A Study of Oribatid Mites as Potential Intermediate Hosts of Anoplocephalid Tapeworms of Tatra chamois and Tatra marmots from the Tatra Mountains, Central Europe, and Report of a New Intermediate Host for Andrya cuniculi, the Parasite of Leporidae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Localities
2.2. Faeces Collection and Coprological Analyses
2.3. Mite Sampling and Morphological Identification
2.4. Molecular Identification and Phylogenetic Analysis
2.5. Statistical Analyses
3. Results
3.1. Coprological Analyses
3.2. Morphological Analyses of Mites
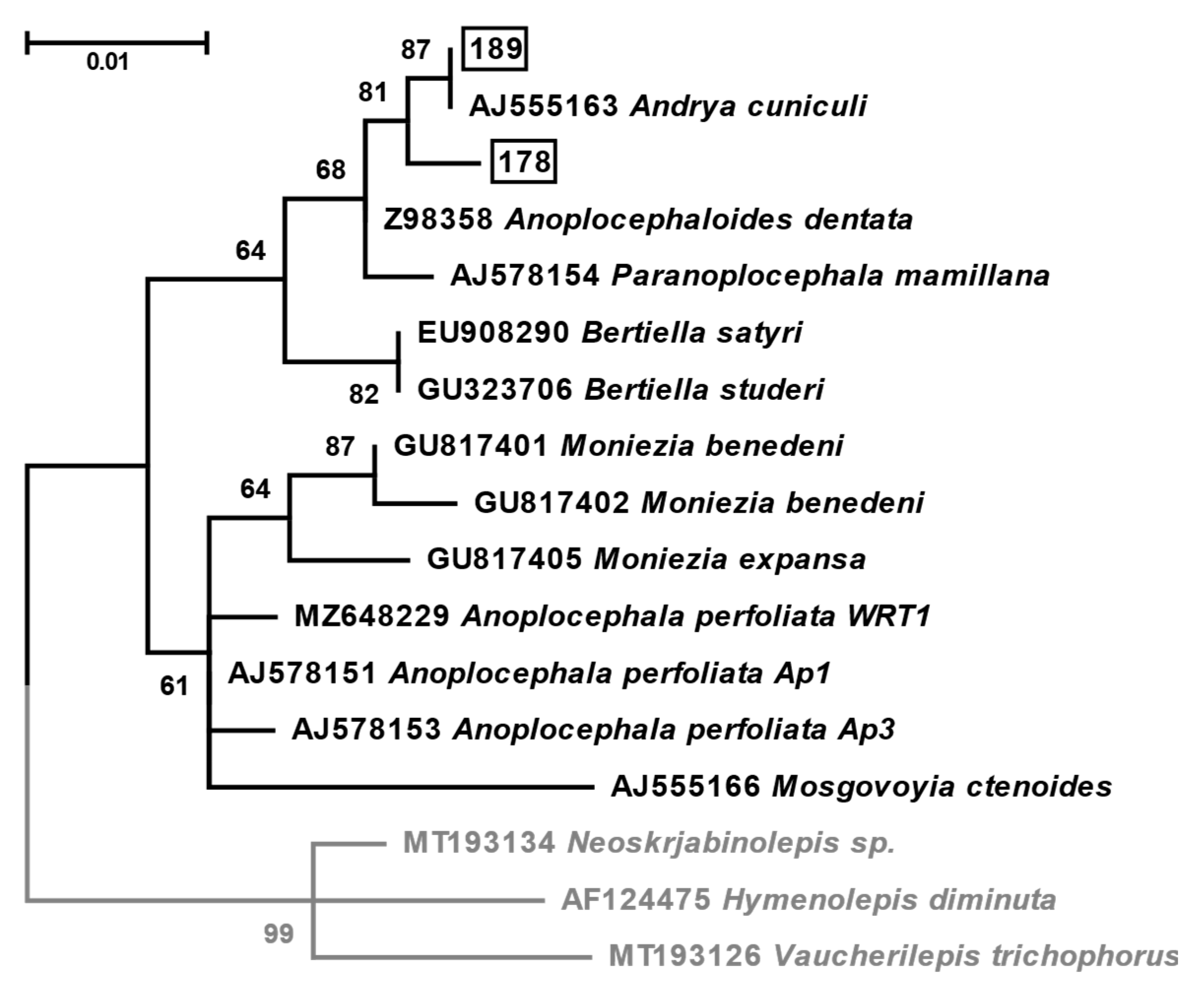
3.3. Molecular Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mituch, J. K helmintofaune raticovej zveri prežúvavej v TANAPe. Ceskoslov. Ochr. Prírody 1969, 8, 239–250. (In Slovak) [Google Scholar]
- Novacký, M.; Chovancová, B. Symboly Tatier. In Tatry–Príroda; Koutná, A., Chovancová, B., Eds.; Baset: Prague, Czech Republic, 2010; pp. 617–639. [Google Scholar]
- Mituch, J. Helmintofauna aves and mammalia. In Proceedings of Zborník Prác o Tatranskom Národnom Parku 16; Martin: Osveta, Slovakia, 1974; pp. 43–64. [Google Scholar]
- Mituch, J.; Hovorka, J.; Hovorka, I.; Tenkáčová, I. Helminty a Helmintocenózy Raticovej Poľovnej Zveri v Ekologických Podmienkach Karpatského Oblúka; Helmintologický Ústav SAV: Košice, Slovakia, 1984; p. 81. (Phase: Final Report). (In Slovak) [Google Scholar]
- Štefančíková, A.; Chovancová, B.; Hájek, B.; Dudiňák, V.; Šnábel, V. Revision of chamois infection by lung nematodes under ecological conditions of national parks of Slovakia with respect to ongoing global climate changes. Helminthologia 2011, 48, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Hurníková, Z.; Miterpáková, M.; Chovancová, B.; Jászayová, A.; Zwijacz-Kozica, T. Pilot research on gastronitestinal parasites of Tatra chamois (Rupicapra rupicapra tatrica). Ann. Agric. Environ. Med. 2022, 29, 513–517. [Google Scholar] [CrossRef]
- Ribarić, P.; Bujanić, M.; Šprem, N.; Kavčić, K.; Sindičić, M.; Konjević, D.; Martinković, F. Gastrointestinal parasites of Alpine chamois (Rupicapra rupicapra rupicapra): Coprology vs. intestine examination. In Book of Abstracts III, Proceedings of the International Rupicapra Symposium, Šprem, Nikica; Agronomski Fakultet Sveučilišta u Zagrebu: Makarska, Croatia, 2021; p. 6. [Google Scholar]
- Hoby, S.; Schwarzenberger, F.; Doherr, M.G.; Robert, N.; Walzer, C. Steroid hormone related male biased parasitism in chamois, Rupicapra rupicapra rupicapra. Vet. Parasitol. 2006, 138, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Morrondo, P.; Vazquez, L.; Diez-Banos, P. Parasitic infections of wild ruminants in Spain with special attention to roe deer and chamois. Parassitologia 2010, 52, 155–158. [Google Scholar]
- Stancampiano, L.; Serra, S.; Battelli, G. Gastro-intestinal nematode infection in four Alpine chamois herds: Influence of host density on helminth egg output. Parassitologia 2001, 43, 123–130. [Google Scholar] [PubMed]
- Kalúz, S.; Ferenčík, J. Pôdne roztoče (Acari) kalamitných plôch vo Vysokých Tatrách. In Pokalamitný Výskum v TANAP-e 2008; Geofyzikálny ústav SAV: Bratislava, Slovakia, 2009; pp. 108–119. [Google Scholar]
- Kocian, J. Štíty a doliny. In Tatry–Príroda; Koutná, A., Chovancová, B., Eds.; Baset: Prague, Czech Republic, 2010; pp. 45–186. [Google Scholar]
- Weigmann, G. Hornmilben (Oribatida): Acari, Actinochaetida; Goecke & Evers: Keltern, Germany, 2006; p. 520. [Google Scholar]
- Subías, L.S. Listado Sistemático, Sinonímico y Biogeográfico de los Ácaros Oribátidos (Acariformes: Oribatida) del Mundo. Graellsia. 60 (Número Extraordinario): 3-305. 17ª Actualización; 2004. Updated in 2020. p. 527. Available online: http://bba.bioucm.es/cont/docs/RO_1.pdf (accessed on 7 October 2022).
- Katz, A.D.; Taylor, S.J.; Davis, M.A. At the confluence of vicariance and dispersal: Phylogeography of carnicolous springtails (Collembola: Arrhopalitidae, Tomoceridae) codistributed across a geologically complex karst landscape in Illinois and Missouri. Ecol. Evol. 2018, 8, 10306–10325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 6 March 2023).
- Reiczigel, J.; Marozzi, M.; Fabian, I.; Rozsa, L. Biostatistics for parasitologists—A primer to Quantitative Parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Simpson, G.L.; Guillaume, F.B.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P. Vegan: Community Ecology Package. 2022. Available online: https://github.com/vegandevs/vegan (accessed on 7 March 2023).
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M. Cluster: “Finding Groups in Data”: Cluster Analysis Extended Rousseeuw et al. 2021. Available online: https://svn.r-project.org/R-packages/trunk/cluster/ (accessed on 7 March 2023).
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.; Weisberg, S.; Price, B.; Friendly, M.; Hong, J. Effects: Effect Displays for Linear, Generalized Linear, and Other Models. 2022. Available online: https://CRAN.R-project.org/package=effects (accessed on 7 March 2023).
- Denegri, G.M. Review of oribatid mites as intermediate hosts of tapeworms of the Anoplocephalidae. Exp. Appl. Acarol. 1993, 17, 567–580. [Google Scholar] [CrossRef]
- Roczeń-Karczmarz, M.; Tomczuk, K. Oribatid mites as vectors of invasive diseases. Acarologia 2016, 56, 613–623. [Google Scholar] [CrossRef] [Green Version]
- Tomczuk, K.; Grzybek, M.; Szczepaniak, K.; Studzińska, M.; Demkowska-Kutrzepa, M.; Roczeń- Karczmarz, M.; Zahrai, A.; Kostro, K.; Junkuszew, A. Factors affecting prevalence and abundance of A. perfoliata infections in horses from south-eastern Poland. Vet. Parasitol. 2017, 246, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Pacek, S.; Seniczak, S.; Graczyk, R.; Chachaj, B.; Seniczak, A. Seasonal dynamics of mites (Acari) in pastures and meadows in Poland, with species analysis of Oribatida. Acarologia 2020, 60, 668–683. [Google Scholar] [CrossRef]
- Stunkard, H.W. The life cycle of Moniezia expansa. Science 1937, 86, 312. [Google Scholar] [CrossRef]
- McAloon, F.M. Oribatid mites as intermediate hosts of Anoplocephala manubriata, cestode of the Asian elephant in India. Exp. Appl. Acarol. 2004, 32, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Kassai, T.; Mahunka, S. Studies on tapeworm in ruminants II: Oribatids as intermediate hosts of Moniezia species. Acta Veterina Hung. 1965, 15, 227–249. [Google Scholar]
- Akrami, M.A.; Saboori, A.; Eslami, A. Observations on oribatid mites (Acari: Oribatida) serving as intermediate hosts of Moniezia expansa (Cestoda: Anoplocephalidae) in Iran. Int. J. Acarol. 2007, 33, 365–369. [Google Scholar] [CrossRef]
- Schuster, R.K.; Coetzee, L. Cysticercoids of Anoplocephala magna (Euccestoda: Anoplocephalidae) experimentally grown in oribatid mites (Acari: Oribatida). Vet. Parasitol. 2012, 190, 285–288. [Google Scholar] [CrossRef]
- Ebermann, E. Oribatiden (Oribatei, Acari) als Zwischenwirte des Murmeltier-Bandwurmes Ctenotaenia marmotae (Frölich, 1802). Parasitol. Res. 1976, 50, 303–312. (In German) [Google Scholar]
- Ahaniazad, M.; Bagheri, M.; Roumi, V.; Akrami, M.A. An efficient and non-destructive DNA extraction method for oribatid mites. Arch. Phytopathol. Plant Prot. 2018, 51, 187–196. [Google Scholar] [CrossRef]
- Sattlerová-Štefančíková, A. Kamzík a Jeho Parazitárne Ochorenia; Press Print, Parazitologický Ústav SAV: Košice, Slovakia, 2005; p. 124. [Google Scholar]
- Mituch, J. Správa o Helmintologickom Vyšetrení Niektorých Cicavcov z Oblasti TANAP-u: Záverečná Správa. Tatranská Lomnica: Výskumná Stanica a Múzeum; Helmintologický Ústav SAV: Košice, Slovakia, 1970; p. 2. (Phase: Final Report). (In Slovak) [Google Scholar]
- Callait, M.P.; Gauthier, D. Parasite adaptations to hibernation in Alpine marmots (Marmota marmota). In Life in the Cold. 11th International Hibernation Symposium; Heldmaier, G., Klingenspor, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 139–146. [Google Scholar]
- Preleuthner, M.; Pinsker, W.; Kruckenhauser, L.; Miller, W.J.; Prosl, H. Alpine marmots in Austria. The present population structure as a result of postglacial distribution history. Acta Theriol. 1995, 3, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Preleuthner, M.; Canderola, S.; Lanfranchi, P.; Prosl, H. Parasiten des Alpenmurmeltieres (Marmota marmota): Systematic, Entwicklung, Verbreitung. Neue Folge Nr. 1999, 146, 77–92. [Google Scholar]
- Prosl, H.; Preleuthner, M.; Bergmann, A. Endoparasites of Marmota marmota in the Tyrolian Alps. In Proceedings of the 1st International Syposium on the Alpine Marmota (Marmota marmot) and Genus Marmot; Basano, B., Durio, P., Gallo Orsi, U., Macchi, E., Eds.; International Marmot Network: Torino, Italy, 1992; pp. 215–216. [Google Scholar]
- Bassano, B.; Sabatier, B.; Rossi, L.; Macchi, E. Parasitic fauna of the digestive tract of Marmota marmota in the western alps. In 1st International Syposium on the Alpine Marmota (Marmota marmot) and Genus Marmot; Bassano, B., Durio, P., Gallo-Orsi, U., Macchi, E., Eds.; International Marmot Network: Torino, Italy, 1992; pp. 13–24. [Google Scholar]
- Callait, M.P. Marmotte alpine et endoparasites: Biologie des parasites et comparaison de l´infestation sur differents sites. In Proceedings of the 4éme Journée d´Étude sur la Marmotte Alpine; Ramousse, R., Le Berre, M., Eds.; International Marmot Network: Lyon, France, 1997; pp. 53–58. [Google Scholar]
- Gortázar, C.; Herrero, J.; García-Serrano, A.; Lucientes, J.; Luco, D.F. Preliminary data on the parasitic fauna of the digestive system of Marmota marmota in the Western Pyrenees. In International Marmot Netwoirk; Le Berre, M., Ramousse, R., Le Guelte, L., Eds.; Biodiversity in Marmots: Moscow, Russia, 1996; pp. 105–108. [Google Scholar]
- Krivopalov, A.; Abramov, S.; Akimova, L.; Barkhatova, A.; Gromov, A.; Konyaev, S.; Lopatina, N.; Sidorovich, A.; Vlasov, E.; Vlasenko, P.; et al. Molecular Characterization of Ctenotaenia marmotae (Frölich, 1802) Railliet, 1893 (Cyclophyllidea: Anoplocephalidae) Parasitizing Rodents of the Genus Marmota and Spermophilus from Eurasia. Diversity 2022, 14, 531. [Google Scholar] [CrossRef]
- Chovancová, B. Súčasná situácia a perspektívy svišťa vrchovského tatranského v TANAP-e. In Proceedings of the Conference Malá zver a jej Životné Prostredie, Košice, Slovakia, 1993; pp. 111–116. (In Slovak). [Google Scholar]
- Tenora, F.; Koubkova, B.; Feliu, C. Redescription of Andrya cuniculi (Blanchard, 1891) (Cestoda: Anoplocephalidae), a parasite of Oryctolagus ciniculus (Lagomorpha) in Spain. Folia Parasitol. 2002, 49, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Foronda, P.; Castillo, A.D.; Abreu, N.; Figueruelo, E.; Piñero, J.; Casanova, J.C. Parasitic helminths of the wild rabbit, Oryctolagus cuniculus, in different bioclimatic zones in Tenerife, Canary Islands. J. Helminthol. 2003, 77, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Rosický, B.; Krátochvíl, J. Drobní savci Tatranského národního parku. Ochr. Přirody X 1955, 2, 34–47. (In Czech) [Google Scholar]
- Mošanský, A. Mammalia. In Proceedings of Zborník Prác o Tatranskom Národnom Parku 16; Martin: Osveta, Slovakia, 1974; pp. 267–294. (In Slovak) [Google Scholar]
- Podobiński, L. Stan zwierzyny w Tatrach w roku 1959 i w latach poprzednich. Wierchy 1961, 29, 137–155. [Google Scholar]
- Podobiński, L. Zwierzęta Tatrańskiego Parku Narodowego w 1966, 1967 i wiosną 1962. Wierchy 1968, 37, 260–270. [Google Scholar]
- Haukisalmi, V.; Wickström, L.M. Morphological characterisation of Andrya Railliet, 1893, Neandrya n.g. and Paranoplocephala Lühe, 1910 (Cestoda: Anoplocephalidae) in rodents and lagomorphs. Syst. Parasitol. 2005, 62, 209–219. [Google Scholar] [CrossRef] [PubMed]



| Localities | LS | VD | TD | DW |
|---|---|---|---|---|
| GPS coordinates | 49°11′369″ N 020°12′974″ E | 49°09′959″ N 020°09′041″ E | 49°13′420″ N 019°54′299″ E | 49°23′291″ N 20°04′836″ E |
| Altitude (m) | 1886–2293 | 1712–1961 | 1795–1951 | 1770–2186 |
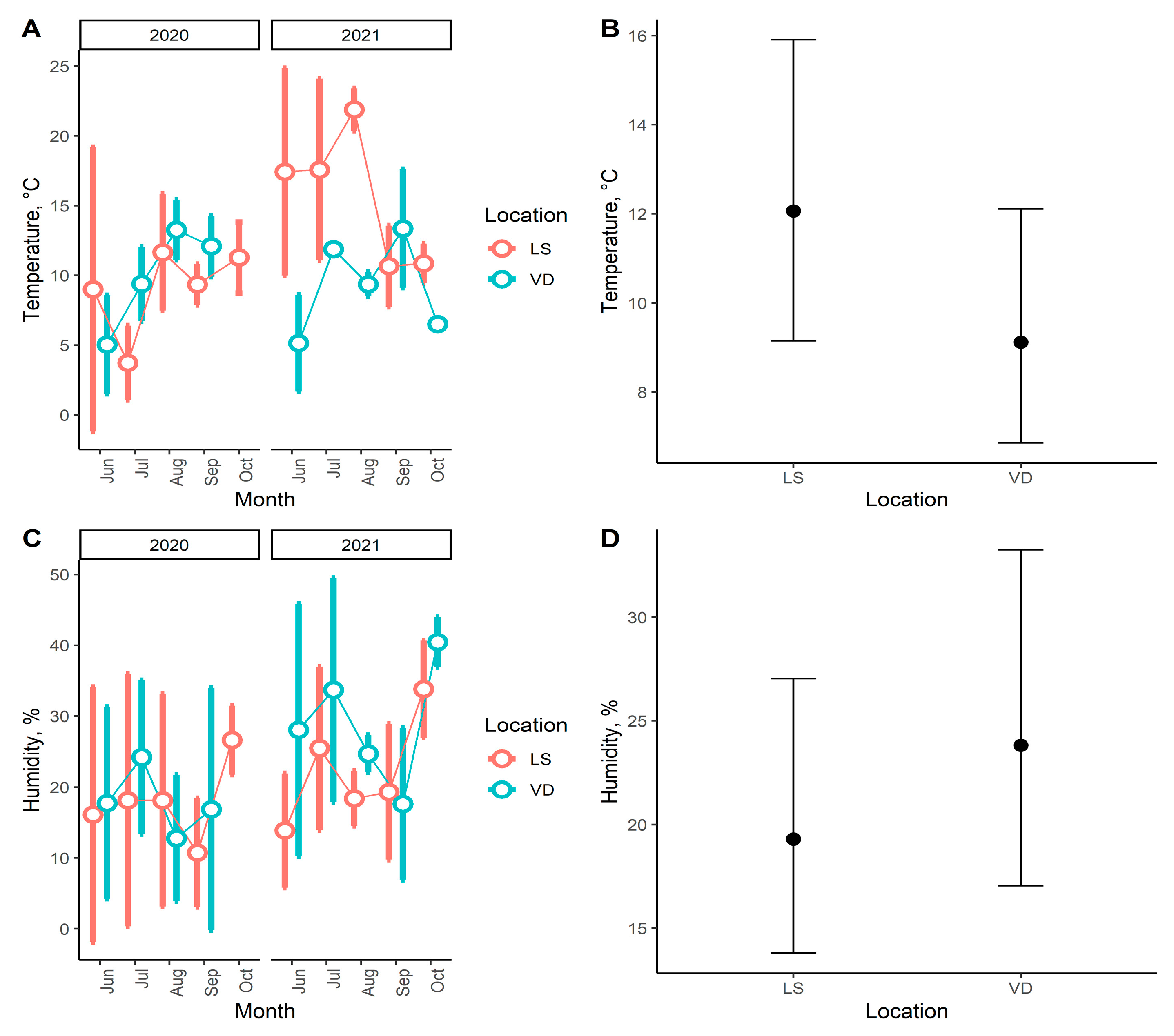
| Average soil temperature | 10.5 °C | 9 °C | 11.3 °C | NA |
| Average soil humidity | 18.4% | 30.3% | 22% | NA |
| Geomorphological subdivision of Mts. | High Tatra | High Tatra | Western Tatra | Polish Tatra |
| Cardinal direction | southeast | south-southwest | southwest | east-northeast |
| Bedrock | granite | granite | limestone | granite |
| Occurrence of the species studied | chamois | chamois/marmot | chamois | chamois |
| Moniezia/Ctenotaenia positivity (%) | 33.6/NA | 16.2/71.1 | 13.1/NA | 43.3/NA |
| 95% CI | 25.5–43.1 | 10.1–24.2/54.1–84.6 | 0.1–16.2 | 25.5–62.6 |
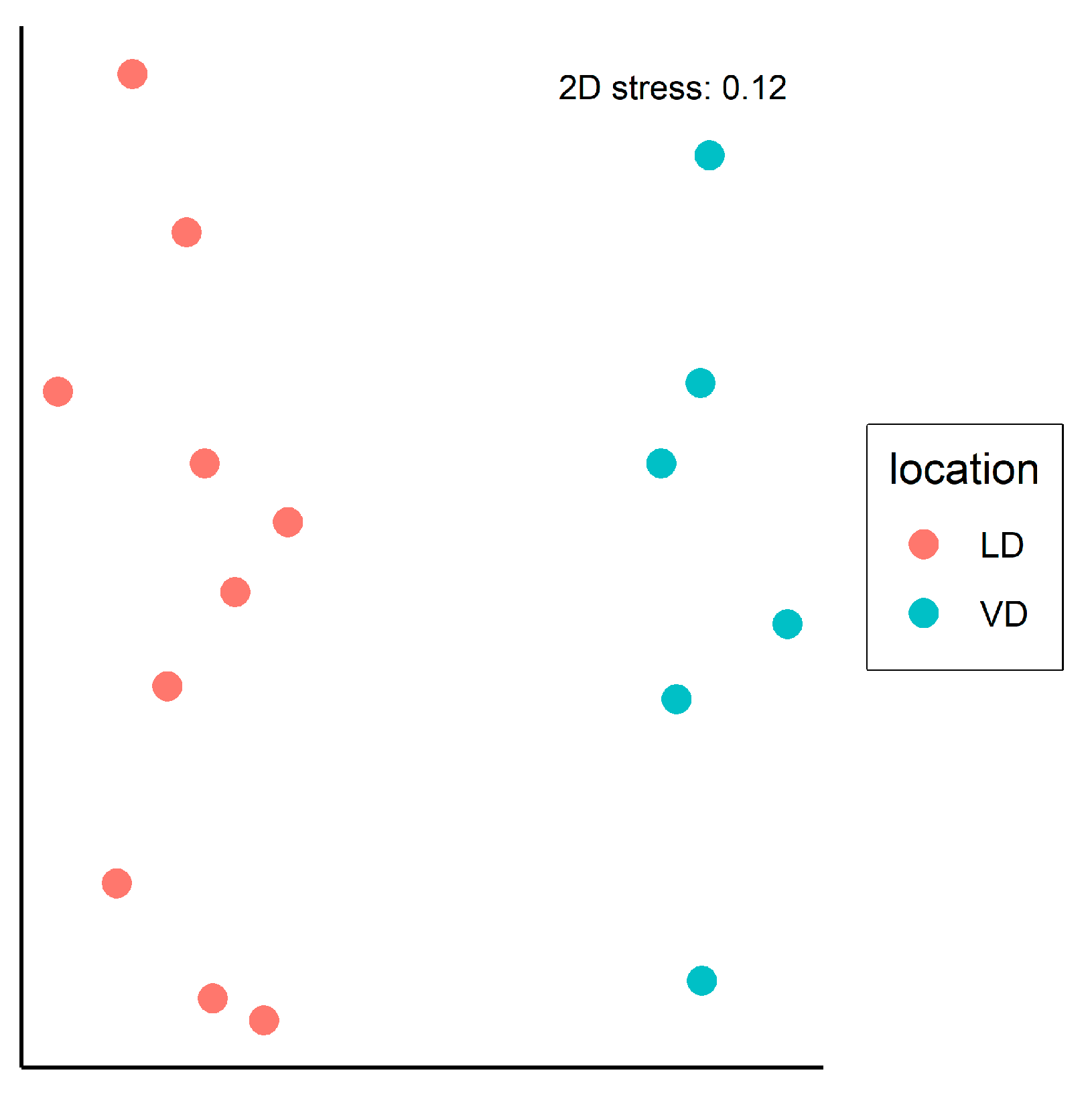
| Oribatid species diversity (No. of taxa) | 72 | 74 | 68 | 50 |
| Abundance (ex.) | 7011 | 6497 | 2930 | 2799 |
| Species | LS | VD | TD | DW |
|---|---|---|---|---|
| Adoristes ovatus (C.L. Koch 1839) | 1 | 10 | - | 6 |
| Achipteria coleoptrata (Linnaeus 1758) | 5 | 40 | 7 | 100 |
| Cepheus cepheiformis (Nicolet 1855) | 9 | - | - | - |
| Ceratoppia bipilis (Hermann 1804) | 16 | 25 | 8 | 36 |
| Ceratozetes gracilis (Michael 1884) | - | 11 | 2 | 1 * |
| Edwardzetes edwardsi (Nicolet 1855) | 1 | 10 * | - | - |
| Hermannia gibba (C.L. Koch 1839) | 372 | 825 | 16 | 65 |
| Liacarus coracinus (C.L. Koch 1841) | 7 | 23 | - | - |
| Liebstadia similis (Michael 1888) | 68 | 45 | 39 | - |
| Oppiella nova (Oudemans 1902) | 2 | 16 | 25 | - |
| Pilogalumna tenuiclava (Berlese 1908) | 100 | 28 | - | 278 |
| Scheloribates laevigatus (C.L. Koch 1835) | 356 | 536 * | 34 | 133 |
| Scheloribates latipes (C.L. Koch 1844) | 34 | 25 | - | 11 |
| Scheloribates pallidulus (C.L. Koch 1841) | - | - | - | 5 |
| Tectocepheusvelatus sarekensis (Trägårdh 1910) | 484 | 519 | 384 * | 161 |
| Trichoribates novus (Sellnick 1928) | - | - | 6 * | 9 |
| Trichoribates trimaculatus (C.L. Koch 1835) | - | 1 | 1 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jászayová, A.; Režnarová, J.; Chovancová, G.; Kostygov, A.Y.; Yurchenko, V.; Antolová, D.; Zwijacz-Kozica, T.; Csanády, A.; Hurníková, Z. A Study of Oribatid Mites as Potential Intermediate Hosts of Anoplocephalid Tapeworms of Tatra chamois and Tatra marmots from the Tatra Mountains, Central Europe, and Report of a New Intermediate Host for Andrya cuniculi, the Parasite of Leporidae. Life 2023, 13, 955. https://doi.org/10.3390/life13040955
Jászayová A, Režnarová J, Chovancová G, Kostygov AY, Yurchenko V, Antolová D, Zwijacz-Kozica T, Csanády A, Hurníková Z. A Study of Oribatid Mites as Potential Intermediate Hosts of Anoplocephalid Tapeworms of Tatra chamois and Tatra marmots from the Tatra Mountains, Central Europe, and Report of a New Intermediate Host for Andrya cuniculi, the Parasite of Leporidae. Life. 2023; 13(4):955. https://doi.org/10.3390/life13040955
Chicago/Turabian StyleJászayová, Alexandra, Jana Režnarová, Gabriela Chovancová, Alexei Yu Kostygov, Vyacheslav Yurchenko, Daniela Antolová, Tomasz Zwijacz-Kozica, Alexander Csanády, and Zuzana Hurníková. 2023. "A Study of Oribatid Mites as Potential Intermediate Hosts of Anoplocephalid Tapeworms of Tatra chamois and Tatra marmots from the Tatra Mountains, Central Europe, and Report of a New Intermediate Host for Andrya cuniculi, the Parasite of Leporidae" Life 13, no. 4: 955. https://doi.org/10.3390/life13040955






