Comparison of Activity and Safety of DSPAα1 and Its N-Glycosylation Mutants
Abstract
1. Introduction
2. Methods and Materials
2.1. Preparation of Plasmids and Strains
2.2. Mutagenesis of DSPAα1
2.3. Expression and Purification of Wild-Type and Mutant DSPAα1
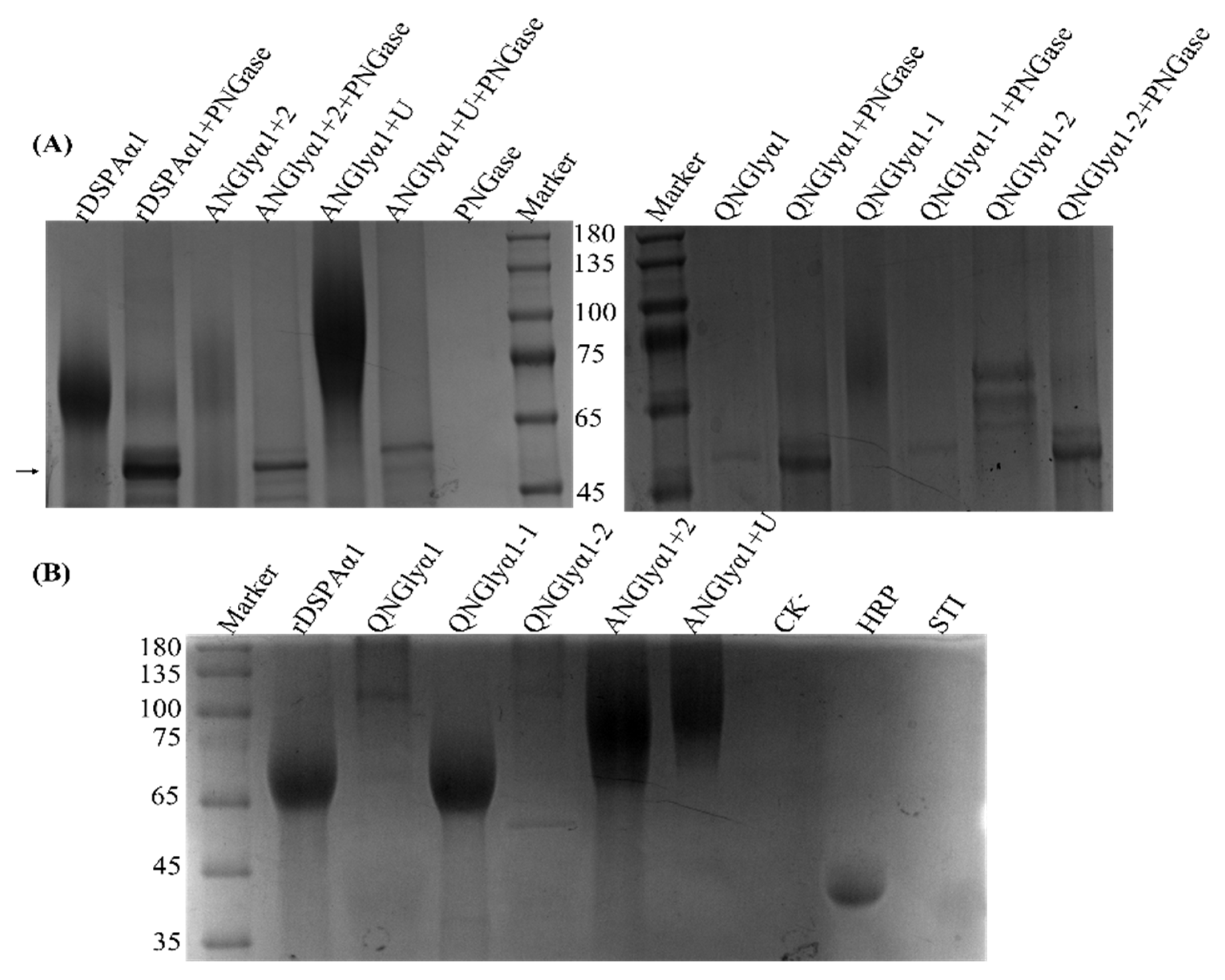
2.4. SDS-PAGE Analysis
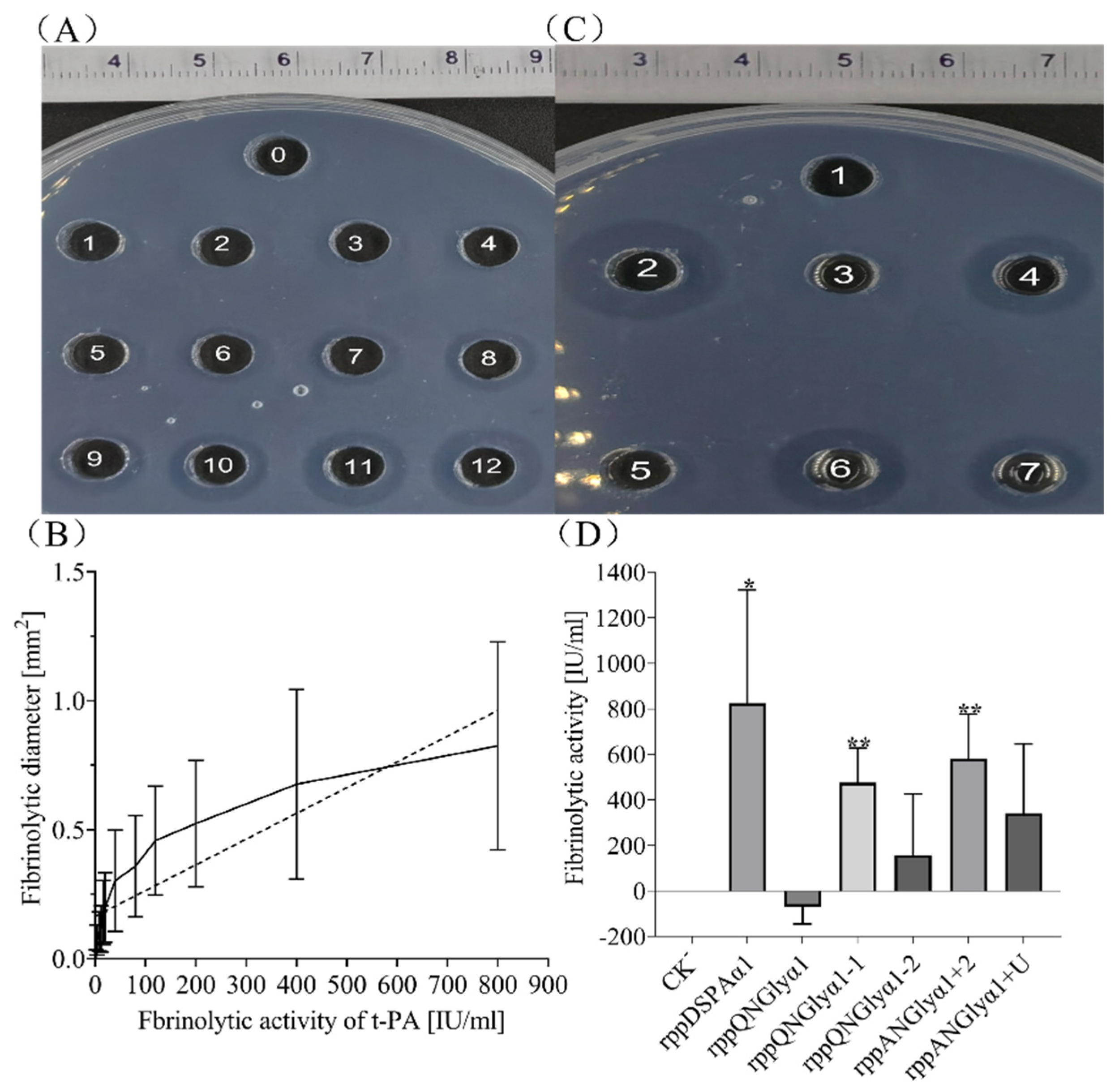
2.5. Fibrin Assay of DSPAα1 Activity
2.6. Kinetics of S-2765 Hydrolysis
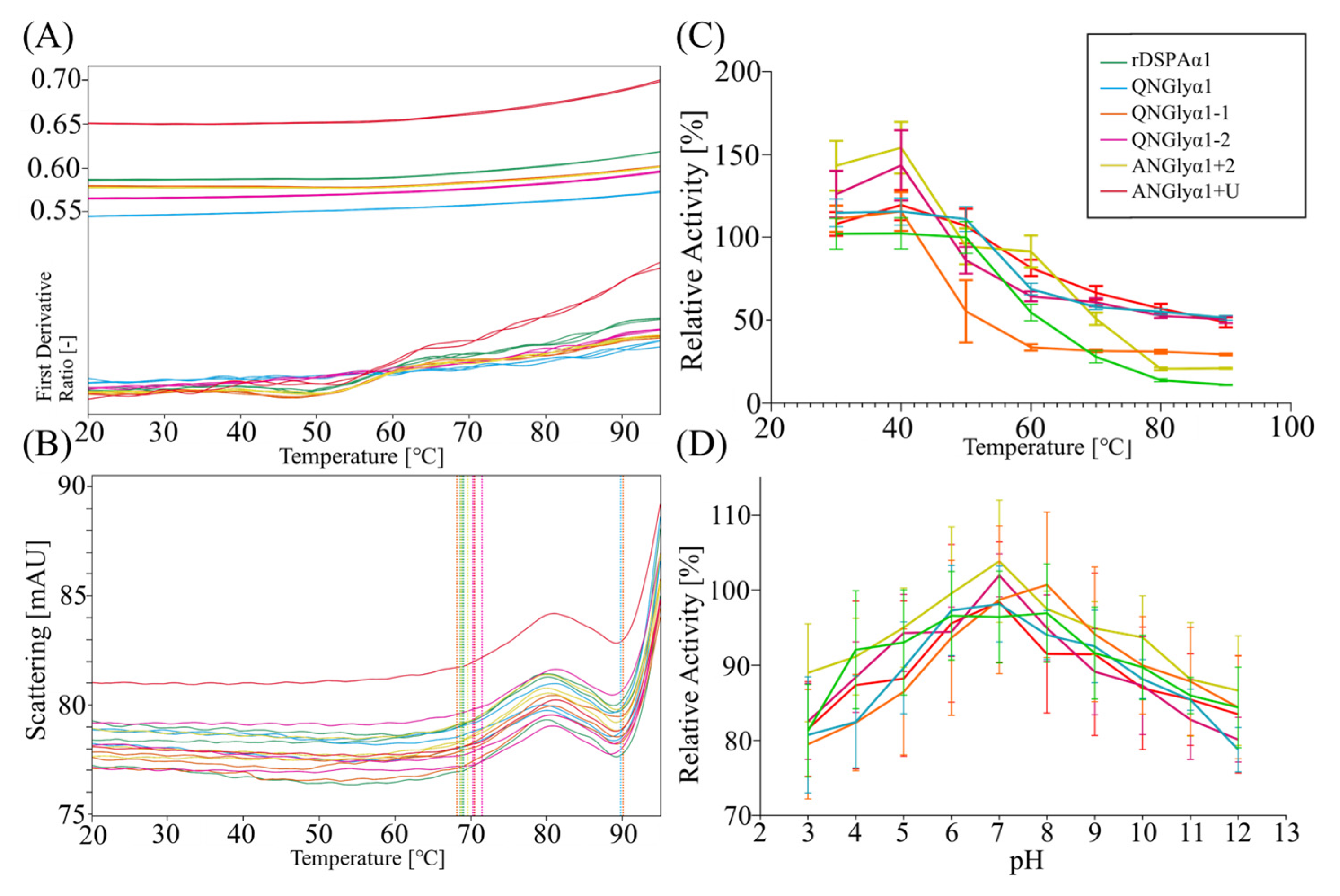
2.7. Detection of Thermal Stability and Acid-Base Stability
2.8. Rat Modelling and Drug Delivery
2.9. Thrombus Detection and Hemostatic Factors Analysis
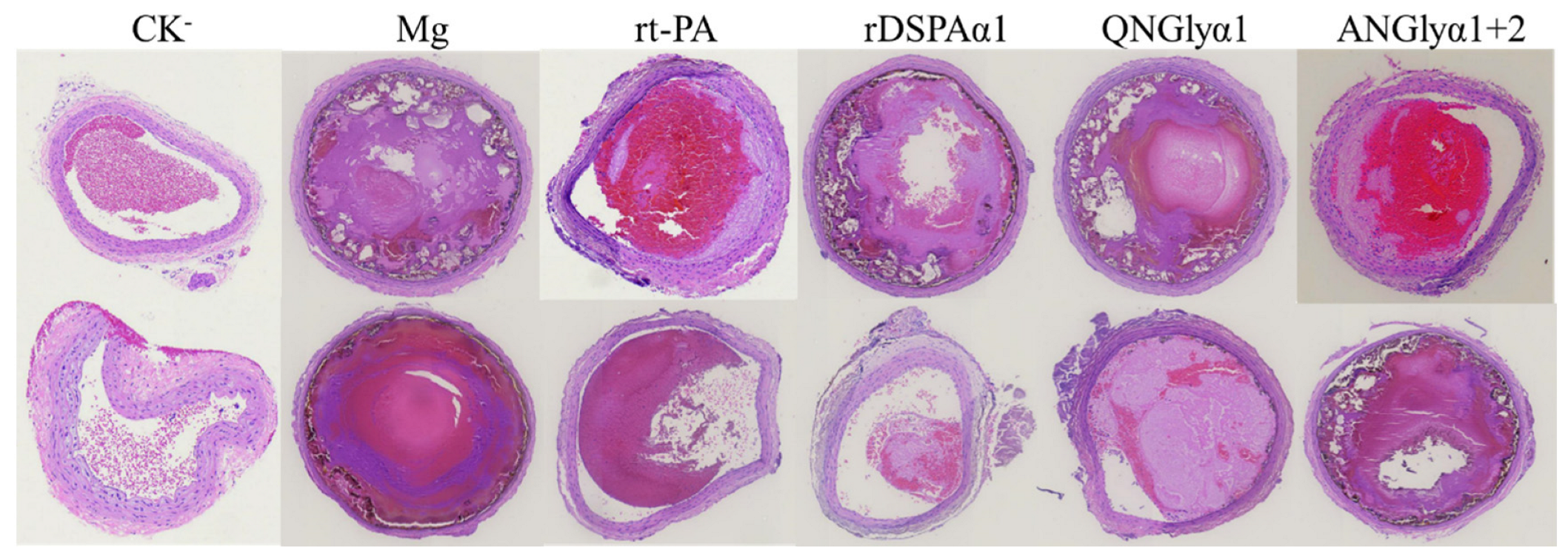
2.10. Thrombopathological Biopsy Analysis
3. Results
3.1. Prediction of N-Glycosylation Sites and Structural Predictive Analysis
3.2. Expression, Purification, and SDS-PAGE Analysis of DSPAα1 and Mutants
3.3. Effect of N-Glycosylation on Thermostability and Acid-Base Tolerance
3.4. Effect of N-Glycosylation on Fibrinolytic Activity and Fibrin Selectivity of DSPAα1 and Its Mutants In Vitro
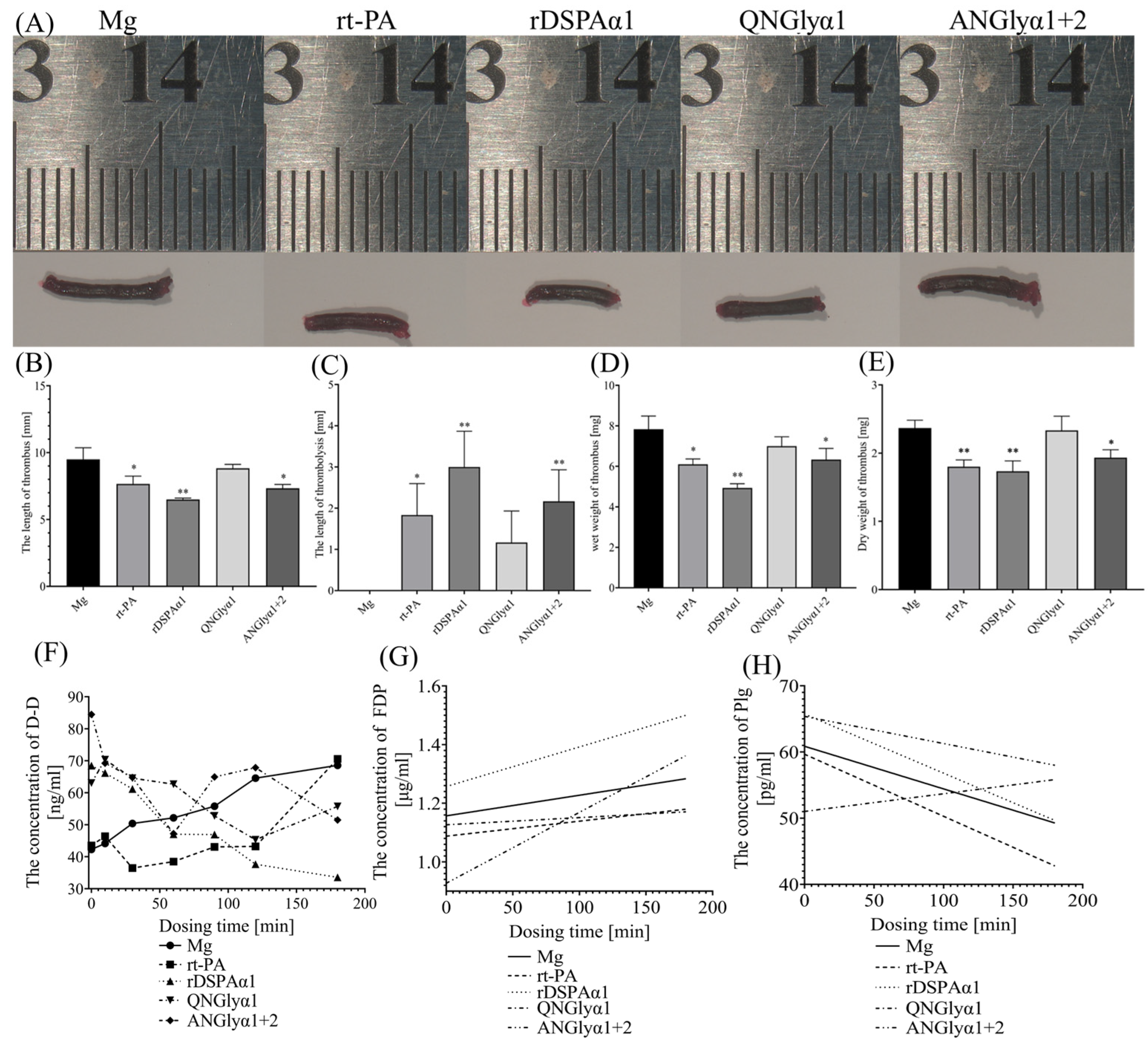
3.5. Effects of N-Glycosylation Mutations on Thrombolytic Effects of DSPAα1 and Its Mutants in Rats
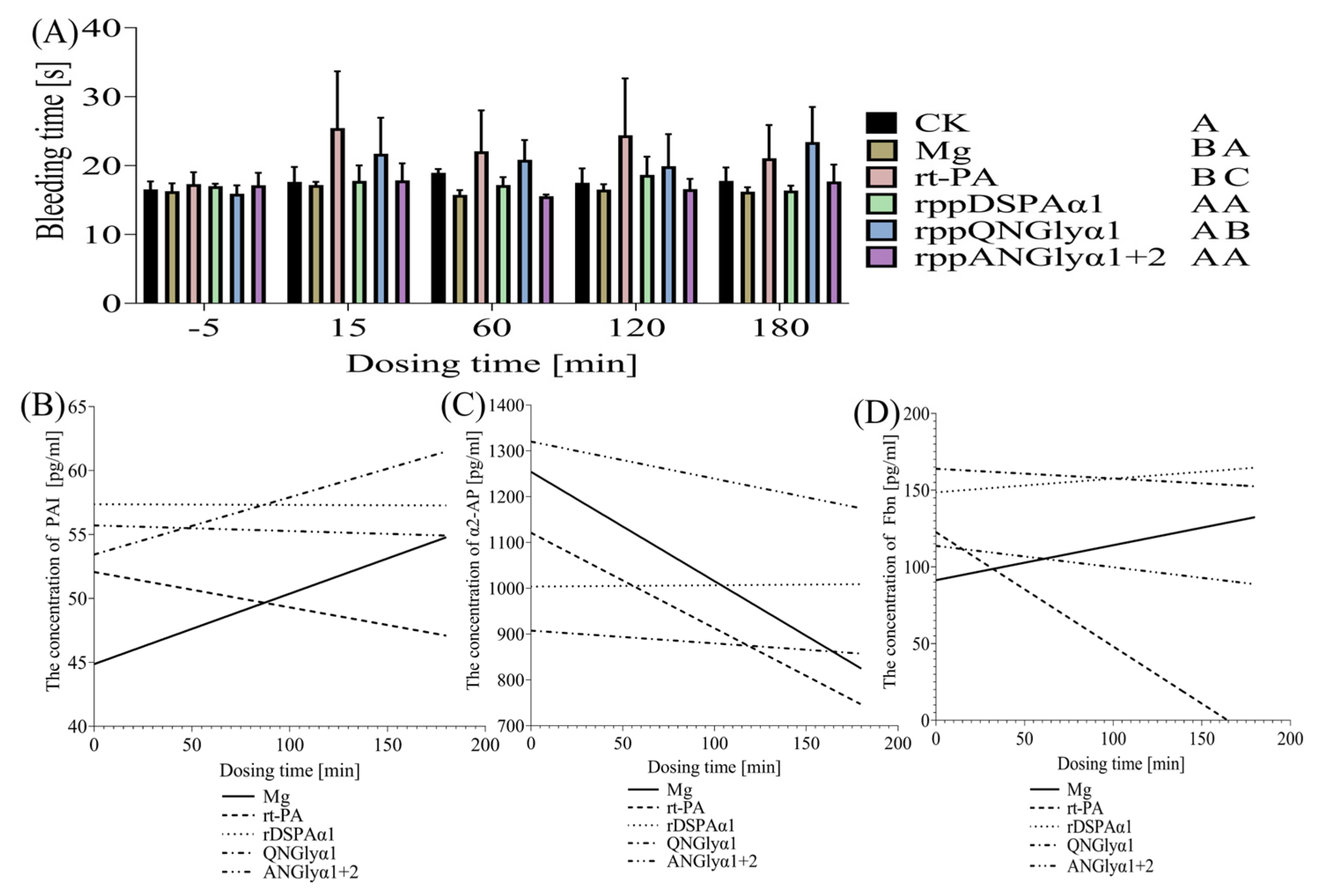
3.6. Effects of N-Glycosylation Mutations on the Thrombolysis Safety of DSPAα1 and Its Mutants in Rats
4. Discussions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Full Name |
| Å | Ångström unit, the smallest unit representing the spatial distance between two sites or atoms |
| D-D | D-dimer |
| F | Fibrin |
| Fbn | Fibrinogen |
| FDP | Fibrin/Fibrinogen Degradation Products |
| HRP | Horseradish peroxidase containing 16% glycoprotein |
| Kcat | Turnover number |
| Km | Michaelis constant |
| MPV | The mean of platelet volume |
| Plg | Plasminogen |
| PLT | The platelet count |
| PDW | The width of platelet distribution |
| PCT | Thrombocytosis |
| PAI | Plasminogen activator inhibitor |
| PNGase F | The most effective enzyme for removing N-linked glycans from glycoproteins |
| rt-PA | Alteplase |
| S-2765 | A chromogenic substrate for determination of Factor Xa activity |
| STI | Soybean Trypsin Inhibitor, unglycosylated |
| SPF | Specific pathogen-free |
| t-PA | Tissue plasminogen activator |
| u-PA | Urokinase-type plasminogen Activator |
| α2-AP | α2-Antiplasmin |
References
- Nadkarni, G.N.; Lala, A.; Bagiella, E.; Chang, H.L.; Moreno, P.R.; Pujadas, E.; Arvind, V.; Bose, S.; Charney, A.W.; Chen, M.D.; et al. Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 1815–1826. [Google Scholar] [CrossRef]
- Whyte, C.S.; Morrow, G.B.; Mitchell, J.L.; Chowdary, P.; Mutch, N.J. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J. Thromb. Haemost. 2020, 18, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, J.; Gao, Z.-G.; Zhang, D.; Zhu, L.; Han, G.W.; Moss, S.M.; Paoletta, S.; Kiselev, E.; Lu, W.; et al. Structure of the human P2Y12 receptor in complex with an antithrombotic drug. Nature 2014, 509, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, K.; Gao, Z.-G.; Paoletta, S.; Zhang, D.; Han, G.W.; Li, T.; Ma, L.; Zhang, W.; Müller, C.E.; et al. Agonist-bound structure of the human P2Y12 receptor. Nature 2014, 509, 119–122. [Google Scholar] [CrossRef]
- Flemmig, M.; Melzig, M.F. Serine-proteases as plasminogen activators in terms of fibrinolysis. J. Pharm. Pharmacol. 2012, 64, 1025–1039. [Google Scholar] [CrossRef]
- Ma, D.; Mizurini, D.M.; Assumpção, T.C.F.; Li, Y.; Qi, Y.; Kotsyfakis, M.; Ribeiro, J.; Monteiro, R.; Francischetti, I.M.B. Desmolaris, a novel factor XIa anticoagulant from the salivary gland of the vampire bat (Desmodus rotundus) inhibits inflammation and thrombosis in vivo. Blood 2013, 122, 4094–4106. [Google Scholar] [CrossRef] [PubMed]
- Carey, H.V.; Martin, S.L.; Horwitz, B.A.; Yan, L.; Bailey, S.M.; Podrabsky, J.; Storz, J.F.; Ortiz, R.M.; Wong, R.P.; Lathrop, D.A. Elucidating nature’s solutions to heart, lung, and blood diseases and sleep disorders. Circ. Res. 2012, 110, 915–921. [Google Scholar] [CrossRef]
- Debens, T. Technology evaluation: Desmoteplase, PAION/Forest. Curr. Opin. Mol. Ther. 2004, 6, 567–575. [Google Scholar]
- Gulba, D.C.; Praus, M.; Witt, W. DSPA alpha—Properties of the plasminogen activators of the vampire bat Desmodus rotundus. Fibrinolysis 1995, 9, 91–96. [Google Scholar] [CrossRef]
- Elmaraezy, A.; Abushouk, A.I.; Saad, S.; Eltoomy, M.; Mahmoud, O.; Hassan, H.M.; Aboelmakarem, A.; Fotoh, A.A.; Althaher, F.; Huy, N.T.; et al. Desmoteplase for Acute Ischemic Stroke: A Systematic Review and Metaanalysis of Randomized Controlled Trials. CNS Neurol. Disord. Drug Targets 2017, 16, 789–799. [Google Scholar] [CrossRef]
- Krätzschmar, J.; Haendler, B.; Langer, G.; Boidol, W.; Bringmann, P.; Alagon, A.; Donner, P.; Schleuning, W.-D. The plasminogen activator family from the salivary gland of the vampire bat Desmodus rotundus: Cloning and expression. Gene 1991, 105, 229–237. [Google Scholar] [CrossRef]
- Bringmann, P.; Gruber, D.; Liese, A.; Toschi, L.; Krätzschmar, J.; Schleuning, W.-D.; Donner, P. Structural features mediating fibrin selectivity of vampire bat plasminogen activators. J. Biol. Chem. 1995, 270, 25596–25603. [Google Scholar] [CrossRef]
- Liberatore, G.T.; Samson, A.; Bladin, C.; Schleuning, W.-D.; Medcalf, R.L. Vampire bat salivary plasminogen activator (desmoteplase): A unique fibrinolytic enzyme that does not promote neurodegeneration. Stroke 2003, 34, 537–543. [Google Scholar] [CrossRef]
- Gardell, S.J.; Duong, L.T.; Diehl, R.E.; York, J.D.; Hare, T.R.; Register, R.B.; Jacobs, J.W.; Dixon, R.A.; Friedman, P.A. Isolation, characterization, and cDNA cloning of a vampire bat salivary plasminogen activator. J. Biol. Chem. 1989, 264, 17947–17952. [Google Scholar] [CrossRef]
- Bergum, P.W.; Gardell, S.J. Vampire bat salivary plasminogen activator exhibits a strict and fastidious requirement for polymeric fibrin as its cofactor, unlike human tissue-type plasminogen activator. A kinetic analysis. J. Biol. Chem. 1992, 267, 17726–17731. [Google Scholar] [CrossRef]
- Gardell, S.J.; Hare, T.R.; Bergum, P.W.; Cuca, G.C.; O’Neill-Palladino, L.; Zavodny, S.M. Vampire bat salivary plasminogen activator is quiescent in human plasma in the absence of fibrin unlike human tissue plasminogen activator. Blood 1990, 76, 2560–2564. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, R.M.A.A.J.F. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: Therapeutic strategies. CNS Neurol. Disord. Drug Targets 2008, 7, 243–253. [Google Scholar] [CrossRef]
- Montoney, M.; Gardell, S.J.; Marder, V.J. Comparison of the bleeding potential of vampire bat salivary plasminogen activator versus tissue plasminogen activator in an experimental rabbit model. Circulation 1995, 91, 1540–1544. [Google Scholar] [CrossRef]
- Mellott, M.J.; Stabilito, I.I.; Holahan, M.A.; Cuca, G.C.; Wang, S.; Li, P.; Barrett, J.S.; Lynch, J.J.; Gardell, S.J. Vampire bat salivary plasminogen activator promotes rapid and sustained reperfusion without concomitant systemic plasminogen activation in a canine model of arterial thrombosis. Arter. Thromb. A J. Vasc. Biol. 1992, 12, 212–221. [Google Scholar] [CrossRef][Green Version]
- Piechowski-Jozwiak, B.; Bogousslavsky, J. Stroke and patent foramen ovale in young individuals. Eur. Neurol. 2013, 69, 108–117. [Google Scholar] [CrossRef]
- Wild, R.; Kowal, J.; Eyring, J.; Ngwa, E.M.; Aebi, M.; Locher, K.P. Structure of the yeast oligosaccharyltransferase complex gives insight into eukaryotic N-glycosylation. Science 2018, 359, 545–550. [Google Scholar] [CrossRef]
- Zuber, C.; Roth, J. N-glycosylation; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Sinclair, A.M.; Elliott, S. Glycoengineering: The effect of glycosylation on the properties of therapeutic proteins. J. Pharm. Sci. 2005, 94, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-H. Protein glycosylation: New challenges and opportunities. J. Org. Chem. 2005, 70, 4219–4225. [Google Scholar] [CrossRef] [PubMed]
- Dingermann, T.; Dingermann, T. Recombinant therapeutic proteins: Production platforms and challenges. Biotechnol. J. 2008, 3, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Girija, U.V.; Furze, C.M.; Gingras, A.R.; Yoshizaki, T.; Ohtani, K.; Marshall, J.E.; Wallis, A.K.; Schwaeble, W.J.; El-Mezgueldi, M.; Mitchell, D.A.; et al. Molecular basis of sugar recognition by collectin-K1 and the effects of mutations associated with 3MC syndrome. BMC Biol. 2015, 13, 27. [Google Scholar] [CrossRef]
- Jiang, B.; Argyros, R.; Bukowski, J.; Nelson, S.; Sharkey, N.; Kim, S.; Copeland, V.; Davidson, R.C.; Chen, R.; Zhuang, J.; et al. Inactivation of a GAL4-like transcription factor improves cell fitness and product yield in glycoengineered Pichia pastoris strains. Appl. Environ. Microbiol. 2015, 81, 260–271. [Google Scholar] [CrossRef]
- Han, Y.; Lei, X.G. Role of glycosylation in the functional expression of an Aspergillus niger phytase (phyA) in Pichia pastoris. Arch. Biochem. Biophys. 1999, 364, 83–90. [Google Scholar] [CrossRef]
- Peng, H.; Wang, M.; Wang, N.; Yang, C.; Guo, W.; Li, G.; Huang, S.; Wei, D.; Liu, D. Different N-Glycosylation Sites Reduce the Activity of Recombinant DSPAα2. Curr. Issues Mol. Biol. 2022, 44, 3930–3947. [Google Scholar] [CrossRef]
- Krätzschmar, J.; Haendler, B.; Bringmann, P.; Dinter, H.; Hess, H.; Donner, P.; Schleuning, W.-D. High-level secretion of the four salivary plasminogen activators from the vampire bat Desmodus rotundus by stably transfected baby hamster kidney cells. Gene 1992, 116, 281–284. [Google Scholar] [CrossRef]
- Petri, T.; Langer, G.; Bringmann, P.; Cashion, L.; Shallow, S.; Schleuning, W.D.; Donner, P. Production of vampire bat plasminogen activator DSPA α1 in CHO and insect cells. J. Biotechnol. 1995, 39, 75–83. [Google Scholar] [CrossRef]
- Liu, Y.; Su, C.; Song, X.; Tang, Y.; Bao, Z. Construction of Pichia pastoris strain expressing salivary plasminogen activator from vampire bat (Desmodus rotundus). Sheng Wu Gong Cheng Xue Bao 2009, 25, 566–574. [Google Scholar]
- Zhang, T.; Zhou, M.; Cai, H.; Yan, K.; Zha, Y.; Zhuang, W.; Liang, J.; Cheng, Y. Identification, purification, and pharmacological activity analysis of Desmodus rotundus salivary plasminogen activator alpha1 (DSPAα1) expressed in transgenic rabbit mammary glands. Transgenic Res. 2022, 31, 149–163. [Google Scholar] [CrossRef]
- Gohlke, M.; Nuck, R.; Kannicht, C.; Grunow, D.; Baude, G.; Donner, P.; Reutter, W. Analysis of site-specific N-glycosylation of recombinant Desmodus rotundus salivary plasminogen activator rDSPA alpha 1 expressed in Chinese hamster ovary cells. Glycobiology 1997, 7, 67–77. [Google Scholar] [CrossRef]
- Renatus, M.; Stubbs, M.T.; Huber, R.; Bringmann, P.; Donner, P.; Schleuning, W.-D.; Bode, W. Catalytic domain structure of vampire bat plasminogen activator: A molecular paradigm for proteolysis without activation cleavage. Biochemistry 1997, 36, 13483–13493. [Google Scholar] [CrossRef]
- Schrödinger, L.; DeLano, W. PyMOL. 2020. Available online: http://www.pymol.org/pymol (accessed on 13 September 2022).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Prasad, S.; Kashyap, R.S.; Deopujari, J.Y.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb. J. 2006, 4, 14. [Google Scholar] [CrossRef]
- Michaelis, L.; Menten, M.L. Die kinetik der invert in wirkung. Biochem. Z 1913, 49, 352. [Google Scholar]
- Kanda, H.; Ling, J.; Tonomura, S.; Noguchi, K.; Matalon, S.; Gu, J.G. TREK-1 and TRAAK Are Principal K+ Channels at the Nodes of Ranvier for Rapid Action Potential Conduction on Mammalian Myelinated Afferent Nerves. Neuron 2019, 104, 960–971.e7. [Google Scholar] [CrossRef]
- Yan, W.; Wu, X.; Zhou, W.; Fong, M.Y.; Cao, M.; Liu, J.; Liu, X.; Chen, C.-H.; Fadare, O.; Pizzo, D.P.; et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nature 2018, 20, 597–609. [Google Scholar] [CrossRef]
- Gardell, S.J.; Ramjit, D.R.; Stabilito, I.I.; Fujita, T.; Lynch, J.J.; Cuca, G.C.; Jain, D.; Wang, S.P.; Tung, J.S.; Mark, G.E. Effective thrombolysis without marked plasminemia after bolus intravenous administration of vampire bat salivary plasminogen activator in rabbits. Circulation 1991, 84, 244–253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krauss, I.R.; Merlino, A.; Randazzo, A.; Novellino, E.; Mazzarella, L.; Sica, F. High-resolution structures of two complexes between thrombin and thrombin-binding aptamer shed light on the role of cations in the aptamer inhibitory activity. Nucleic Acids Res. 2012, 40, 8119–8128. [Google Scholar] [CrossRef]
- Tekin, M.; Chioza, B.A.; Matsumoto, Y.; Diaz-Horta, O.; Cross, H.E.; Duman, D.; Kokotas, H.; Moore-Barton, H.L.; Sakoori, K.; Ota, M.; et al. SLITRK6 mutations cause myopia and deafness in humans and mice. J. Clin. Investig. 2013, 123, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, E.; Manzano, R.G.; Cottage, A.; Hawker, K.; Aparicio, S. Evolution of plasminogen-related growth factors (HGF/SF and HGF1/MSP). Ciba Found Symp. 1997, 212, 24–35; discussion 35–45. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Du, W.W.; Lyu, J.; Dong, J.; Zhang, C.; Yang, W.; He, A.; Kwok, Y.S.S.; Ma, J.; Wu, N.; et al. Enhanced breast cancer progression by mutant p53 is inhibited by the circular RNA circ-Ccnb1. Cell Death Differ. 2018, 25, 2195–2208. [Google Scholar] [CrossRef]
- Tsujikawa, M.; Okabayashi, K.; Morita, M.; Tanabe, T. Secretion of a variant of human single-chain urokinase-type plasminogen activator without an N-glycosylation site in the methylotrophic yeast, Pichia pastoris and characterization of the secreted product. Yeast 1996, 12, 541–553. [Google Scholar] [CrossRef]
- Boleij, M.; Pabst, M.; Neu, T.R.; van Loosdrecht, M.C.M.; Lin, Y. Identification of Glycoproteins Isolated from Extracellular Polymeric Substances of Full-Scale Anammox Granular Sludge. Environ. Sci. Technol. 2018, 52, 13127–13135. [Google Scholar] [CrossRef]
- Levine, P.M.; Balana, A.T.; Sturchler, E.; Koole, C.; Noda, H.; Zarzycka, B.; Daley, E.J.; Truong, T.T.; Katritch, V.; Gardella, T.J.; et al. O-GlcNAc Engineering of GPCR Peptide-Agonists Improves Their Stability and in Vivo Activity. J. Am. Chem. Soc. 2019, 141, 14210–14219. [Google Scholar] [CrossRef]
- Boulaftali, Y.; Ho-Tin-Noe, B.; Pena, A.; Loyau, S.; Venisse, L.; François, D.; Richard, B.; Arocas, V.; Collet, J.-P.; Jandrot-Perrus, M.; et al. platelet protease nexin-1, a serpin that strongly influences fibrinolysis and thrombolysis. Circulation 2011, 123, 1326–1334. [Google Scholar] [CrossRef]
- Beyene, G.; Chauhan, R.D.; Ilyas, M.; Wagaba, H.; Fauquet, C.M.; Miano, D.; Alicai, T.; Taylor, N.J. A Virus-Derived Stacked RNAi Construct Confers Robust Resistance to Cassava Brown Streak Disease. Front. Plant Sci. 2017, 7, 2052. [Google Scholar] [CrossRef]
- Payne, H.; Ponomaryov, T.; Watson, S.P.; Brill, A. Mice with a deficiency in CLEC-2 are protected against deep vein thrombosis. Blood 2017, 129, 2013–2020. [Google Scholar] [CrossRef]
- Kahler, Z.P.; Kline, J.A. Standardizing the D-dimer Assay: Proposing the d-dimer International Managed Ratio. Clin. Chem. 2015, 61, 776–778. [Google Scholar] [CrossRef]
- Yamakawa, K.; Ogura, H.; Fujimi, S.; Morikawa, M.; Ogawa, Y.; Mohri, T.; Nakamori, Y.; Inoue, Y.; Kuwagata, Y.; Tanaka, H.; et al. Recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: A multicenter propensity score analysis. Intensiv. Care Med. 2013, 39, 644–652. [Google Scholar] [CrossRef]
- Larsen, C.C.; Kligman, F.; Tang, C.; Kottke-Marchant, K.; Marchant, R.E. A biomimetic peptide fluorosurfactant polymer for endothelialization of ePTFE with limited platelet adhesion. Biomaterials 2007, 28, 3537–3548. [Google Scholar] [CrossRef]
- Wolf, D.; Hohmann, J.-D.; Wiedemann, A.; Bledzka, K.; Blankenbach, H.; Marchini, T.; Gutte, K.; Zeschky, K.; Bassler, N.; Hoppe, N.; et al. Binding of CD40L to Mac-1’s I-domain involves the EQLKKSKTL motif and mediates leukocyte recruitment and atherosclerosis—But does not affect immunity and thrombosis in mice. Circ. Res. 2011, 109, 1269–1279. [Google Scholar] [CrossRef]
- French, D.; Hamilton, L.H.; Mattano, L.A.; Sather, H.N.; Devidas, M.; Nachman, J.B.; Relling, M.V. A PAI-1 (SERPINE1) polymorphism predicts osteonecrosis in children with acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood 2008, 111, 4496–4499. [Google Scholar] [CrossRef]
- Singh, S.; Houng, A.K.; Reed, G.L. Venous stasis-induced fibrinolysis prevents thrombosis in mice: Role of α2-antiplasmin. Blood 2019, 134, 970–978. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Tolstrup, J.S.; Jakobsen, M.U.; Heitmann, B.L.; Grønbæk, M.; O’Reilly, E.; Bälter, K.; Goldbourt, U.; Hallmans, G.; Knekt, P.; et al. Alcohol intake and risk of coronary heart disease in younger, middle-aged, and older adults. Circulation 2010, 121, 1589–1597. [Google Scholar] [CrossRef]
- Price, J.L.; Shental-Bechor, D.; Dhar, A.; Turner, M.J.; Powers, E.T.; Gruebele, M.; Levy, Y.; Kelly, J.W. Context-dependent effects of asparagine glycosylation on Pin WW folding kinetics and thermodynamics. J. Am. Chem. Soc. 2010, 132, 15359–15367. [Google Scholar] [CrossRef]
- Morant, M.; Jørgensen, K.; Schaller, H.; Pinot, F.; Møller, B.L.; Werck-Reichhart, D.; Bak, S. CYP703 is an ancient cytochrome P450 in land plants catalyzing in-chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen. Plant Cell 2007, 19, 1473–1487. [Google Scholar] [CrossRef]
- Jacobs, P.P.; Geysens, S.; Vervecken, W.; Contreras, R.; Callewaert, N. Engineering complex-type N-glycosylation in Pichia pastoris using GlycoSwitch technology. Nat. Protoc. 2009, 4, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery—New applications on the horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef] [PubMed]




| Administration | Before | After | |||||
|---|---|---|---|---|---|---|---|
| Sampling time (min) | −5.000 | 10.000 | 30.000 | 60.000 | 90.000 | 120.000 | 180.000 |
| blood volume (μL) | 300.000 | 300.000 | 300.000 | 300.000 | 300.000 | 300.000 | 300.000 |
| TBT (min) | −5.000 | 10.000 | 30.000 | 60.000 | 90.000 | 120.000 | 180.000 |
| Enzyme | Motifs | Modification | Mutation Sites | N-Glycosylation Sites |
|---|---|---|---|---|
| rDSPAα1 | N153-N184(A186)-K368-N398 | / | / | N153-N398 |
| QNGlyα1 | Q153-N184(A186)-K368-Q398 | Deletion | N153Q N398Q | / |
| QNGlyα1-1 | Q153-N184(A186)-K368-N398 | Deletion | N153Q | N398 |
| QNGlyα1-2 | N153-N184(A186)-K368-Q398 | Deletion | N398Q | N153 |
| ANGlyα1 + 2 | N153-N184(T186)-K368-N398 | Addition | A186T | N153-N184-N398 |
| ANGlyα1 + U | N153-N184(A186)-N368-N398 | Addition | K368N | N153-N368-N398 |
| Enzyme | Cofactor | Kcat ×103 | Km mM | Kcat/Km mM−1S−1 | Stimulation Factor | Ratio Fbn/Fbg |
|---|---|---|---|---|---|---|
| rDSPAα1 | None | 0.094 ± 0.001 | 2.709 ± 0.155 | 34.830 ± 2.350 | 1.000 | |
| Fbg | 0.097 ± 0.000 | 0.343 ± 0.015 | 282.240 ± 13.070 | 8.100 | ||
| Fbn | 0.259 ± 0.024 | 0.045 ± 0.010 | 5883.440 ± 983.570 | 168.900 | 20.800 | |
| QNGlyα1 | None | 0.084 ± 0.004 | 1.380 ± 0.257 | 62.240 ± 10.690 | 1.000 | |
| Fbg | 0.118 ± 0.012 | 0.369 ± 0.077 | 328.740 ± 62.750 | 5.300 | ||
| Fbn | 0.165 ± 0.017 | 0.763 ± 0.085 | 216.420 ± 5.840 | 3.500 | 0.700 | |
| QNGlyα1-1 | None | 0.088 ± 0.001 | 2.258 ± 0.046 | 38.800 ± 0.780 | 1.000 | |
| Fbg | 0.144 ± 0.019 | 0.493 ± 0.005 | 292.580 ± 1.780 | 7.500 | ||
| Fbn | 0.149 ± 0.001 | 0.448 ± 0.030 | 333.760 ± 20.680 | 8.600 | 1.100 | |
| QNGlyα1-2 | None | 0.138 ± 0.008 | 1.764 ± 0.111 | 78.240 ± 0.600 | 1.000 | |
| Fbg | 0.126 ± 0.004 | 1.320 ± 0.033 | 95.230 ± 3.970 | 1.200 | ||
| Fbn | 0.124 ± 0.008 | 0.153 ± 0.016 | 812.780 ± 54.960 | 10.400 | 8.500 | |
| ANGlyα1 + 2 | None | 0.089 ± 0.001 | 3.688 ± 0.318 | 24.280 ± 1.990 | 1.000 | |
| Fbg | 0.144 ± 0.003 | 0.577 ± 0.024 | 248.960 ± 6.330 | 10.300 | ||
| Fbn | 0.168 ± 0.027 | 0.052 ± 0.005 | 3257.040 ± 561.250 | 134.100 | 13.100 | |
| ANGlyα1 + U | None | 0.082 ± 0.002 | 0.674 ± 0.067 | 122.880 ± 13.680 | 1.000 | |
| Fbg | 0.096 ± 0.005 | 0.453 ± 0.129 | 221.760 ± 60.490 | 1.800 | ||
| Fbn | 0.097 ± 0.001 | 0.168 ± 0.028 | 590.130 ± 98.900 | 4.800 | 2.700 |
| Samples | P1 | P2 | P3 | P4 | P5 | P6 | Total Score |
|---|---|---|---|---|---|---|---|
| CK | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| Mg | 20.000 ± 0.000 | 20.000 ± 0.000 | 19.375 ± 0.354 | 19.375 ± 0.354 | 18.750 ± 0.463 | 16.875 ± 0.744 | 114.375 ± 0.835 |
| rt-PA | 15.833 ± 1.329 | 16.667 ± 1.211 | 12.500 ± 0.837 | 14.167 ± 0.753 | 10.833 ± 1.169 | 20.000 ± 0.000 | 90.000 ± 2.366 |
| rDSPAα1 | 9.167 ± 1.169 | 14.167 ± 0.983 | 7.500 ± 0.548 | 7.500 ± 0.548 | 5.000 ± 0.000 | 18.333 ± 0.516 | 61.667 ± 1.966 |
| QNGlyα1 | 15.833 ± 0.753 | 20.000 ± 0.000 | 13.333 ± 0.516 | 17.500 ± 0.547 | 20.000 ± 0.000 | 16.667 ± 0.816 | 103.333 ± 0.816 |
| ANGlyα1 + 2 | 16.667 ± 0.816 | 20.000 ± 0.000 | 14.167 ± 0.753 | 15.000 ± 0.632 | 18.333 ± 0.516 | 8.333 ± 0.816 | 92.500 ± 1.049 |
| PLT (109/L) | MPV | PDW (%) | PCT | p Value | ||
|---|---|---|---|---|---|---|
| CK | No dosing | 67.000 ± 7.211 | 9.400 ± 0.265 | 14.167 ± 0.462 | 0.062 ± 0.005 | |
| 15 min after dosing | 65.000 ± 1.732 | 9.633 ± 0.058 | 13.833 ± 0.306 | 0.062 ± 0.001 | p > 0.05 | |
| 1 h after dosing | 60.333 ± 4.163 | 9.700 ± 0.265 | 14.133 ± 0.643 | 0.062 ± 0.005 | p > 0.05 | |
| Mg | No dosing | 830.667 ± 37.072 | 7.700 ± 0.520 | 8.933 ± 0.666 | 0.640 ± 0.016 | |
| 15 min after dosing | 826.333 ± 50.143 | 7.600 ± 0.300 | 8.833 ± 0.551 | 0.628 ± 0.021 | p > 0.05 | |
| 1 h after dosing | 746.000 ± 42.673 | 7.600 ± 0.265 | 8.800 ± 0.520 | 0.567 ± 0.008 | p < 0.005 | |
| rt-PA | No dosing | 872.667 ± 105.945 | 6.833 ± 0.058 | 7.567 ± 0.231 | 0.594 ± 0.078 | |
| 15 min after dosing | 716.333 ± 106.931 | 7.133 ± 0.153 | 7.767 ± 0.404 | 0.512 ± 0.066 | p > 0.05 | |
| 1 h after dosing | 576.333 ± 42.595 | 6.933 ± 0.416 | 7.900 ± 0.721 | 0.401 ± 0.052 | p < 0.005 | |
| rDSPAα1 | No dosing | 728.333 ± 73.173 | 7.367 ± 0.153 | 8.333 ± 0.231 | 0.537 ± 0.065 | |
| 15 min after dosing | 585.667 ± 71.654 | 7.133 ± 0.231 | 8.133 ± 0.451 | 0.417 ± 0.038 | p < 0.0001 | |
| 1 h after dosing | 724.333 ± 50.817 | 7.000 ± 0.100 | 7.867 ± 0.289 | 0.506 ± 0.028 | p > 0.05 | |
| QNGlyα1 | No dosing | 860.667 ± 25.146 | 6.567 ± 0.115 | 7.300 ± 0.000 | 0.563 ± 0.028 | |
| 15 min after dosing | 772.667 ± 54.775 | 7.333 ± 0.289 | 8.167 ± 0.751 | 0.547 ± 0.067 | p > 0.05 | |
| 1 h after dosing | 498.000 ± 21.284 | 6.900 ± 0.265 | 7.867 ± 0.493 | 0.357 ± 0.031 | p < 0.005 | |
| ANGlyα1 + 2 | No dosing | 768.000 ± 120.104 | 6.967 ± 0.503 | 7.900 ± 0.721 | 0.532 ± 0.048 | |
| 15 min after dosing | 572.333 ± 18.502 | 7.100 ± 0.436 | 7.900 ± 0.889 | 0.405 ± 0.014 | p > 0.05 | |
| 1 h after dosing | 712.667 ± 16.010 | 7.033 ± 0.643 | 7.900 ± 0.889 | 0.502 ± 0.055 | p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Wang, N.; Wang, M.; Yang, C.; Guo, W.; Li, G.; Huang, S.; Wei, D.; Liu, D. Comparison of Activity and Safety of DSPAα1 and Its N-Glycosylation Mutants. Life 2023, 13, 985. https://doi.org/10.3390/life13040985
Peng H, Wang N, Wang M, Yang C, Guo W, Li G, Huang S, Wei D, Liu D. Comparison of Activity and Safety of DSPAα1 and Its N-Glycosylation Mutants. Life. 2023; 13(4):985. https://doi.org/10.3390/life13040985
Chicago/Turabian StylePeng, Huakang, Nan Wang, Mengqi Wang, Caifeng Yang, Wenfang Guo, Gangqiang Li, Sumei Huang, Di Wei, and Dehu Liu. 2023. "Comparison of Activity and Safety of DSPAα1 and Its N-Glycosylation Mutants" Life 13, no. 4: 985. https://doi.org/10.3390/life13040985
APA StylePeng, H., Wang, N., Wang, M., Yang, C., Guo, W., Li, G., Huang, S., Wei, D., & Liu, D. (2023). Comparison of Activity and Safety of DSPAα1 and Its N-Glycosylation Mutants. Life, 13(4), 985. https://doi.org/10.3390/life13040985






