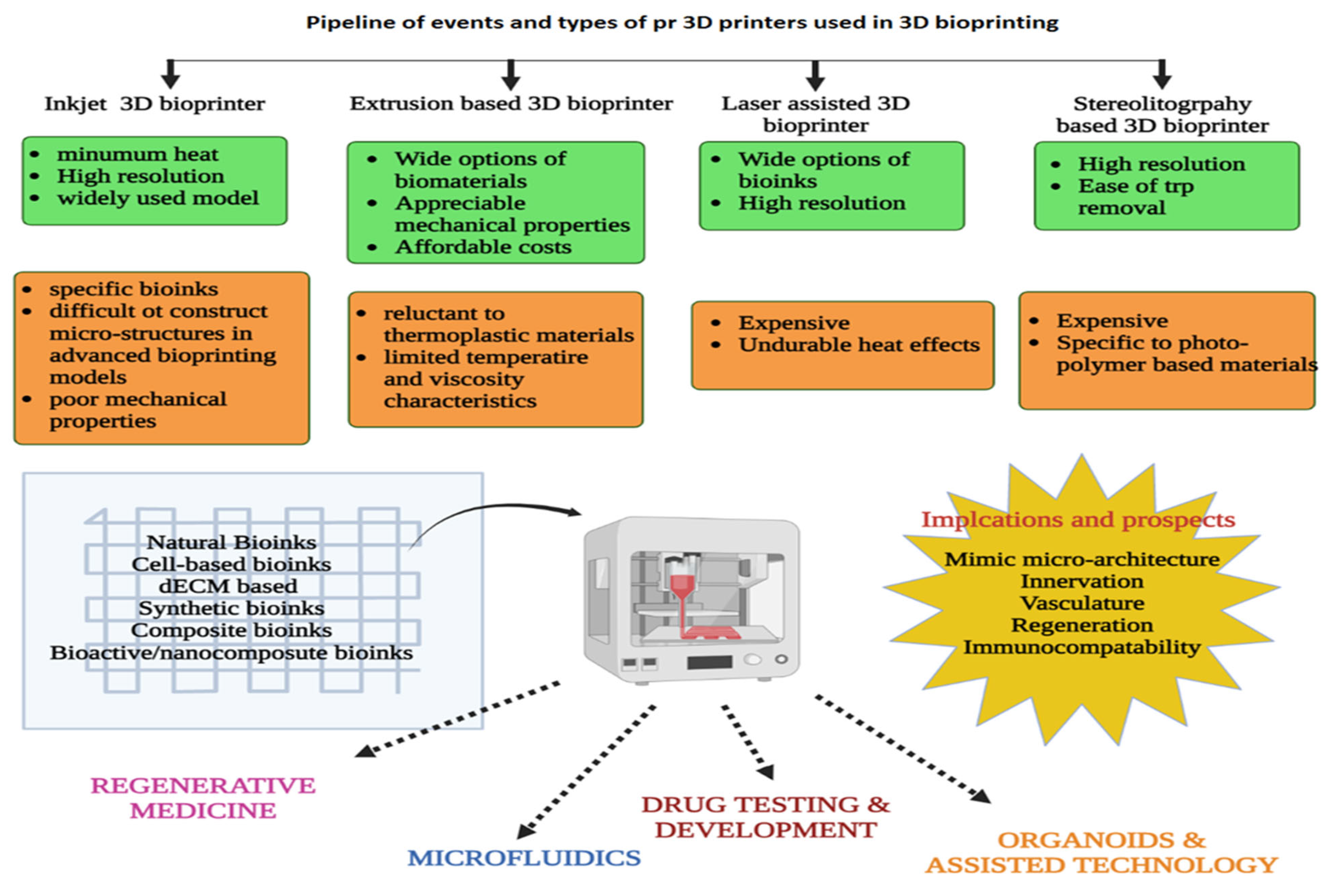
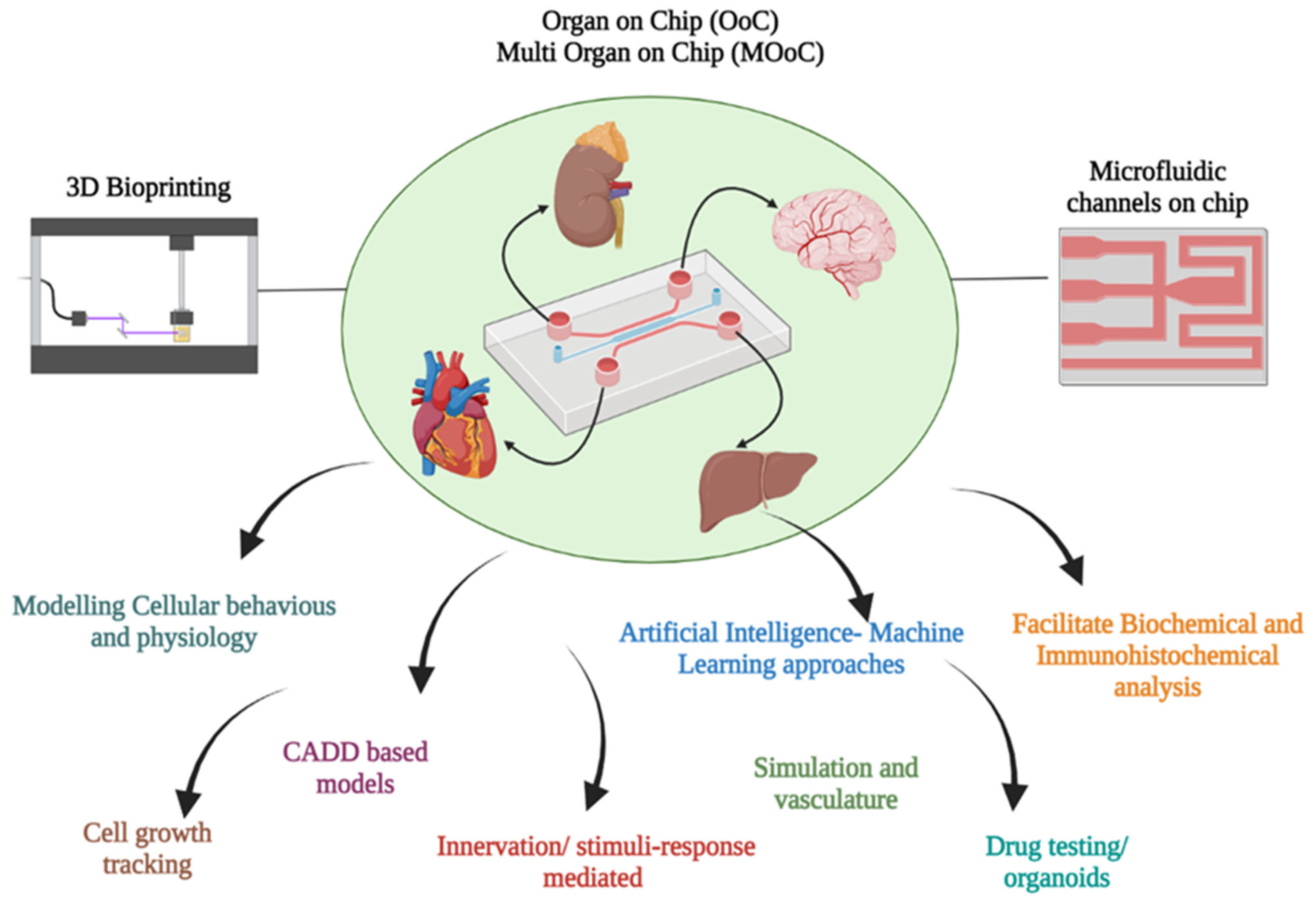
| S. No | Type of Tissue | Clinical Models | Reference |
|---|---|---|---|
| 1. | Skin tissue | Primary human dermal keratinocytes were fabricated with dermal equivalents and epidermis-like structures were developed | [62] |
| 2. | Skin tissue | Free-form fabrication (FFF) 3D bioprinting technique was adopted to engineer human plasma-derived bilayered skin using human fibroblasts (hFBs) and keratinocytes (hKCs) retrieved from skin biopsies were implanted in immunodeficient athymic mice | [63] |
| 3. | Skin tissue | 3D-BP pigmented human skin constructs were engineered with keratinocytes, melanocytes and fibroblasts obtained from donors, and they exhibited similar constitutive pigmentation as the skin donors | [64] |
| 4. | Neural tissue | Improved spinal cord regeneration, reduced scar and offered elongated nerve fibers in vivo (transection rat SCI model) using porous scaffolds | [65] |
| 5. | Neural tissue | Polyurethane-based NSCs-based 3D-BP constructs using fused deposition showed improved motor function and survival rate in adult zebrafish with induced traumatic brain injury (TBI) models | [66] |
| 6. | Neural tissue | Gellan gum fabricated with oligodendrocyte-like cell scaffolds implanted in the injured tissues showed less inflammation in hemisection rat spinal cord injury (SCI) model | [67] |
| 7. | Bone tissue | 3D-BP models influenced bone tissue regeneration (BTR) in calvarial bone defects in animal models | [68] |
| 8. | Bone tissue | Biodegradable 3D printed scaffolds with controlled release of deferoxamine and developed through a layer-by-layer assembly technique improved angiogenesis and osteogenesis and augumented bone development and reconstruction in animal models | [69] |
| 9. | Bone tissue | Nanoclay-based 3D printed scaffolds stimulated vascular ingrowth in ex vivo conditions and produced bone mineral tissue in vivo models | [54] |
| 10. | Cardiac tissue | Epicardial application of human cardiac-derived progenitor cells (hCMPCs) in a 3D-printed gelatin/hyaluronic acid patch significantly improved cardiac function after myocardial infarction in mouse model | [70] |
| 11. | Cardiac tissue | 3D printed complex tissue patch tailored with stem cell-laden decellularized extracellular matrix bioinks worked well for cardiac repair in mouse models Improved cell migration to the infected area, improved cardiac function and reduced cardiac hypertrophy and fibrosis. | [71] |
| 12. | Cardiac tissue | 3D bioprinted cardiac tissues with recombinant human tropoelastin were assessed in in vivo models. They further stimulated negligible inflammatory response and showed effective biodegradation in vivo in subcutaneously implanted rats | [55] |
| Bioinks | Type of Cell Lines | Bioprinted Material | Bioprinter | Reference |
|---|---|---|---|---|
| Protein-Based Bio-Inks | ||||
| Collagen | Encapsulated keratinocytes and fibroblasts | 3D skin tissue | Laser-based 3D bioprinters | Koch et al. [84] |
| Collagen droplets | Smooth muscle cells (SMCs) | Valve-based droplet ejector system | Skin tissue | Moon et al. [85] |
| Collagen–agarose blend | Mesenchymal stem cells (MSCs) | Extrusion-based | Skin tissue | Duarte Campos et al. [86] |
| Gelatin-alginate composite | Pre-osteoblasts and Human adipose tissue-derived stem cells (ASCS) | 3D tubular bone constructs | 3D porous cellular blocks | Yeo et al. [87] |
| Gelatin-alginate composite | Encapsulate myoblasts | 3D tubular bone constructs | Soft tissue constructs | Zhang et al. [88] |
| Gelatin/alginate bioink with hydroxyapatite (HAp) | Encapsulate myoblasts | 3D tubular bone constructs | Syringe tip heaters in extrusion printers | Wüst et al. [89] |
| Gelatin-alginate composite + | Encapsulated smooth muscle cells (SMCs) in valve root part and valve leaflet interstitial cells | Cell-laden aortic valve conduits | Extrusion-based bioprinter | Duan et al. [90] |
| Agarose, Alginate, GelMA, and BioINK | Articular cartilage- resident chondroprogenitor cells (ACPCs) | Cartilage tissue constructs | Extrusion-based bioprinting system (3D bioplotter) | Levato et al. [91] |
| Fibrin and alginate | Encapsulated chondrocytes | Cartilage tissue | Inkjet bioprinter | Nakamura et al. [92] |
| Fibrinogen | Encapsulated endothelial colony-forming cells (ECFCs) were in a fibrinogen-HA mixture | 3D assembly of multi-cellular arrays. | Laser-based 3D bioprinters | Gruene et al. [93] |
| fibrinogen with PEG or a PEG-geIatin mixture | Human microvascular endothelial cells (HMVEC) | Micro-vasculature networks | Thermal inkjet printing technology | Cui and Boland [94] |
| Silk fibroin and gelatin | primary chondrocytes from porcine | Cartilage tissue constructs | Micro-extrusion bioprinter | Singh et al. [95] |
| Hyaluronic acid (hyaluronan, HA) | Human bone marrow-derived mesenchymal stromal cells (MSCs) | Cartilage tissue constructs | Extruder and microvalve-based print | Hauptstein et al. [96] |
| Hyaluronic acid methacrylate (HAMA) | Pancreatic extracellular matrix (pECM) | 3D-printed islet organoid | Extrusion-based bioprinting system | Wang et al. [97] |
| dECM-based bioinks | ||||
| Silk-dECM construct + TGF-β encapsulated | Decellularized extracellular matrix (SF-dECM bioinks) mixed with bone marrow mesenchymal stem cells (BMSCs) | Cartilage tissue | Extrusion-based bioprinting system | Zhang et al. [98] |
| dECM-based bioinks | Decellularized adipose, heart, and cartilage tissue structures | Various tissues | Multi-head tissue-organ building system (MtoBS) | Pati et al. [53] |
| GelMA with liver dECM. | Encapsulated human-induced hepatocytes (hiHep cells) | Liver tissue | DLP (digital light processing) is a 3D printing technology | Mao et al. [99] |
| dECM-based bioink | Primary ovarian Cells | 3D Primary ovarian cell-laden | Bio-architect 3D bioprinter | Zheng et al. [100] |
| Ru/SPS with dECM | Human turbinate mesenchymal stromal cells (hTMSCs) | Various tissues | Extrusion-based printing and DLP bioprinting system | Kim et al. [101] |
| Matrigel with alginate | Vascular endothelial growth factor (VEGF) | Human endothelial progenitor cells (EPCs) laden constricts | Pneumatic dispensing system enabled | Poldervaart et al. [102] |
| Synthetic polymer-based bioink | ||||
| Graphene–polyurethane composite | Neural stem cells | Tissue constructs for neural tissue | Conventional bio-printer | Huang et al. [103] |
| Silicon, ceramic, cellulose, metal, and carbon-based nano materials. | Respective cell-laden hydrogels | Bone and cartilage tissue constructs | Multi-head tissue-organ building system (MtoBS) | Cai et al. [104] |
| Albumen/Na Alg composite | Human umbilical vein endothelial cells | Organ 3D printing | Extrusion-based 3D bioprinting system | Liu et al. [105] |
| ECM/AMP hydrogel containing 2% octapeptide FEFEFKFK | Encapsulated dental pulp stem cells (DPSCs) | Cranio-maxillofacial Bone Tissue | Microvalve bioprinting | Dubey et al. [106] |
| Poly(vinyl alcohol) (PVA) | Nanocomposite PVA/GO-HAp | Artificial cartilage re-constructs | Extrusion-based 3D printing | Meng et al. [107] |
| Au NPs with thiol-modified hyaluronic acid and gelatin (AuNP-sECMs) | NIH-3T3 cells | Vascular | Extrusion-based printing | Skardal et al. [108] |
| AgNPs in hydrogels | Chondrocytes | Cyborg organs/cartilage | Extrusion-based printing | Hassan et al. [109] |
| Magnetic iron oxide nanoparticles | Porcine aortic endothelial cells | Vasculature networks | Hybrid nano- printing system | Yildirim and Arslan-Yildiz [110] |
| Cryo bioink | Red blood cells | Vasculature | Extrusion-based printing | El Assal et al. [111] |
| Genetically modified phage | MC3T3-E1 | Bones | Extrusion-based printing | Deo et al. [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pushparaj, K.; Balasubramanian, B.; Pappuswamy, M.; Anand Arumugam, V.; Durairaj, K.; Liu, W.-C.; Meyyazhagan, A.; Park, S. Out of Box Thinking to Tangible Science: A Benchmark History of 3D Bio-Printing in Regenerative Medicine and Tissues Engineering. Life 2023, 13, 954. https://doi.org/10.3390/life13040954
Pushparaj K, Balasubramanian B, Pappuswamy M, Anand Arumugam V, Durairaj K, Liu W-C, Meyyazhagan A, Park S. Out of Box Thinking to Tangible Science: A Benchmark History of 3D Bio-Printing in Regenerative Medicine and Tissues Engineering. Life. 2023; 13(4):954. https://doi.org/10.3390/life13040954
Chicago/Turabian StylePushparaj, Karthika, Balamuralikrishnan Balasubramanian, Manikantan Pappuswamy, Vijaya Anand Arumugam, Kaliannan Durairaj, Wen-Chao Liu, Arun Meyyazhagan, and Sungkwon Park. 2023. "Out of Box Thinking to Tangible Science: A Benchmark History of 3D Bio-Printing in Regenerative Medicine and Tissues Engineering" Life 13, no. 4: 954. https://doi.org/10.3390/life13040954
APA StylePushparaj, K., Balasubramanian, B., Pappuswamy, M., Anand Arumugam, V., Durairaj, K., Liu, W.-C., Meyyazhagan, A., & Park, S. (2023). Out of Box Thinking to Tangible Science: A Benchmark History of 3D Bio-Printing in Regenerative Medicine and Tissues Engineering. Life, 13(4), 954. https://doi.org/10.3390/life13040954









